Abstract
Biocatalysis has the potential to address the need for more sustainable organic synthesis routes. Protein engineering can tune enzymes to perform in cascade reactions and for efficient synthesis of enantiomerically enriched compounds, using both natural and new-to-nature reaction pathways. This review highlights recent achievements in biocatalysis, especially the development of novel enzymatic syntheses to access versatile small molecule intermediates and complex biomolecules. Biocatalytic strategies for the degradation of persistent pollutants and approaches for biomass valorization are also discussed. The transition of chemical synthesis to a greener future will be accelerated by implementing enzymes and engineering them for high performance and new activities.
Introduction
Biocatalytic transformations are becoming an essential part of the synthetic chemist’s armamentarium, and these greener and more sustainable catalysts are transforming chemical process landscapes [1,2]. Enzymes can produce valuable compounds with high yields and exquisite selectivities, providing cost benefits compared to traditional organic synthesis and reducing waste production. The tunability and evolvability of biocatalysts, using protein engineering techniques such as directed evolution, mean that efficient catalysts can be generated for key synthetic steps [3,4]. The chemical transformations catalyzed by enzymes are not limited to those found in nature, and thus exciting new trajectories such as strategies for the degradation of persistent pollutants and polymers as well as the atomefficient synthesis of complex biomolecules are enabled. Here we highlight some of the latest advancements in biocatalysis, focusing on unique attributes that have been harnessed in industry and academic research (Fig. 1). We aim to showcase the ever-increasing opportunities that enzymes offer in devising solutions to long-standing challenges in organic synthesis.
Figure 1.
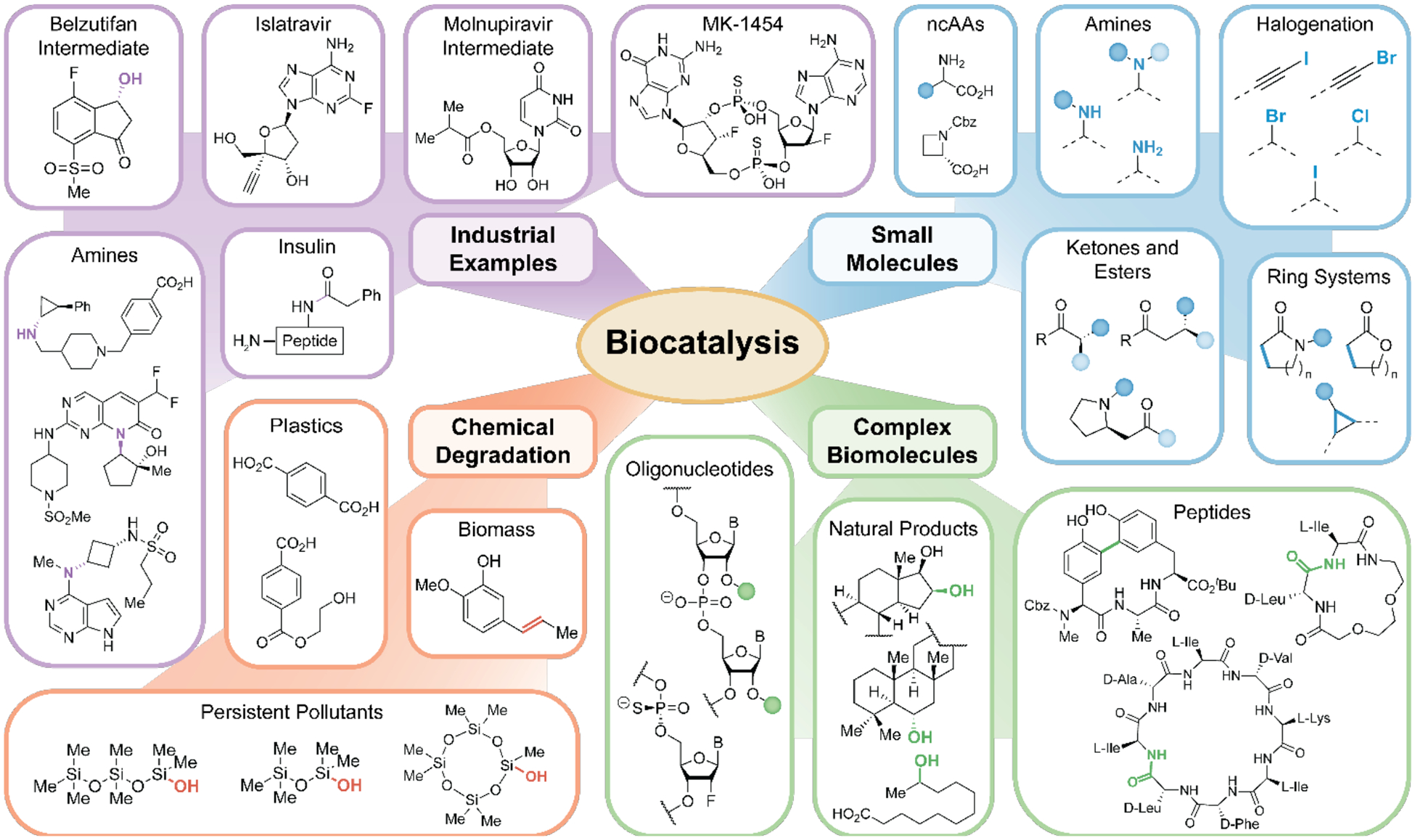
Examples of chemoenzymatic approaches that pave the way to selectively construct synthetic targets in the pharmaceutical industry as well as in the context of small molecule and complex biomolecule synthesis. In addition to biosynthesis, biocatalysts provide new strategies for pollutant degradation and biomass valorization.
