Abstract
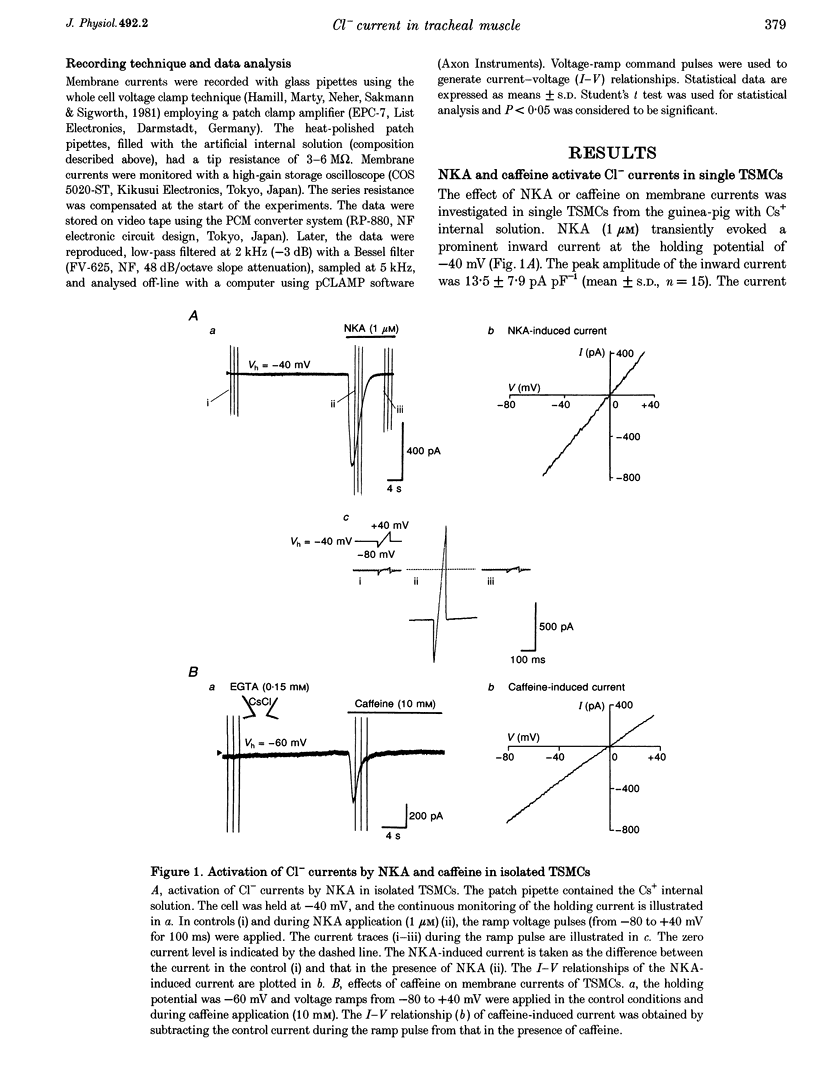
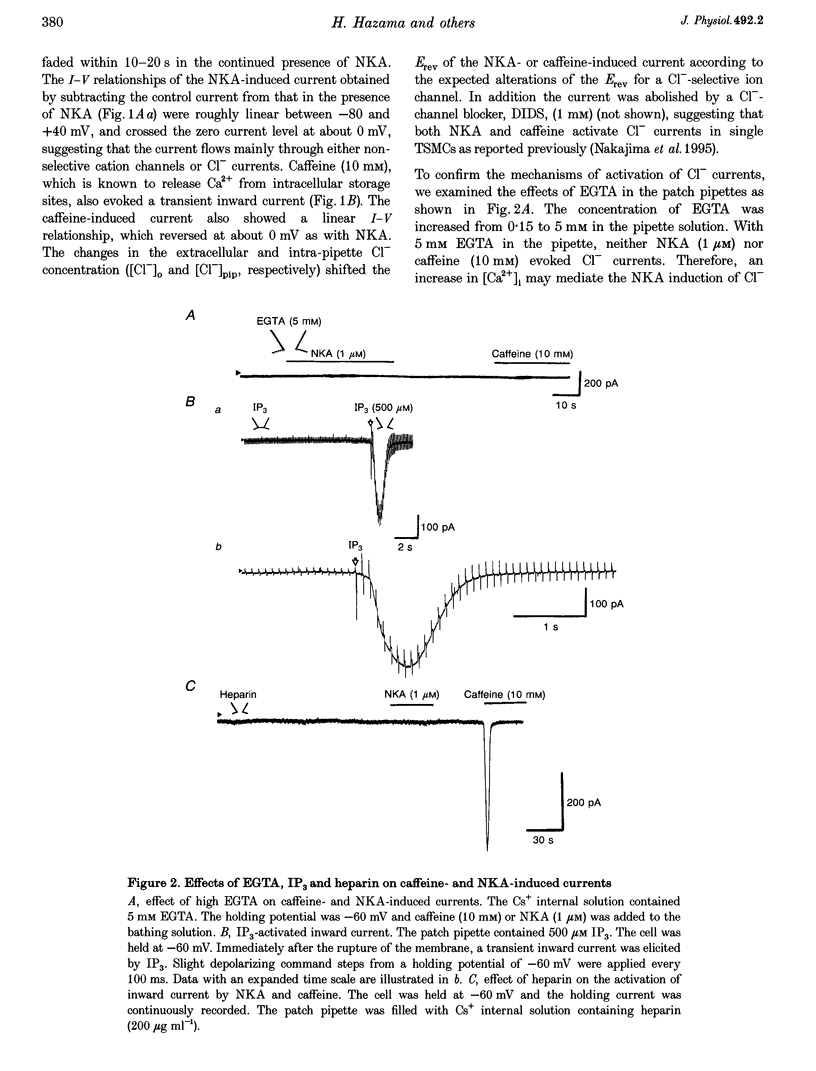
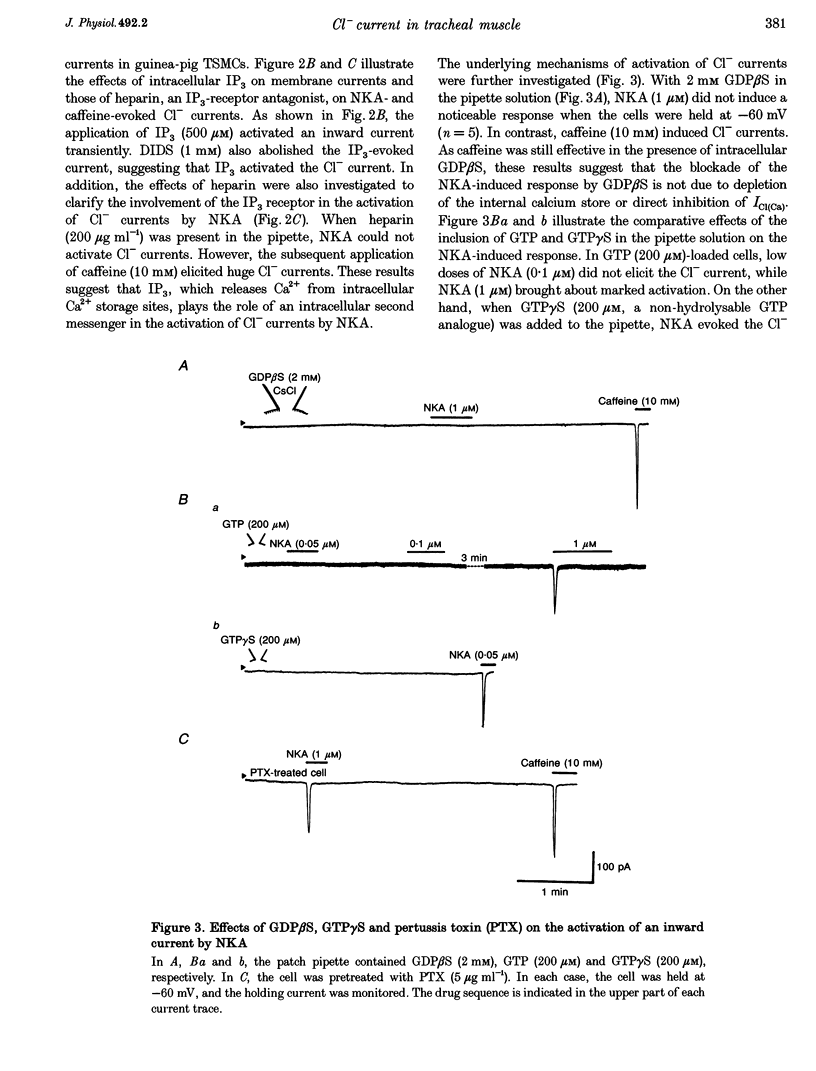
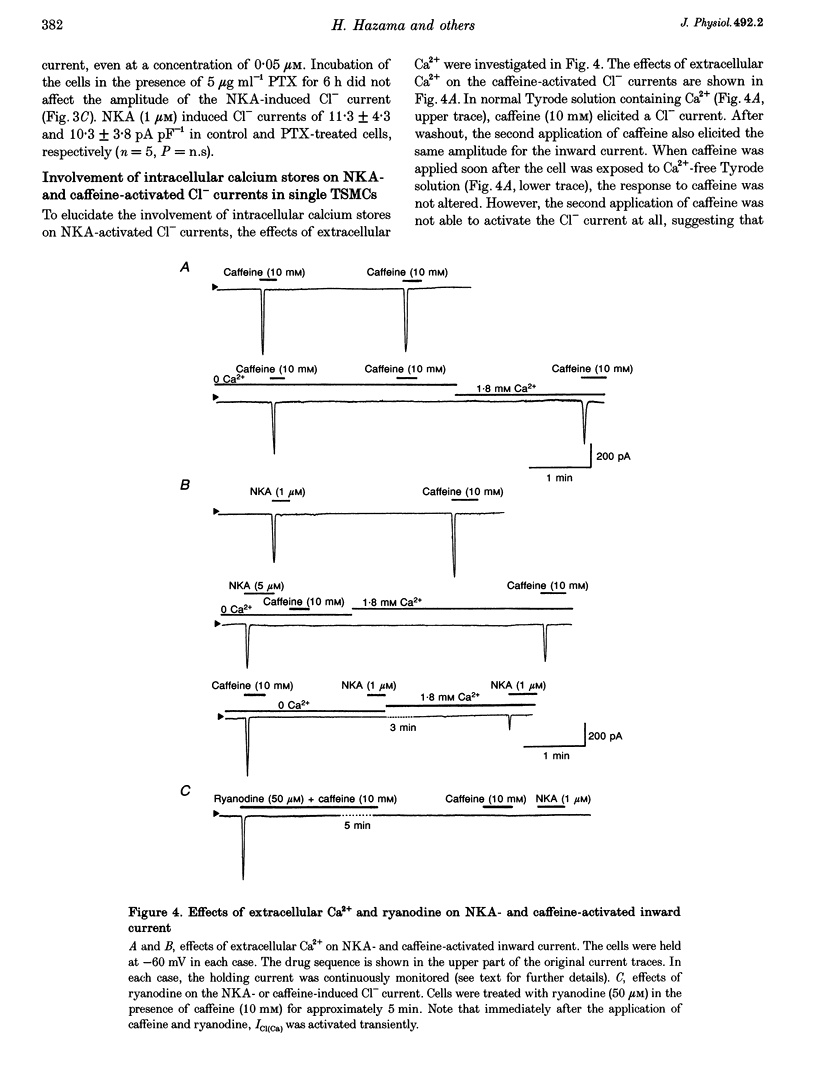
1. Membrane currents were recorded by a patch clamp technique in guinea-pig tracheal myocytes, using the whole cell mode with Cs(+) internal solution. 2. Both neurokinin A (NKA, 1 mu M) and caffeine (10 mM) evoked Ca(2+)-activated Cl- currents (I[Cl(Ca)]) transiently. In Ca(2+)-free bathing solution, the first application of NKA or caffeine elicited I[Cl(Ca)] but the second application of these substances failed to activate it. In addition, pretreatment with ryanodine in the presence of caffeine abolished the response to both NKA and caffeine whilst heparin (200 mu g ml(-1)) only blocked the NKA-induced response. I[Cl(Ca)] was also elicited by inositol 1,4,5-trisphosphate (IP(3)). 3. Command voltage pulses positive to 0 mV from a holding potential of -60 mV activated the voltage-dependent L-type Ca2+ current (I(Ca,L)) and late outward current. Upon repolarization to the holding potential, slowly decaying inward tail currents were recorded. The outward current during the depolarizing pulses and the inward tail current were enhanced by Bay K 8644, but completely blocked by Cd2+ or nifedipine. Replacement of external Ca2+ with Ba2+, removal of Ca2+ from the bath solution, or inclusion of EGTA (5 mM) in the patch pipette, also led to abolition of these currents, indicating that they were Ca2+ dependent, and that Ca2+ influx due to I(Ca,L) activated the currents. 