Abstract
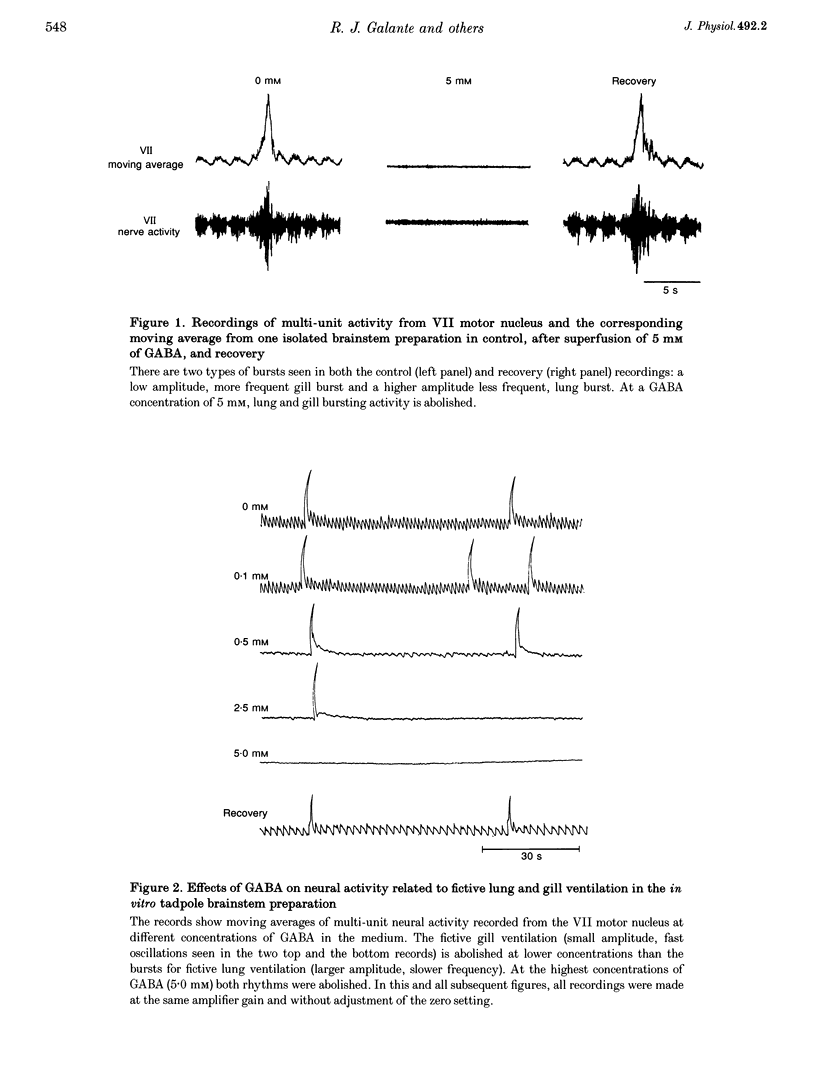
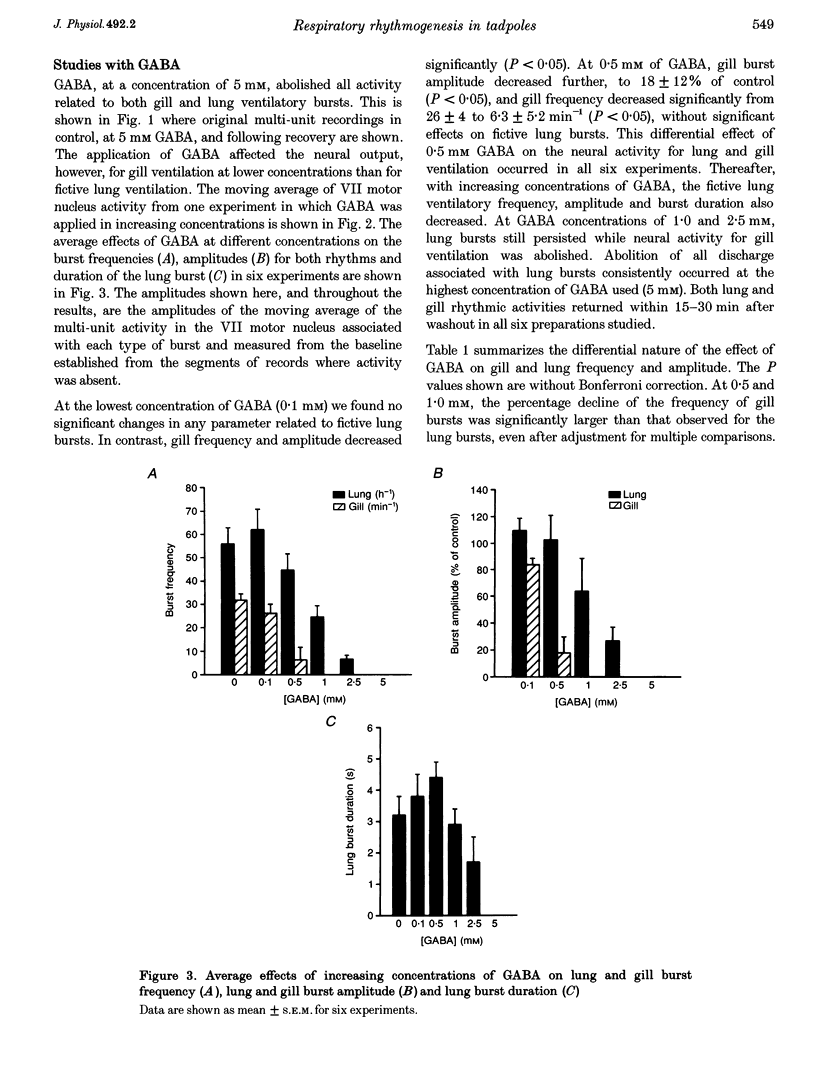
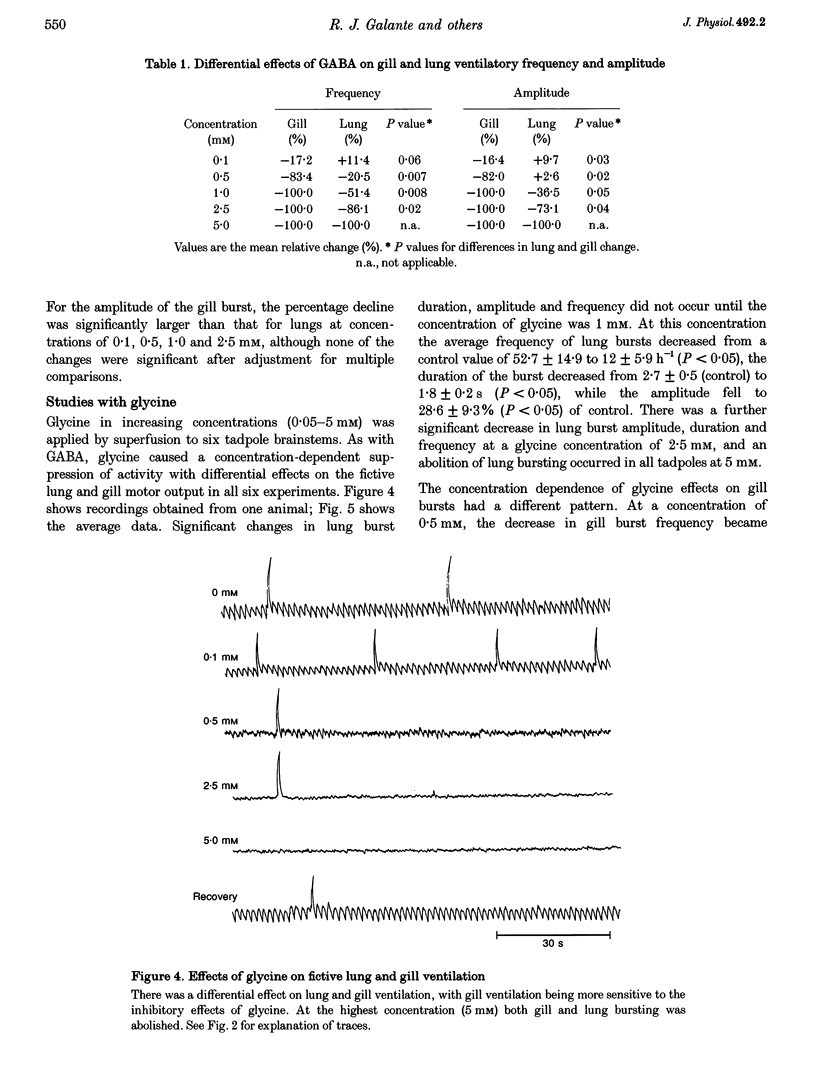
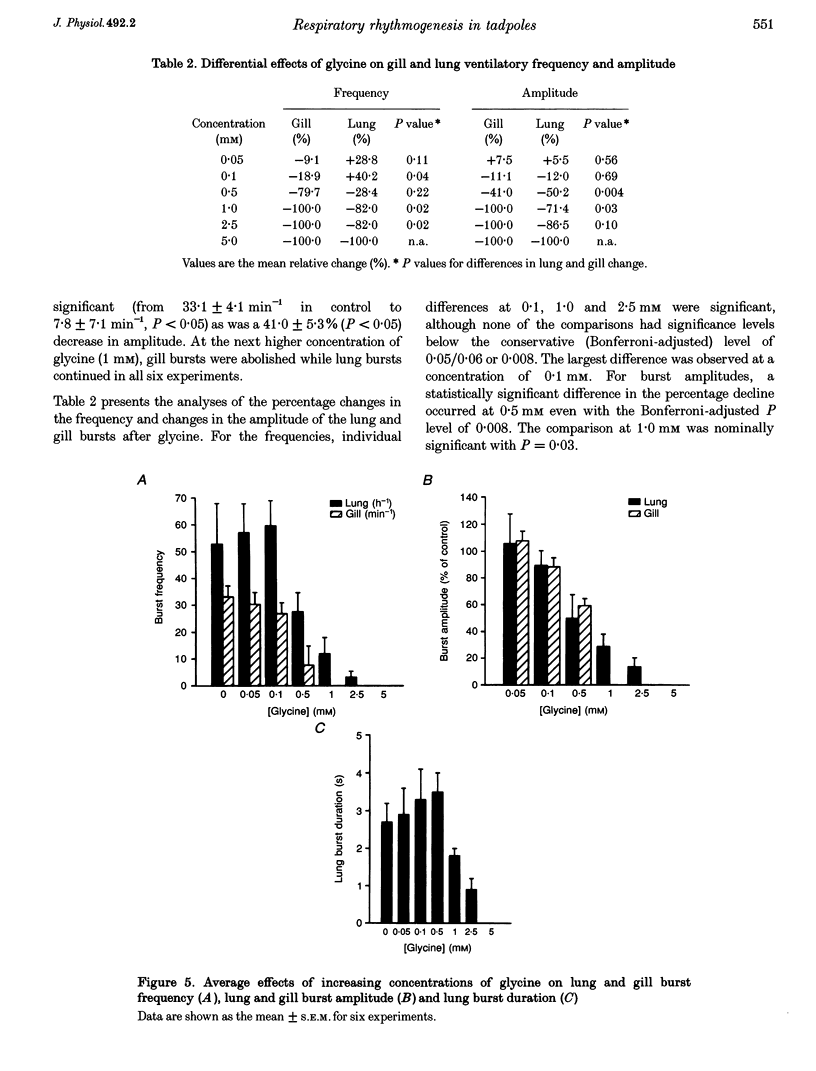
1. The isolated brainstem of larval Rana catesbeiana maintained in vitro generates neural bursts that correspond to the lung and gill ventilatory activity generated in the intact specimen. To investigate the role of chloride channel-dependent inhibitory mechanisms mediated by GABA(A) and/or glycine receptors on fictive lung and gill ventilation, we superfused the isolated brainstems with agonists, antagonists (bicuculline and/or strychnine) or a chloride-free solution while recording multi-unit activity from the facial motor nucleus. 2. Superfusion with the agonists (GABA or glycine) produced differential effects on frequency, amplitude and duration of the neural bursts related to lung and gill ventilation. At a GABA or glycine concentration of 1.0 mM, fictive gill bursts were abolished while fictive lung bursts persisted, albeit with reduced amplitude and frequency. 