Application of Biocatalysts in the Pharmaceutical Industry
The pharmaceutical industry has recognized the valuable role enzymes can play in manufacturing bioactive molecules, leveraging their advantages to circumvent obstacles encountered in traditional synthetic processes. This appreciation stems primarily from enzymes’ remarkably high chemo-, regio-, enantio-, and substrate selectivities, which can enable multiple enzymes to work together in the same pot (‘cascade’ reactions) and make complex molecules with few side products, much as they do inside living cells. In this section, we will highlight recent examples that demonstrate the power of biocatalysts for the synthesis of enantioenriched building blocks, target molecules, or active pharmaceutical ingredients (APIs).
Biocatalysts confer high regio- and enantioselectivity.
Enzyme properties can be tuned for industrial applications by engineering the protein sequence. Recent work by Merck researchers addressed challenges associated with α-ketoglutarate-dependent dioxygenases (α-KGD) on manufacturing scale, including low total turnover number (TTN), aerobic reaction conditions, low stability, enzyme inactivation by self-hydroxylation, and overoxidation of unnatural substrates. An engineered α-KGD replaced five synthetic steps with a direct enzymatic hydroxylation to produce chiral intermediate 2 from 1 used in the synthesis of belzutifan in high enantioselectivity and preparative yield (Fig. 2a) [*5]. Compared to heme-dependent oxygenases, α-KGDs require only iron in combination with α-ketoglutarate and do not necessitate complex cofactors or co-expression of reductase domains. Enzymes’ high selectivity also enables them to target specific functional groups among others, and this selectivity can be engineered by directed evolution. Fryszkowska and coworkers engineered acylases for improved bioconjugation of insulin (consisting of both α-and β-peptides covalently bound by disulfide bonds). This facilitated the selective acylation of an internal or terminal amine, as well as selective hydrolysis of the phenylacetyl group [6].
Figure 2.