4. When [Cl(-)](O) or [Cl(-)](i) was changed, the reversal potential (E(rev)) of the Ca2+-activated currents shifted, thus behaving like a Cl(-)-selective ion channel as predicted by the Nernst equation. DIDS (1 mM) completely abolished the currents, also suggesting that they were I[Cl(Ca)]. 5. NKA (1 mu M) and caffeine (30 mM) transiently activated I[Cl(Ca)], and after that both agents markedly reduced I[Cl(Ca)] induced by I(Ca,L). This is probably due to sarcoplasmic reticulum (SR) Ca2+ release induced by NKA or caffeine, followed by inhibition of the Ca(2+)-induced Ca2+ release from the SR. 6. The present results indicate that I[Cl(Ca)] can be activated by SR Ca2+ release due to NKA or caffeine (through IP(3) or ryanodine receptors) as well as by Ca2+ influx due to I(Ca,L). It also suggests that activation of I[Cl(Ca)] by NKA may be mediated by the production of IP(3), which releases Ca2+ from the SR.
Full text
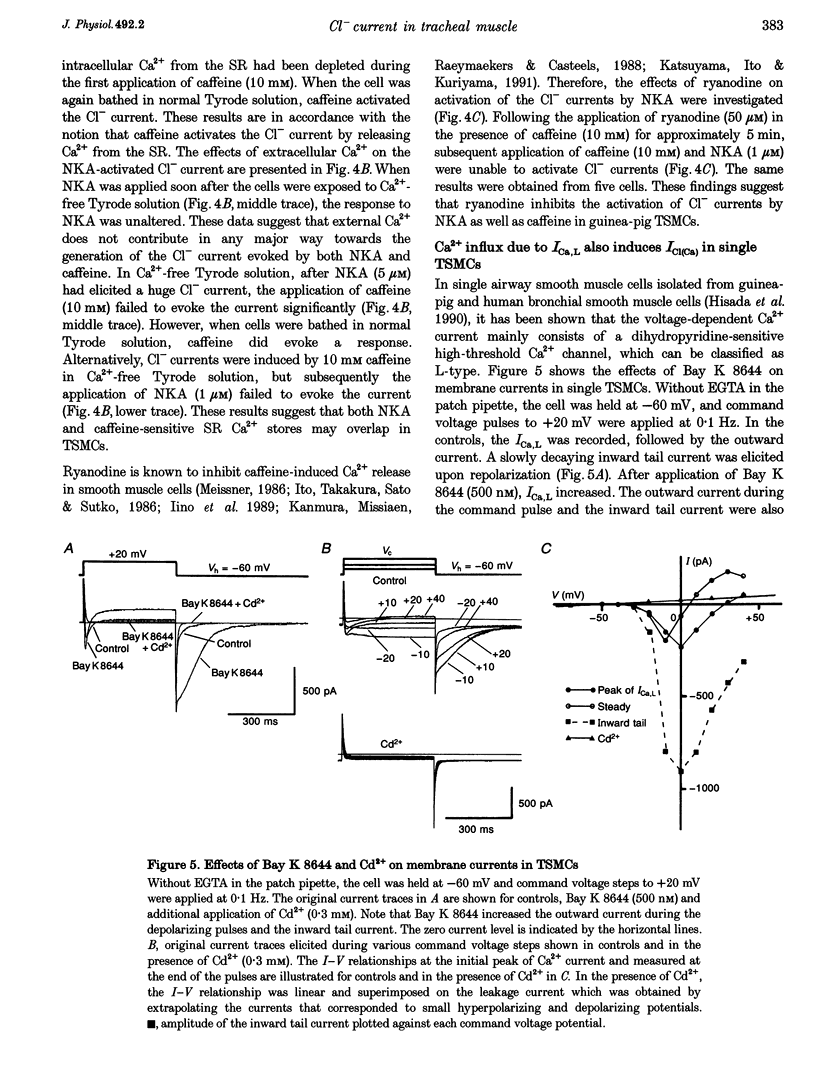
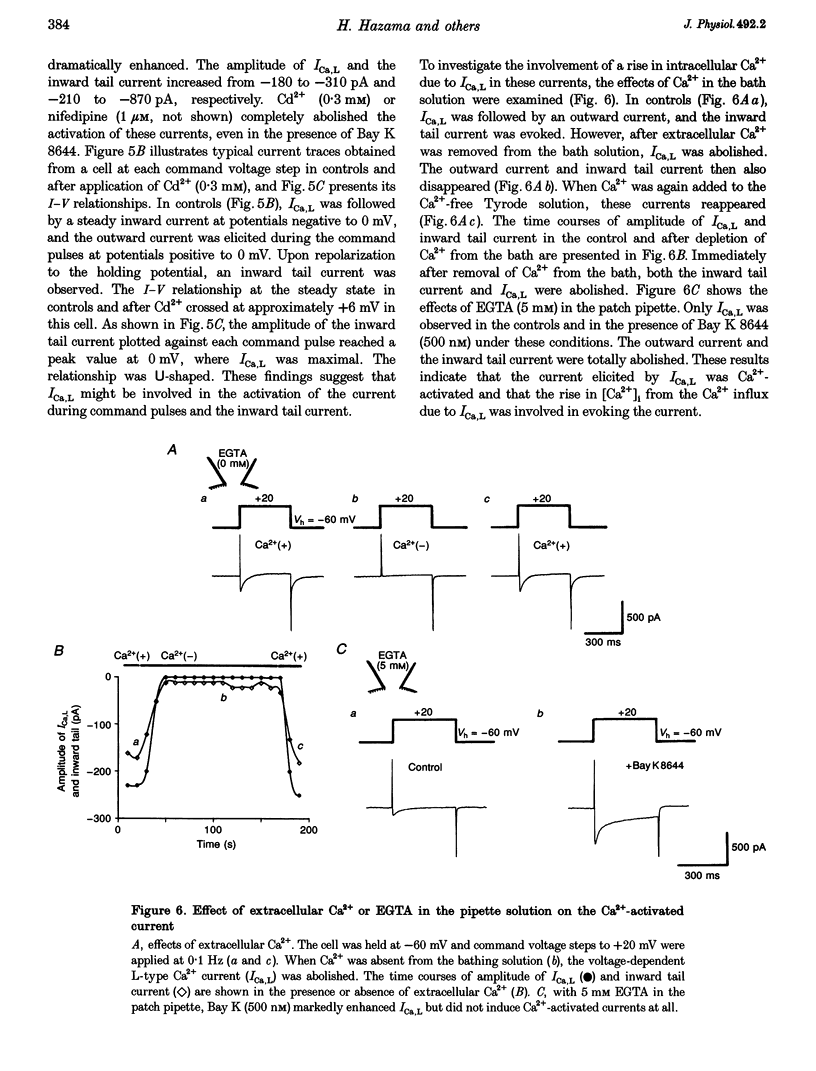
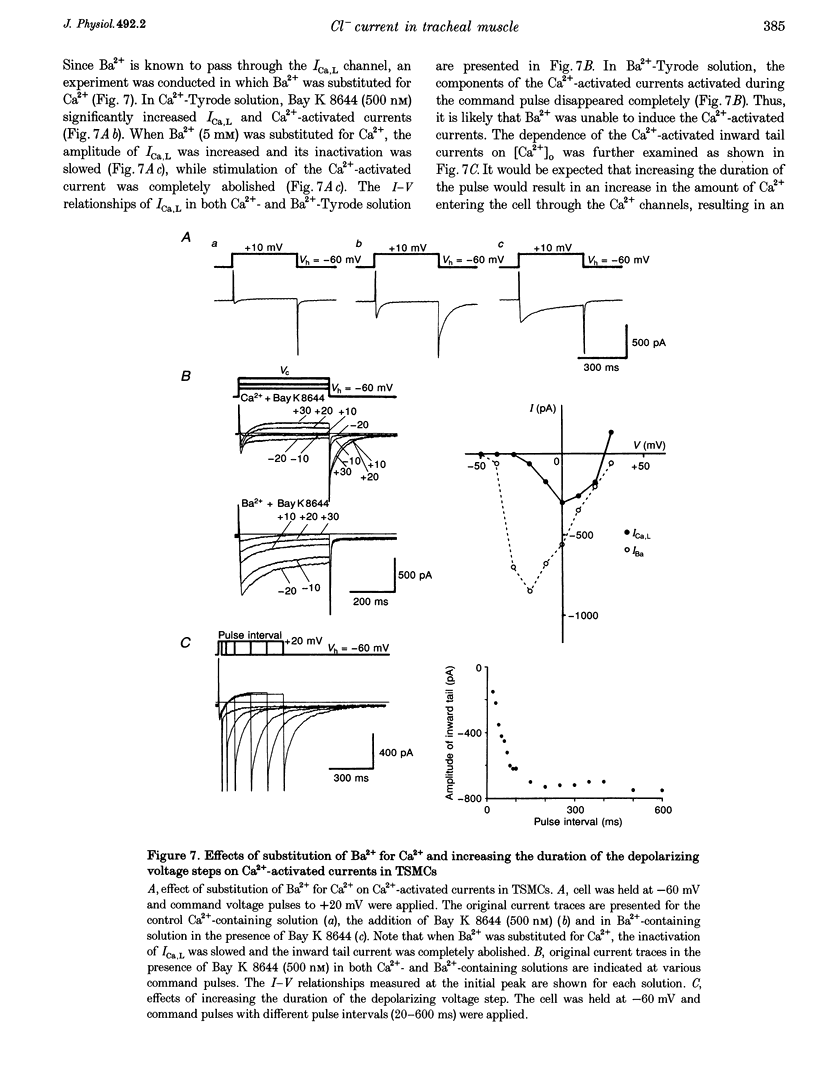
PDF

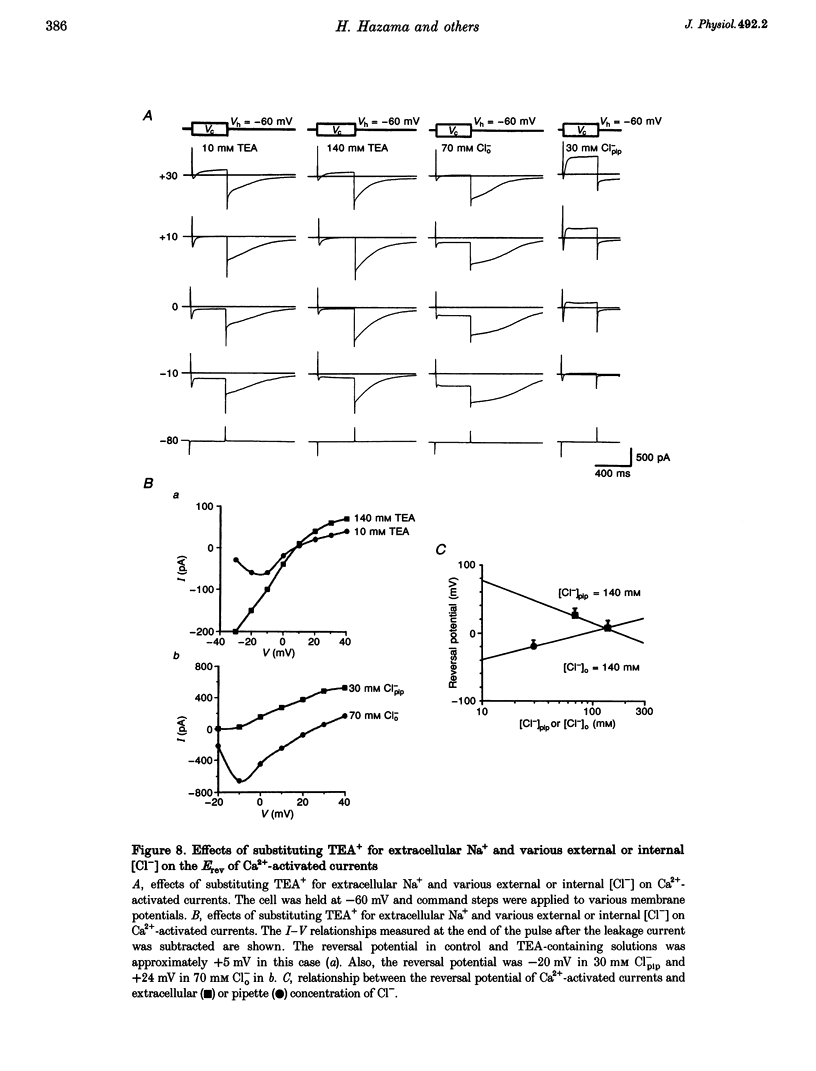
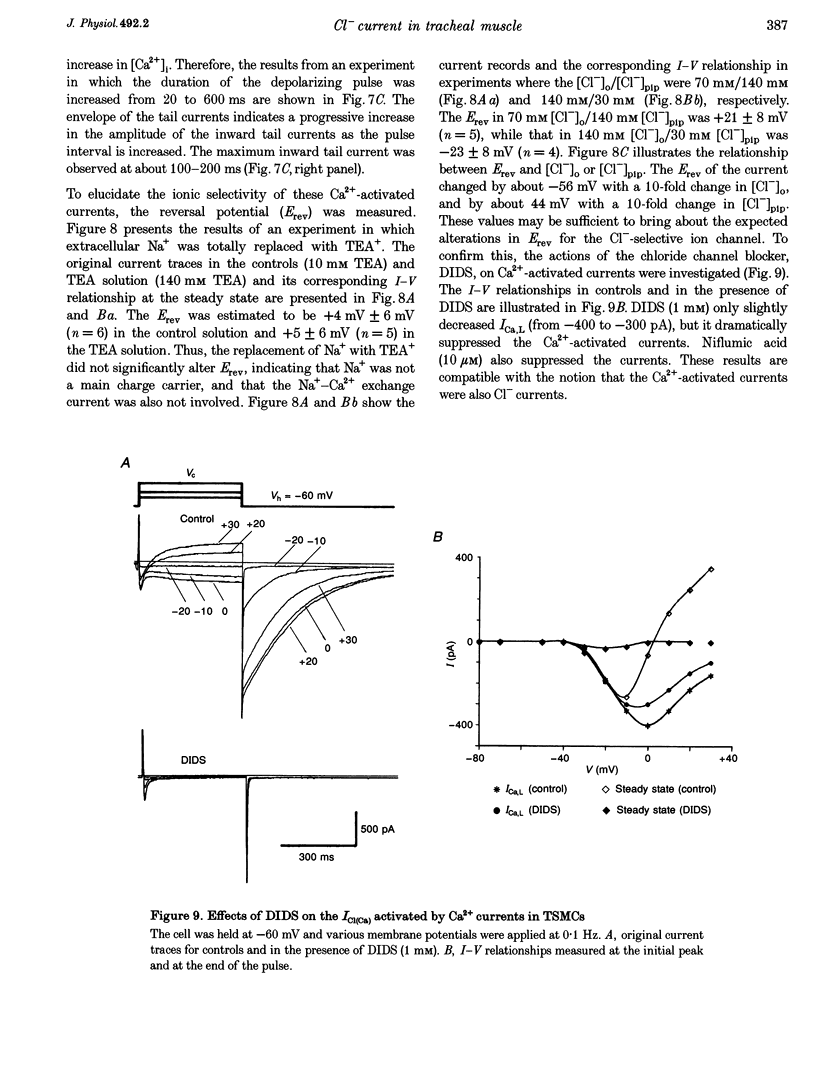
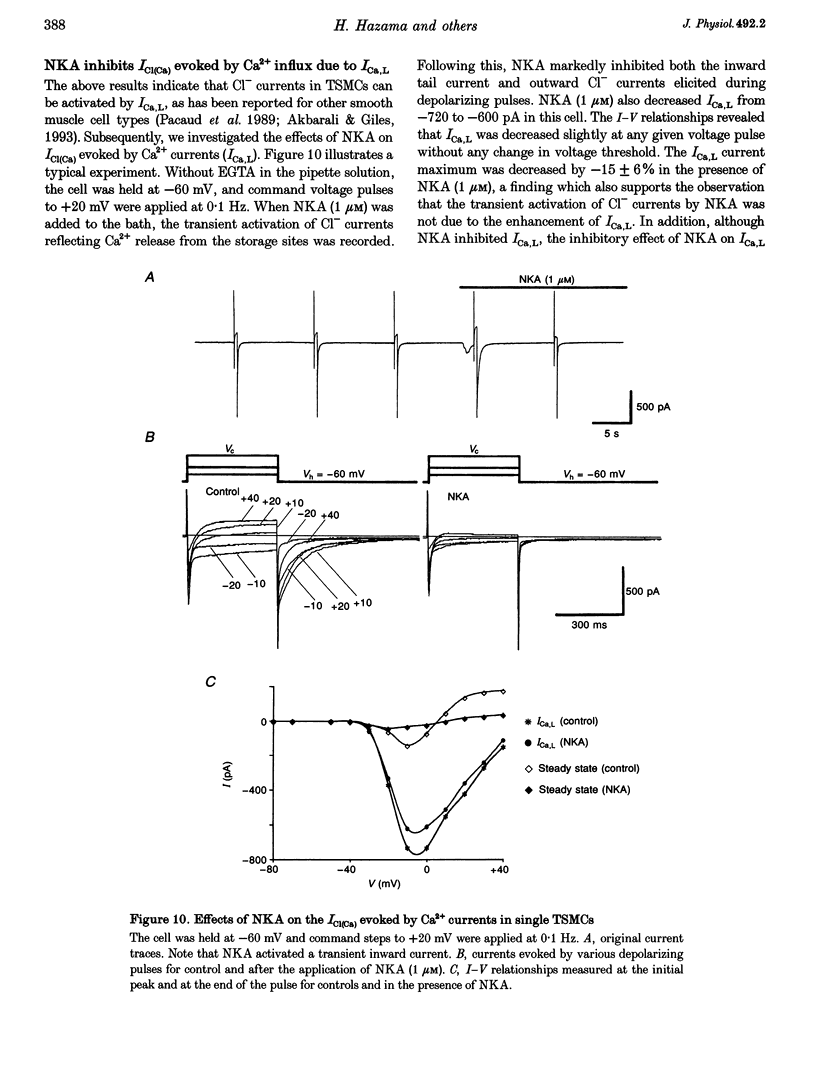
