Abstract
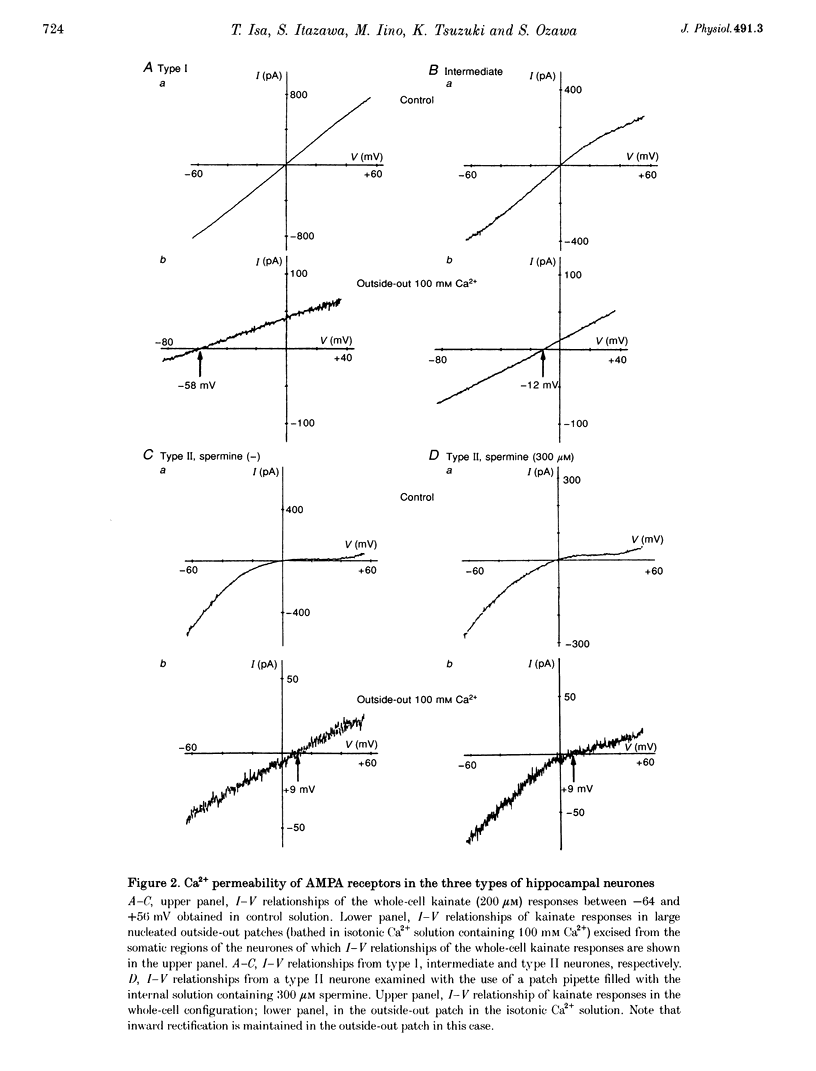
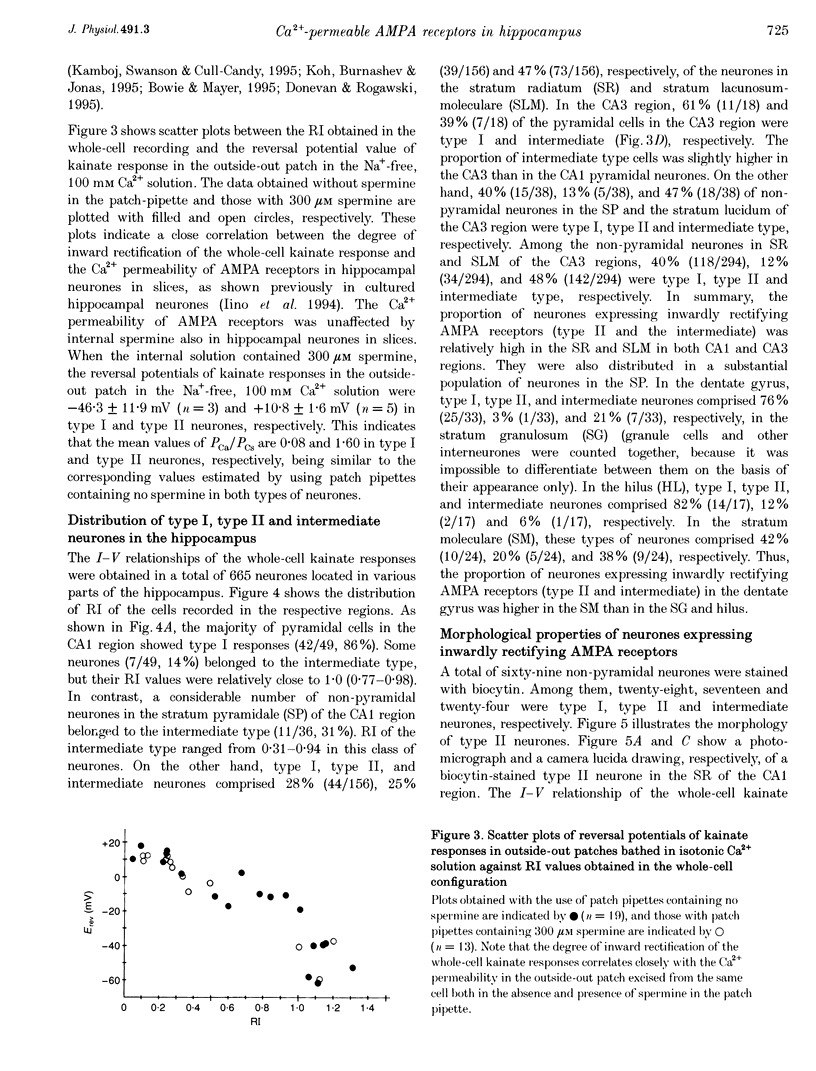
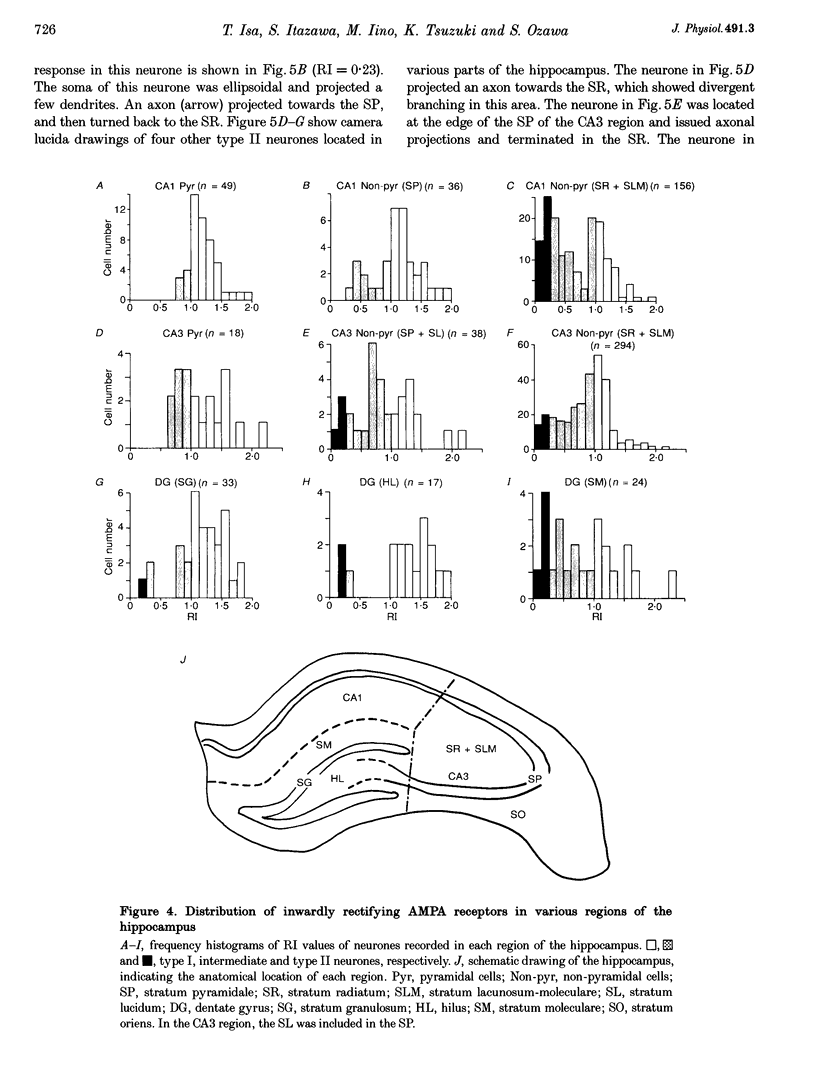
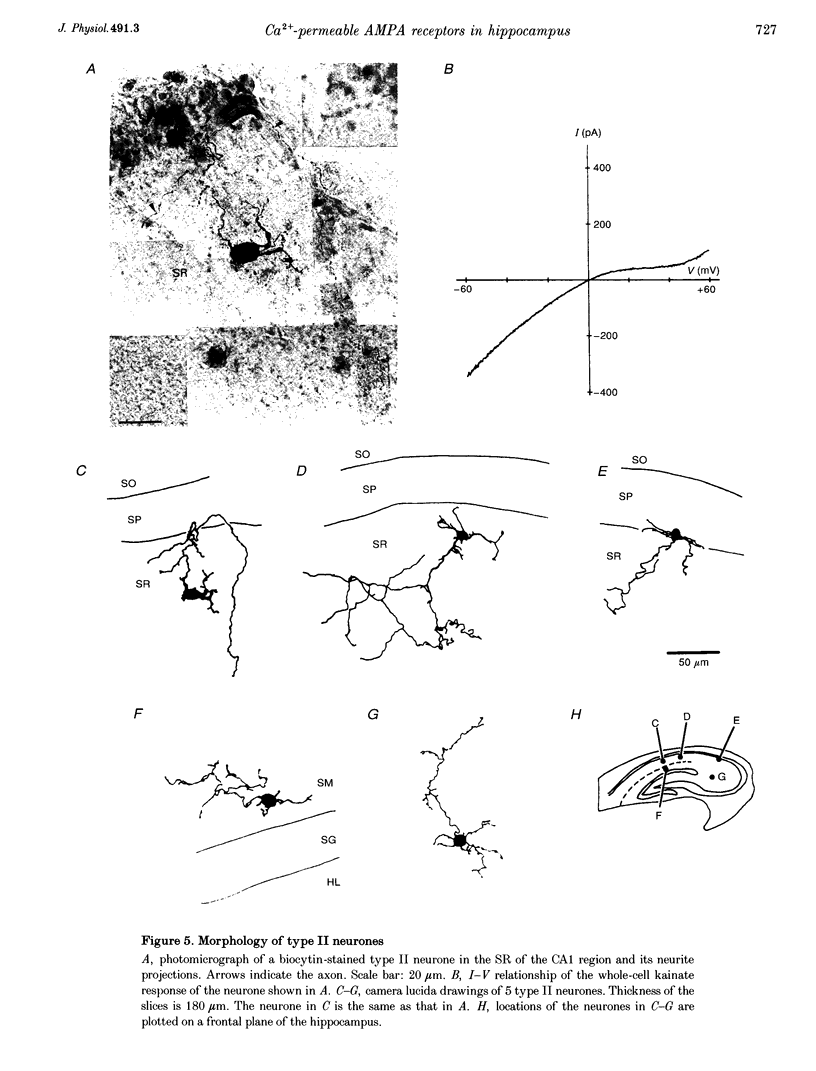
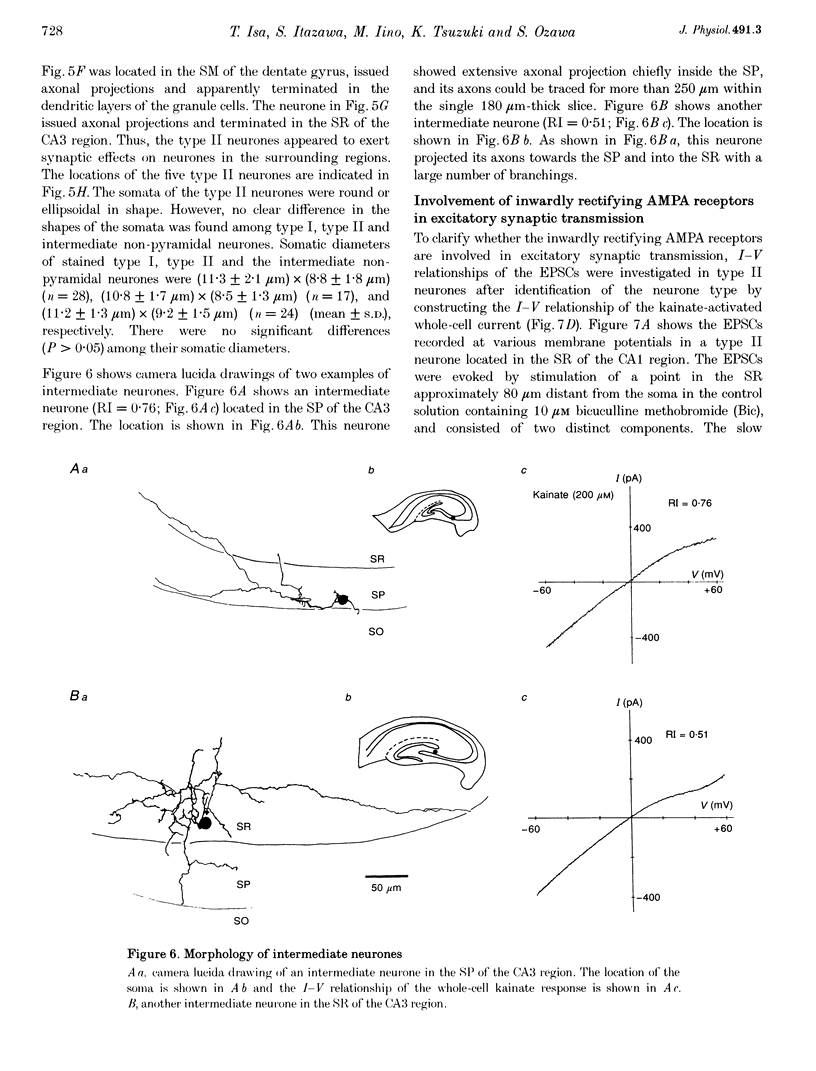
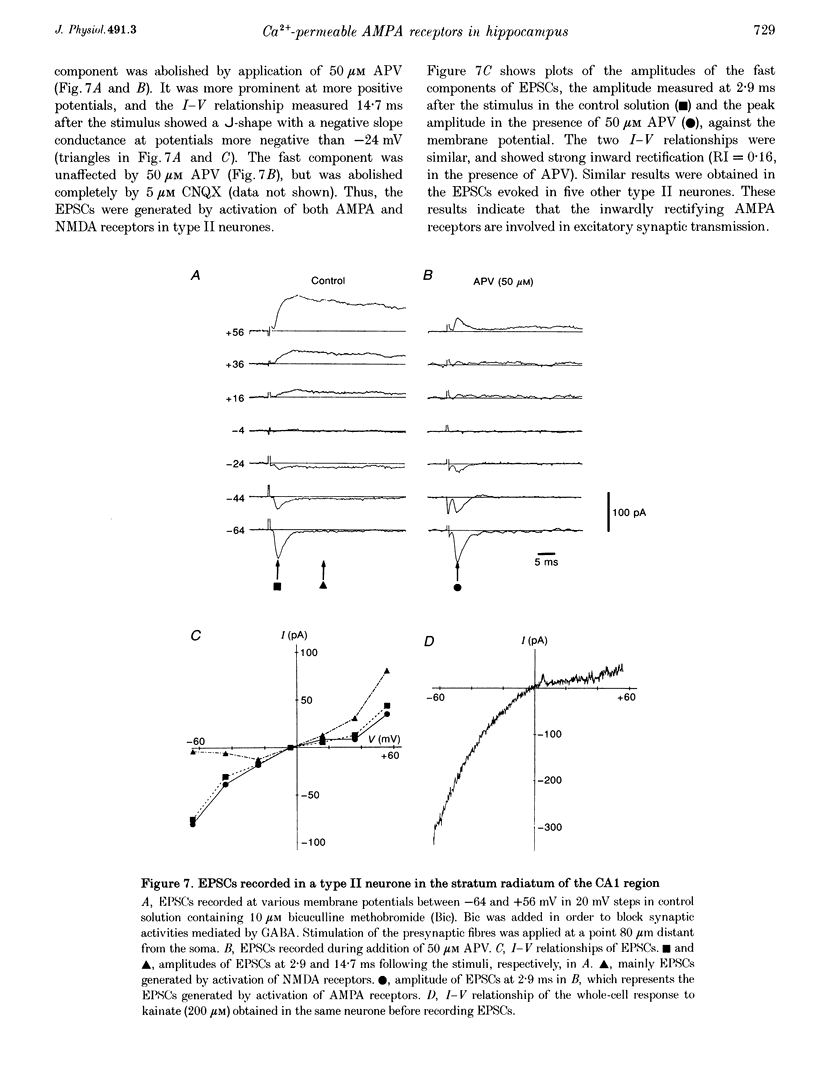
1. Current-voltage (I-V) relationships and Ca2+ permeability of receptor channels activated by bath application of kainate, a non-desensitizing agonist of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, were examined in various types of neurones in hippocampal slices of 5- to 13-day-old rats by using the tight-seal patch clamp recording technique. 2. Three types of responses were observed: type I response with outwardly rectifying I-V relationship, type II response with I-V relationship of marked inward rectification, and intermediate response with I-V relationship of weaker inward rectification. Neurones with type I, type II and intermediate I-V relationships of kainate responses were referred to as type I, type II and intermediate neurones, respectively. 