3. At the lowest concentrations used (1.0-2.5 microM), the GABA(A) receptor antagonist bicuculline produced an increase in the frequency of lung bursts. At higher concentrations (5.0-2.0 microM) bicuculline produced non-specific excitatory effects. The glycine antagonist strychnine, at concentrations lower than 5.0 microM, caused a progressive decrease in the frequency and amplitude of the gill bursts and eventually abolished the rhythmic activity. At higher concentrations (7.5 microM), non-specific excitatory effects occurred. Superfusion with bicuculline (10 microM) and strychnine (5 microM) combined abolished the neural output for gill ventilation but increased the frequency, amplitude and duration of lung bursts. 4. Superfusion with Cl(-)-free solution also abolished the rhythmic neural bursts associated with gill ventilation, while it significantly increased the amplitude (228 +/- 51%; P < 0.05) (mean +/- S.E.M.) and duration of the lung bursts (3.5 +/- 0.1 to 35.3 +/- 3.7 s; P < 0.05) and improved the regularity of their occurrence. 5. We conclude that different neural systems generate rhythmic activity for lung and gill ventilation. Chloride-mediated inhibition may be essential for generation of neural bursts associated with gill ventilation. In contrast, the burst associated with lung ventilation can be generated in the absence of Cl(-)-mediated inhibition although the latter plays a role in shaping the normal lung burst.