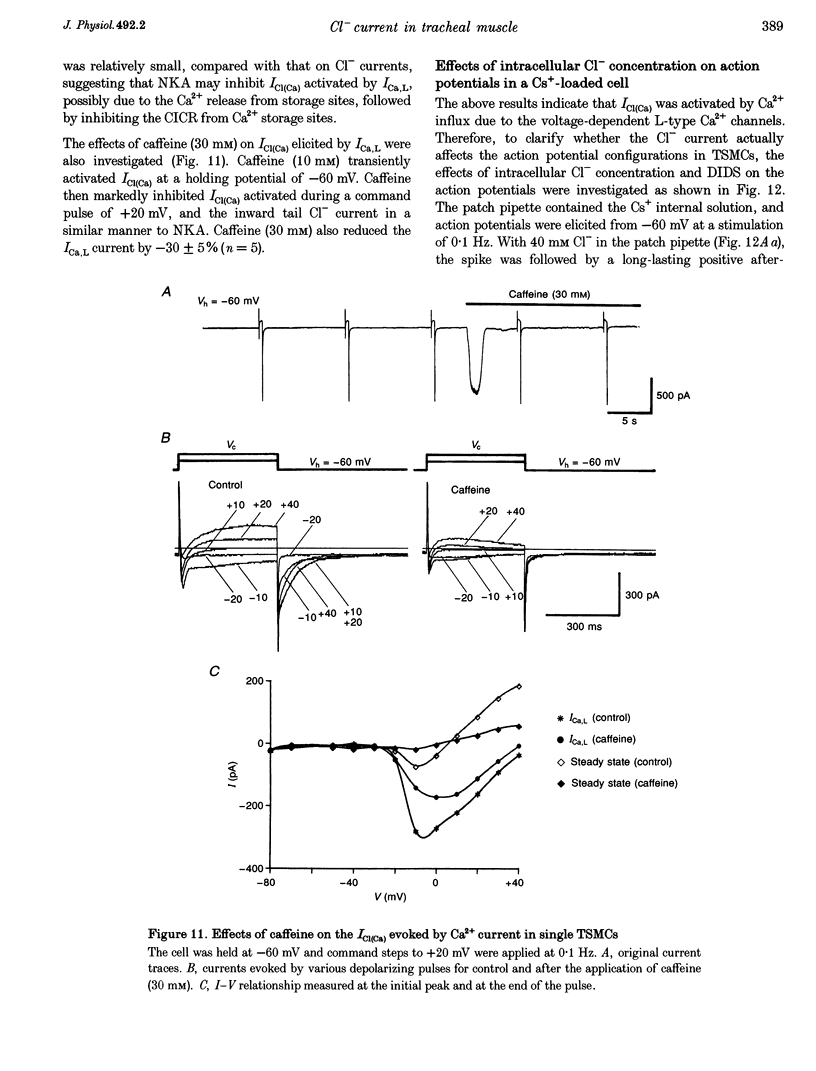
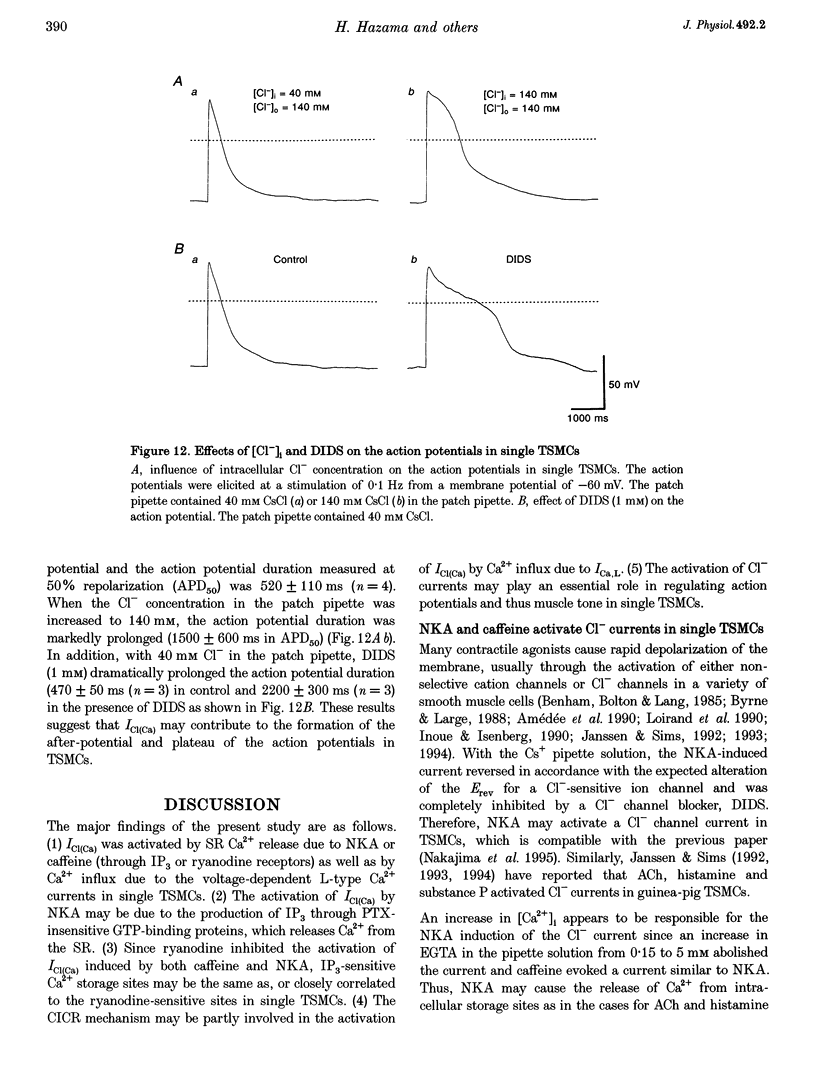



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akasu T., Nishimura T., Tokimasa T. Calcium-dependent chloride current in neurones of the rabbit pelvic parasympathetic ganglia. J Physiol. 1990 Mar;422:303–320. doi: 10.1113/jphysiol.1990.sp017985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarali H. I., Giles W. R. Ca2+ and Ca(2+)-activated Cl- currents in rabbit oesophageal smooth muscle. J Physiol. 1993 Jan;460:117–133. doi: 10.1113/jphysiol.1993.sp019462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amédée T., Large W. A., Wang Q. Characteristics of chloride currents activated by noradrenaline in rabbit ear artery cells. J Physiol. 1990 Sep;428:501–516. doi: 10.1113/jphysiol.1990.sp018224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. J., Chung K. F., Page C. P. Inflammatory mediators and asthma. Pharmacol Rev. 1988 Mar;40(1):49–84. [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985 Jul 25;316(6026):345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- Bristow D. R., Curtis N. R., Suman-Chauhan N., Watling K. J., Williams B. J. Effects of tachykinins on inositol phospholipid hydrolysis in slices of hamster urinary bladder. Br J Pharmacol. 1987 Jan;90(1):211–217. doi: 10.1111/j.1476-5381.1987.tb16842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. Membrane ionic mechanisms activated by noradrenaline in cells isolated from the rabbit portal vein. J Physiol. 