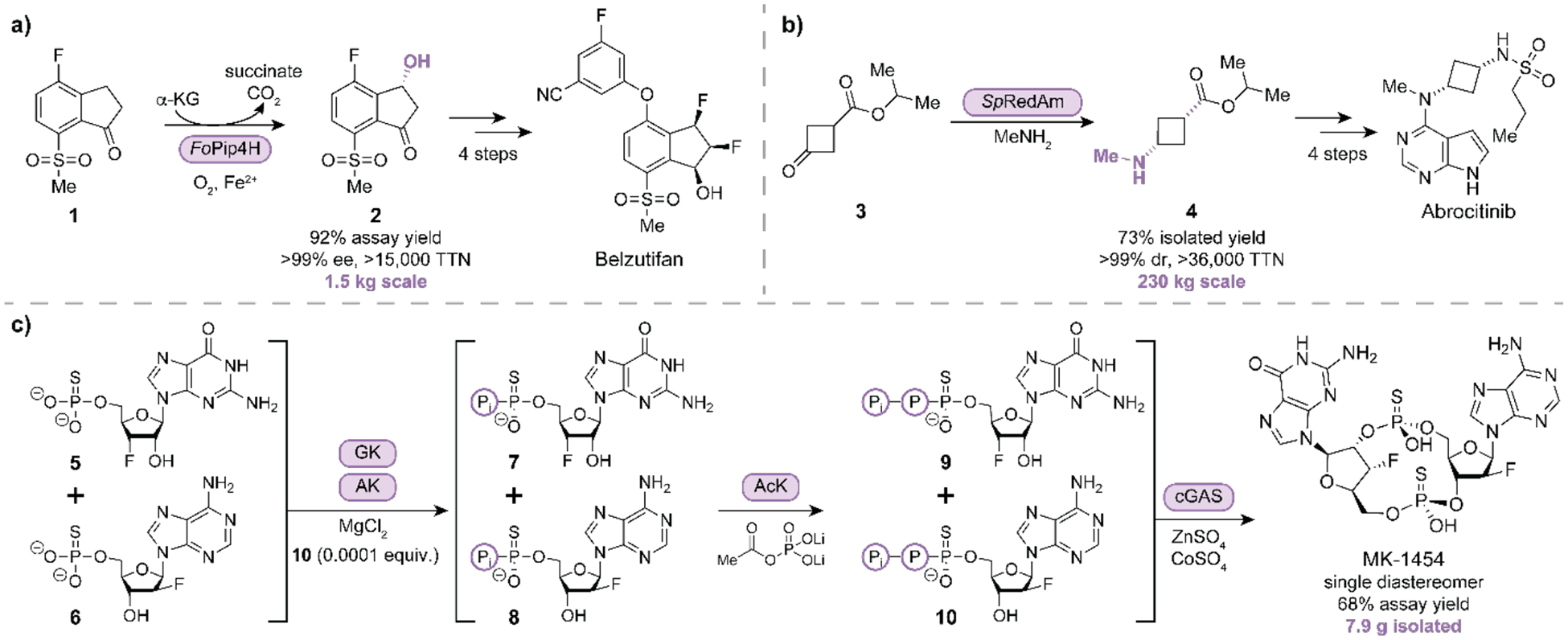
Examples of biocatalysis used to produce APIs. (a) Stereoselective hydroxylation of 1 with α-KGD FoPip4H (2 g/L) to generate enantioenriched intermediate 2 for the production of belzutifan [5]. (b) Biocatalytic reductive amination of ketone 3 catalyzed by SpRedAm (1.9 g/L) for the synthesis of enantioenriched amine building block 4 on 230 kg scale for the synthesis of abrocitinib [9]. (c) An enzymatic cascade to access MK-1454 uses four enzymes and circumvents intermediate purification steps [14]. In one pot, guanylate kinase (GK, 0.1 g/L) and adenylate kinase (AK, 0.1 g/L) phosphorylate 5 and 6, respectively to yield intermediates 7 and 8 which are then phosphorylated by the same acetate kinase (AcK, 75 mg/L). After subsequent addition of cyclic guanosine-adenosine synthase (cGAS, 2.2 g/L), the final cyclization between 9 and 10 yields MK-1454. P = −PO3−, Pi = −PO42−.
Large scale production of APIs.
Imine reductases (IREDs) and reductive aminases (RedAms) are well known for their scalability and have been successfully applied on ton scale for the synthesis of chiral amines from ketones [7]. Researchers at GlaxoSmithKline (GSK) engineered an IRED to perform a stereoselective reductive amination via kinetic resolution of a racemic mixture of trans-phenylcyclo-propylamine (>38,000-fold greater TTN compared to the wild-type enzyme), reducing the generated waste by half – process mass index (PMI) improved from 355 to 178 [8]. Researchers at Pfizer improved on a prior chemoenzymatic synthesis of cis-cyclobutyl-N-methylamine intermediate 4. Initially, a car-bonyl-containing substrate was converted into the corresponding amine with a transaminase, followed by chemical alkylation of the primary amine with iodomethane. Augmenting this preliminary process, they combined transamination and alkylation into a single enzyme-catalyzed reductive amination with methyl amine and a RedAm to selectively form the cis aminated cyclobutane 4 in 73% isolated yield from the corresponding carbonyl 3, a >200-fold increase compared to the wild-type enzyme (Fig. 2b) [*9]. This step was optimized for large scale to afford 230 kg of the product. Cumulative batch processes generated >3.5 megatons of chiral intermediate 4 as the succinate salt for the synthesis of abrocitinib. Another RedAm was used to simplify production of an enantioenriched intermediate by directly installing a protected benzylamine on kilogram scale, with a substrate loading of 50 g/L, 98% ee, and 43% conversion [10,11]. These examples showcase the utility of IREDs and RedAms for chemical manufacturing on ton scale, due to their inherent stability, high activities, and broad applicability for installing amine functional groups. Future efforts need to focus on identifying and developing more enzyme families capable of performing useful transformations on industrial scale.