Full text
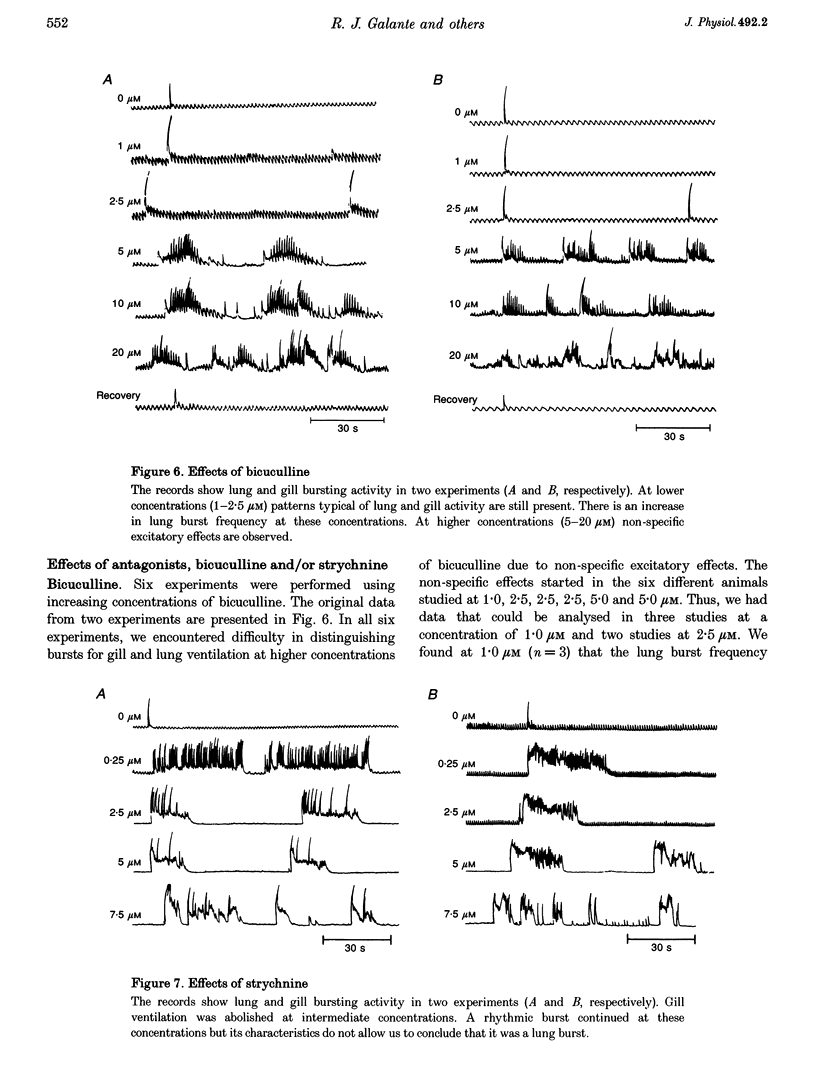
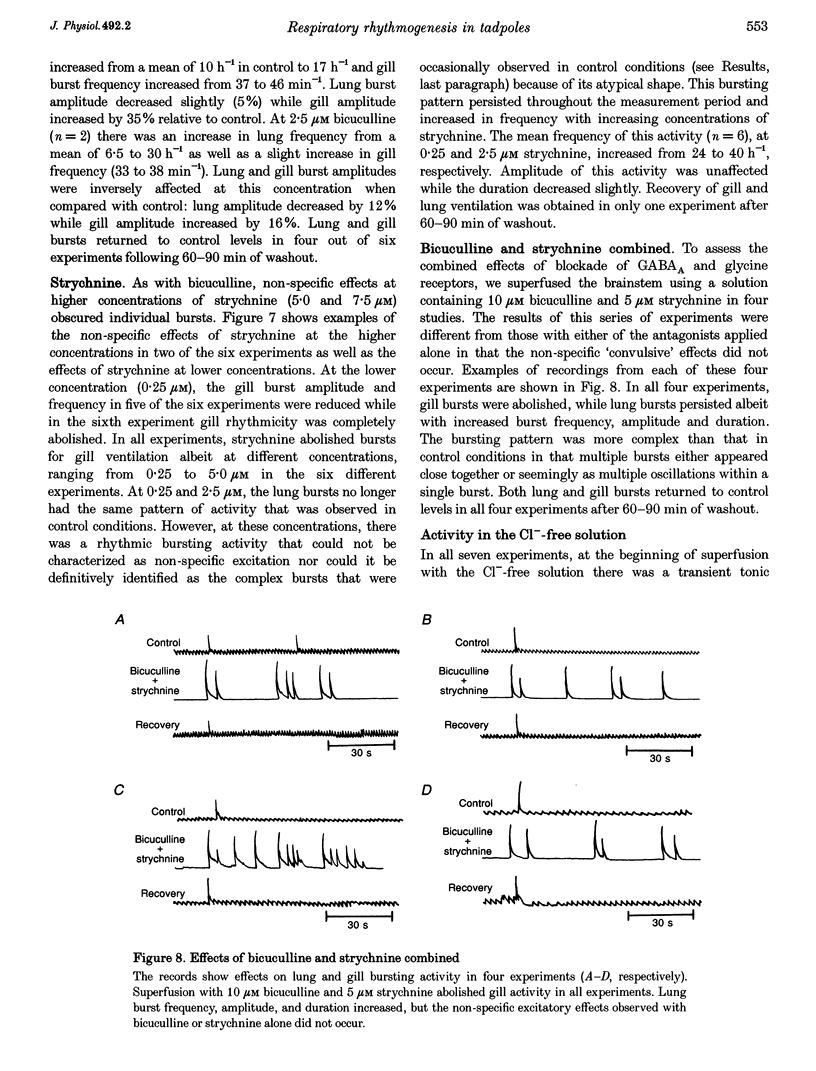
PDF



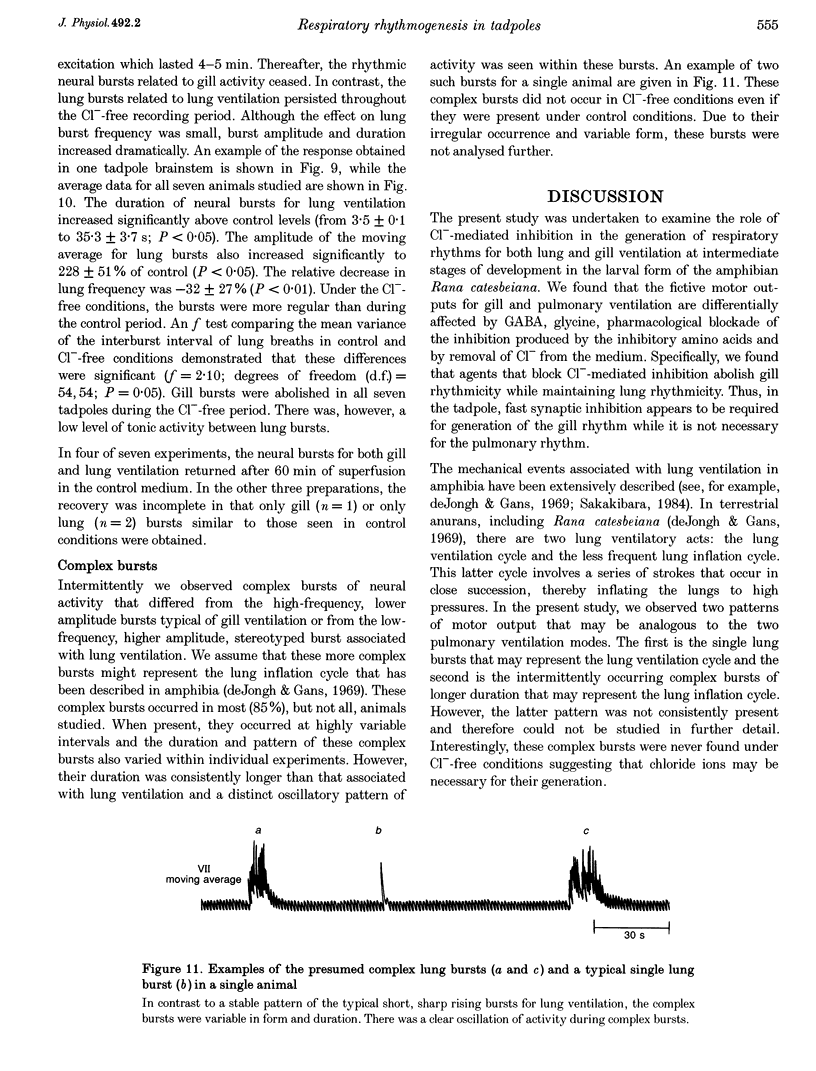



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballantyne D., Richter D. W. Post-synaptic inhibition of bulbar inspiratory neurones in the cat. J Physiol. 1984 Mar;348:67–87. doi: 10.1113/jphysiol.1984.sp015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggren W. W., West N. H. Changing respiratory importance of gills, lungs and skin during metamorphosis in the bullfrog Rana catesbeiana. Respir Physiol. 1982 Feb;47(2):151–164. doi: 10.1016/0034-5687(82)90108-6. [DOI] [PubMed] [Google Scholar]
- Byrne J. H., Koester J. Respiratory pumping: neuronal control of a centrally commanded behavior in Aplysia. Brain Res. 1978 Mar 17;143(1):87–105. doi: 10.1016/0006-8993(78)90754-0. [DOI] [PubMed] [Google Scholar]
- Christoffersen C. R., Skibsted L. H. Calcium ion activity in physiological salt solutions: influence of anions substituted for chloride. Comp Biochem Physiol A Comp Physiol. 1975 Oct 1;52(2):317–322. doi: 10.1016/s0300-9629(75)80094-6. [DOI] [PubMed] [Google Scholar]