1988 Oct;404:557–573. doi: 10.1113/jphysiol.1988.sp017306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Raeymaekers L. The action of acetylcholine and catecholamines on an intracellular calcium store in the smooth muscle cells of the guinea-pig taenia coli. J Physiol. 1979 Sep;294:51–68. doi: 10.1113/jphysiol.1979.sp012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J., Caprilli R., Lund G. F. Electric slow waves in circular muscle of cat colon. Am J Physiol. 1969 Sep;217(3):771–776. doi: 10.1152/ajplegacy.1969.217.3.771. [DOI] [PubMed] [Google Scholar]
- Clapp L. H., Vivaudou M. B., Singer J. J., Walsh J. V., Jr Substance P, like acetylcholine, augments one type of Ca2+ current in isolated smooth muscle cells. Pflugers Arch. 1989 Mar;413(5):565–567. doi: 10.1007/BF00594191. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Giles W., Shimoni Y. Slow inward tail currents in rabbit cardiac cells. J Physiol. 1989 Oct;417:447–463. doi: 10.1113/jphysiol.1989.sp017812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandordy B. M., Frossard N., Rhoden K. J., Barnes P. J. Tachykinin-induced phosphoinositide breakdown in airway smooth muscle and epithelium: relationship to contraction. Mol Pharmacol. 1988 May;33(5):515–519. [PubMed] [Google Scholar]