3. Permeability of Ca2+ ions was estimated by the reversal potential of kainate response in the outside-out patch in Na(+)-free extracellular solution containing 100 mM Ca2+. The reversal potentials were -44.4 +/- 14.0 mV (mean +/- S.D.) for type I (n = 7), +11.8 +/- 3.6 mV for type II (n = 5), and -8.7 +/- 7.4 mV for the intermediate neurones (n = 7). The values of PCa/PCs, the ratios of the permeability coefficients of Ca2+ and Cs+, estimated according to the constant-field equation were 0.08 for type I, 1.71 for type II, and 0.50 for the intermediate neurones. 4. Type II and intermediate responses were observed mainly in non-pyramidal neurones in various areas of the hippocampus, most frequently observed in the stratum molecular of the dentate gyrus and in the stratum radiatum and the stratum lacunosum-molecular of both the CA1 and CA3 regions. Both type II and intermediate neurones stained with biocytin had round- or ellipsoidal-shaped somata and issued divergent axonal projections to the surrounding structures. 5. Excitatory postsynaptic currents (EPSCs) recorded in type II neurones had 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX)-sensitive fast and D-2-amino-5-phosphonovalerate (APV)-sensitive slow components. The I-V relationship of the fast component showed a strong inward rectification, indicating that inwardly rectifying AMPA receptors are involved in excitatory synaptic transmission.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaschke M., Keller B. U., Rivosecchi R., Hollmann M., Heinemann S., Konnerth A. A single amino acid determines the subunit-specific spider toxin block of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate/kainate receptor channels. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6528–6532. doi: 10.1073/pnas.90.14.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bochet P., Audinat E., Lambolez B., Crépel F., Rossier J., Iino M., Tsuzuki K., Ozawa S. Subunit composition at the single-cell level explains functional properties of a glutamate-gated channel. Neuron. 1994 Feb;12(2):383–388. doi: 10.1016/0896-6273(94)90279-8. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Geiger J. R., Melcher T., Koh D. S., Sakmann B., Seeburg P. H., Jonas P., Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995 Jul;15(1):193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Hollmann M., Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Iino M., Mochizuki S., Ozawa S. Relationship between calcium permeability and rectification properties of AMPA receptors in cultured rat hippocampal neurons. Neurosci Lett. 1994 May 23;173(1-2):14–16. doi: 10.1016/0304-3940(94)90139-2. [DOI] [PubMed] [Google Scholar]
- Iino M., Ozawa S., Tsuzuki K. Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol. 1990 May;424:151–165. doi: 10.1113/jphysiol.1990.sp018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J., Morales M., Ibarz J. M., Somohano F. Rectification properties and Ca2+ permeability of glutamate receptor channels in hippocampal cells. Eur J Neurosci. 1994 Jul 1;6(7):1080–1088. doi: 10.1111/j.1460-9568.1994.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Malenka R. C., Kauer J. A. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990 Apr;70(2):513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Iino M., Abe M. Excitatory synapse in the rat hippocampus in tissue culture and effects of aniracetam. Neurosci Res. 1991 Oct;12(1):72–82. doi: 10.1016/0168-0102(91)90101-4. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Iino M., Tsuzuki K. Two types of kainate response in cultured rat hippocampal neurons. J Neurophysiol. 1991 Jul;66(1):2–11. doi: 10.1152/jn.1991.66.1.2. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Iino M. Two distinct types of AMPA responses in cultured rat hippocampal neurons. Neurosci Lett. 1993 Jun 11;155(2):187–190. doi: 10.1016/0304-3940(93)90704-o. [DOI] [PubMed] [Google Scholar]
- Spruston N., Jonas P., Sakmann B. Dendritic glutamate receptor channels in rat hippocampal CA3 and CA1 pyramidal neurons. J Physiol. 1995 Jan 15;482(Pt 2):325–352. doi: 10.1113/jphysiol.1995.sp020521. [DOI] [PMC free article] [PubMed] [Google Scholar]