- Cooke I. R., Berger P. J. Precursor of respiratory pattern in the early gestation mammalian fetus. Brain Res. 1990 Jul 9;522(2):333–336. doi: 10.1016/0006-8993(90)91479-z. [DOI] [PubMed] [Google Scholar]
- Ezure K. Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Prog Neurobiol. 1990;35(6):429–450. doi: 10.1016/0301-0082(90)90030-k. [DOI] [PubMed] [Google Scholar]
- Farber J. P. Medullary inspiratory activity during opossum development. Am J Physiol. 1988 Apr;254(4 Pt 2):R578–R584. doi: 10.1152/ajpregu.1988.254.4.R578. [DOI] [PubMed] [Google Scholar]
- Feldman J. L., Smith J. C. Cellular mechanisms underlying modulation of breathing pattern in mammals. Ann N Y Acad Sci. 1989;563:114–130. doi: 10.1111/j.1749-6632.1989.tb42194.x. [DOI] [PubMed] [Google Scholar]
- Feldman J. L., Smith J. C., Ellenberger H. H., Connelly C. A., Liu G. S., Greer J. J., Lindsay A. D., Otto M. R. Neurogenesis of respiratory rhythm and pattern: emerging concepts. Am J Physiol. 1990 Nov;259(5 Pt 2):R879–R886. doi: 10.1152/ajpregu.1990.259.5.R879. [DOI] [PubMed] [Google Scholar]