- Haeusler G., Richards J. G., Thorens S. Noradrenaline contractions in rabbit mesenteric arteries skinned with saponin. J Physiol. 1981 Dec;321:537–556. doi: 10.1113/jphysiol.1981.sp014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hisada T., Kurachi Y., Sugimoto T. Properties of membrane currents in isolated smooth muscle cells from guinea-pig trachea. Pflugers Arch. 1990 Apr;416(1-2):151–161. doi: 10.1007/BF00370237. [DOI] [PubMed] [Google Scholar]
- Iino M. Calcium-induced calcium release mechanism in guinea pig taenia caeci. J Gen Physiol. 1989 Aug;94(2):363–383. doi: 10.1085/jgp.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Isenberg G. Acetylcholine activates nonselective cation channels in guinea pig ileum through a G protein. Am J Physiol. 1990 Jun;258(6 Pt 1):C1173–C1178. doi: 10.1152/ajpcell.1990.258.6.C1173. [DOI] [PubMed] [Google Scholar]
- Ito K., Takakura S., Sato K., Sutko J. L. Ryanodine inhibits the release of calcium from intracellular stores in guinea pig aortic smooth muscle. Circ Res. 1986 May;58(5):730–734. doi: 10.1161/01.res.58.5.730. [DOI] [PubMed] [Google Scholar]
- Itoh T., Kajiwara M., Kitamura K., Kuriyama H. Roles of stored calcium on the mechanical response evoked in smooth muscle cells of the porcine coronary artery. J Physiol. 