- Haji A., Takeda R., Remmers J. E. Evidence that glycine and GABA mediate postsynaptic inhibition of bulbar respiratory neurons in the cat. J Appl Physiol (1985) 1992 Dec;73(6):2333–2342. doi: 10.1152/jappl.1992.73.6.2333. [DOI] [PubMed] [Google Scholar]
- Hayashi F., Lipski J. The role of inhibitory amino acids in control of respiratory motor output in an arterially perfused rat. Respir Physiol. 1992 Jul;89(1):47–63. doi: 10.1016/0034-5687(92)90070-d. [DOI] [PubMed] [Google Scholar]
- Just J. J., Gatz R. N., Crawford E. C., Jr Changes in respiratory functions during metamorphosis of the bullfrog, Rana catesbeiana. Respir Physiol. 1973 Apr;17(3):276–282. doi: 10.1016/0034-5687(73)90002-9. [DOI] [PubMed] [Google Scholar]
- Kinkead R., Filmyer W. G., Mitchell G. S., Milsom W. K. Vagal input enhances responsiveness of respiratory discharge to central changes in pH/CO2 in bullfrogs. J Appl Physiol (1985) 1994 Oct;77(4):2048–2051. doi: 10.1152/jappl.1994.77.4.2048. [DOI] [PubMed] [Google Scholar]
- Kogo N., Perry S. F., Remmers J. E. Neural organization of the ventilatory activity in the frog, Rana catesbeiana. I. J Neurobiol. 1994 Sep;25(9):1067–1079. doi: 10.1002/neu.480250904. [DOI] [PubMed] [Google Scholar]