1982 Jan;322:107–125. doi: 10.1113/jphysiol.1982.sp014026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen L. J., Sims S. M. Acetylcholine activates non-selective cation and chloride conductances in canine and guinea-pig tracheal myocytes. J Physiol. 1992;453:197–218. doi: 10.1113/jphysiol.1992.sp019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen L. J., Sims S. M. Histamine activates Cl- and K+ currents in guinea-pig tracheal myocytes: convergence with muscarinic signalling pathway. J Physiol. 1993 Jun;465:661–677. doi: 10.1113/jphysiol.1993.sp019699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen L. J., Sims S. M. Substance P activates Cl- and K+ conductances in guinea-pig tracheal smooth muscle cells. Can J Physiol Pharmacol. 1994 Jun;72(6):705–710. doi: 10.1139/y94-100. [DOI] [PubMed] [Google Scholar]
- Katsuyama H., Ito S., Itoh T., Kuriyama H. Effects of ryanodine on acetylcholine-induced Ca2+ mobilization in single smooth muscle cells of the porcine coronary artery. Pflugers Arch. 1991 Nov;419(5):460–466. doi: 10.1007/BF00370789. [DOI] [PubMed] [Google Scholar]
- Korn S. J., Bolden A., Horn R. Control of action potentials and Ca2+ influx by the Ca(2+)-dependent chloride current in mouse pituitary cells. J Physiol. 1991 Aug;439:423–437. doi: 10.1113/jphysiol.1991.sp018674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton P. D., Burke E. P., Sanders K. M. Participation of Ca currents in colonic electrical activity. Am J Physiol. 1989 Sep;257(3 Pt 1):C451–C460. doi: 10.1152/ajpcell.1989.257.3.C451. [DOI] [PubMed] [Google Scholar]