- Kogo N., Remmers J. E. Neural organization of the ventilatory activity in the frog, Rana catesbeiana. II. J Neurobiol. 1994 Sep;25(9):1080–1094. doi: 10.1002/neu.480250905. [DOI] [PubMed] [Google Scholar]
- Liao G-S, Kubin L., Galante R. J., Fishman A. P., Pack A. I. Respiratory activity in the facial nucleus in an in vitro brainstem of tadpole, Rana catesbeiana. J Physiol. 1996 Apr 15;492(Pt 2):529–544. doi: 10.1113/jphysiol.1996.sp021327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon B. R. A functional analysis of the aquatic and aerial respiratory movements of an African lungfish, Protopterus aethiopicus, with reference to the evolution of the lung-ventilation mechanism in vertebrates. J Exp Biol. 1969 Nov;51(2):407–430. doi: 10.1242/jeb.51.2.407. [DOI] [PubMed] [Google Scholar]
- Miller J. P., Selverston A. I. Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. II. Oscillatory properties of pyloric neurons. J Neurophysiol. 1982 Dec;48(6):1378–1391. doi: 10.1152/jn.1982.48.6.1378. [DOI] [PubMed] [Google Scholar]
- Ogilvie M. D., Gottschalk A., Anders K., Richter D. W., Pack A. I. A network model of respiratory rhythmogenesis. Am J Physiol. 1992 Oct;263(4 Pt 2):R962–R975. doi: 10.1152/ajpregu.1992.263.4.R962. [DOI] [PubMed] [Google Scholar]
- Onimaru H., Arata A., Homma I. Firing properties of respiratory rhythm generating neurons in the absence of synaptic transmission in rat medulla in vitro. Exp Brain Res. 1989;76(3):530–536. doi: 10.1007/BF00248909. [DOI] [PubMed] [Google Scholar]
- Onimaru H., Homma I. Respiratory rhythm generator neurons in medulla of brainstem-spinal cord preparation from newborn rat. Brain Res. 1987 Feb 17;403(2):380–384. doi: 10.1016/0006-8993(87)90080-1. [DOI] [PubMed] [Google Scholar]
- Paton J. F., Ramirez J. M., Richter D. W. Mechanisms of respiratory rhythm generation change profoundly during early life in mice and rats. Neurosci Lett. 1994 Mar 28;170(1):167–170. doi: 10.1016/0304-3940(94)90265-8. [DOI] [PubMed] [Google Scholar]
- Rovainen C. M. Generation of respiratory activity by the lamprey brain exposed to picrotoxin and strychnine, and weak synaptic inhibition in motoneurons. Neuroscience. 1983 Nov;10(3):875–882. doi: 10.1016/0306-4522(83)90225-7. [DOI] [PubMed] [Google Scholar]
- Rovainen C. M. Neural control of ventilation in the lamprey. Fed Proc. 1977 Sep;36(10):2386–2389. [PubMed] [Google Scholar]