- Leijten P. A., van Breemen C. The effects of caffeine on the noradrenaline-sensitive calcium store in rabbit aorta. J Physiol. 1984 Dec;357:327–339. doi: 10.1113/jphysiol.1984.sp015502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loirand G., Pacaud P., Mironneau C., Mironneau J. GTP-binding proteins mediate noradrenaline effects on calcium and chloride currents in rat portal vein myocytes. J Physiol. 1990 Sep;428:517–529. doi: 10.1113/jphysiol.1990.sp018225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem. 1986 May 15;261(14):6300–6306. [PubMed] [Google Scholar]
- Nakajima T., Hazama H., Hamada E., Omata M., Kurachi Y. Ionic basis of neurokinin-A-induced depolarization in single smooth muscle cells isolated from guinea-pig trachea. Pflugers Arch. 1995 Aug;430(4):552–562. doi: 10.1007/BF00373892. [DOI] [PubMed] [Google Scholar]
- Nishimura T., Akasu T., Tokimasa T. A slow calcium-dependent chloride current in rhythmic hyperpolarization in neurones of the rabbit vesical pelvic ganglia. J Physiol. 1991 Jun;437:673–690. doi: 10.1113/jphysiol.1991.sp018618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacaud P., Loirand G., Lavie J. L., Mironneau C., Mironneau J. Calcium-activated chloride current in rat vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 1989 Apr;413(6):629–636. doi: 10.1007/BF00581813. [DOI] [PubMed] [Google Scholar]
- Pacaud P., Loirand G., Mironneau C., Mironneau J. Opposing effects of noradrenaline on the two classes of voltage-dependent calcium channels of single vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 1987 Nov;410(4-5):557–559. doi: 10.1007/BF00586539. [DOI] [PubMed] [Google Scholar]
- Sanders K. M., Smith T. K. Enteric neural regulation of slow waves in circular muscle of the canine proximal colon. J Physiol. 1986 Aug;377:297–313. doi: 10.1113/jphysiol.1986.sp016188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small R. C. Electrical slow waves and tone of guinea-pig isolated trachealis muscle: effects of drugs and temperature changes. Br J Pharmacol. 1982 Sep;77(1):45–54. doi: 10.1111/j.1476-5381.1982.tb09267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. P., Supplisson S., Torres R., Sachs G., Mayer E. Characterization of large-conductance chloride channels in rabbit colonic smooth muscle. J Physiol. 1992 Mar;448:355–382. doi: 10.1113/jphysiol.1992.sp019046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt A. C., Gibbons W. R. Calcium-activated chloride current in rabbit ventricular myocytes. Circ Res. 1991 Feb;68(2):424–437. doi: 10.1161/01.res.68.2.424. [DOI] [PubMed] [Google Scholar]