- Russell D. F. Respiratory pattern generation in adult lampreys (Lampetra fluviatilis): interneurons and burst resetting. J Comp Physiol A. 1986 Jan;158(1):91–102. doi: 10.1007/BF00614523. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y. The pattern of respiratory nerve activity in the bullfrog. Jpn J Physiol. 1984;34(2):269–282. doi: 10.2170/jjphysiol.34.269. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Ellenberger H. H., Ballanyi K., Richter D. W., Feldman J. L. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991 Nov 1;254(5032):726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed N. I., Bulloch A. G., Lukowiak K. In vitro reconstruction of the respiratory central pattern generator of the mollusk Lymnaea. Science. 1990 Oct 12;250(4978):282–285. doi: 10.1126/science.2218532. [DOI] [PubMed] [Google Scholar]
- Thompson K. J. Organization of inputs to motoneurons during fictive respiration in the isolated lamprey brain. J Comp Physiol A. 1985 Oct;157(3):291–302. doi: 10.1007/BF00618119. [DOI] [PubMed] [Google Scholar]
- Vinay L., Grillner S. Spino-bulbar neurons convey information to the brainstem about different phases of the locomotor cycle in the lamprey. Brain Res. 1992 Jun 5;582(1):134–138. doi: 10.1016/0006-8993(92)90327-6. [DOI] [PubMed] [Google Scholar]