One-pot enzymatic cascades.
The high chemoselectivity of biocatalysts can suppress unproductive side reactions and allow multiple reactions to occur in a single pot. This typically results in a process with high atom economy, good step efficiency, limited waste generation, and no need for purification between chemical steps. Examples from Merck include extraordinary enzymatic systems for synthesis of APIs such as complex cyclic dinucleotides and nucleosides. Impressive demonstrations of enzyme cascade syntheses of nucleosides islatravir and molnupiravir have generated the excitement and interest biocatalysis deserves [12,13]. Another example is the cascade synthesis of a stimulator of interferon genes (STING) protein activator, MK-1454, currently in clinical trials [*14]. The original synthesis of this cyclic dinucleotide required nine synthetic steps – Merck researchers streamlined the synthesis into three concatenated biocatalytic reactions. The cascade consisted of two enzymatic phosphorylation events requiring three engineered kinases to produce activated thiotriphosphorylated nucleotides 9 and 10 (Fig. 2c).
The final step involved an engineered cyclic guanosine-adenosine synthase (cGAS) and a bimetallic system (Zn2+ and Co2+) necessary for the stereocontrolled cyclization of 9 and 10 to produce MK-1454. With these reported enzymatic cascades, API production was achieved in fewer steps with less waste generated during synthesis and purification, improving the PMI. Protein engineering enabled inefficient, naturally occurring enzymes to become competent and robust catalysts with improved activity and diastereoselectivity.
Frontiers of Biocatalysis for Small Molecule Synthesis
Academic research groups are expanding the repertoire of biocatalysis by discovering novel enzyme-catalyzed natural product syntheses and developing non-natural transformations using engineered enzymes. Especially, new chiral synthetic intermediates have been accessed efficiently and stereoselectively using biocatalytic strategies. These novel and creative reaction designs are primed to be useful resources to consider in developing biocatalytic routes that produce fine chemicals and pharmaceuticals, after further improvement of process relevant properties such as enzyme stability, activity, and selectivity at scale.
Amination strategies to access important synthetic targets.
The efficient and stereoselective introduction of amine functional handles into target molecules is a fundamental synthetic task, given their importance in bioactive molecules. New-to-nature heme-containing enzymes have been shown to achieve direct amination by catalyzing C–H bond and alkene functionalization reactions. Engineered Pyrobaculum arsenaticum protoglobin (ParPgb) variants can use inexpensive hydroxylamine hydrochloride (NH2OH·HCl) as a nitrene precursor, generating water as the sole byproduct (Fig. 3a) [*15]. In the directed evolution campaign to develop these new enzymes, it was observed that the KM values for hydroxylamine decreased (5.4 mM to 0.30 mM), suggesting higher affinity of the aminating reagent with the heme enzyme resulting in improved activity (at low substrate concentrations). Also, an increase in the turnover number (kcat) by 180-fold from the initial variant improved overall yield. The ability of enzymes to utilize hydroxylamine as an aminating reagent was then expanded to the biocatalytic conversion of boronic acids into the corresponding amines [16]. As another example, Zhao and coworkers engineered flavin mononucleotide (FMN)-dependent ene-reductases to use N–O based reagents as amine precursors in the enantioselective hydroamination of alkenes under visible light [17]. The authors indicate that further directed evolution of the biocatalyst is required to improve on the low enzyme activity and expand the substrate scope.
Figure 3.
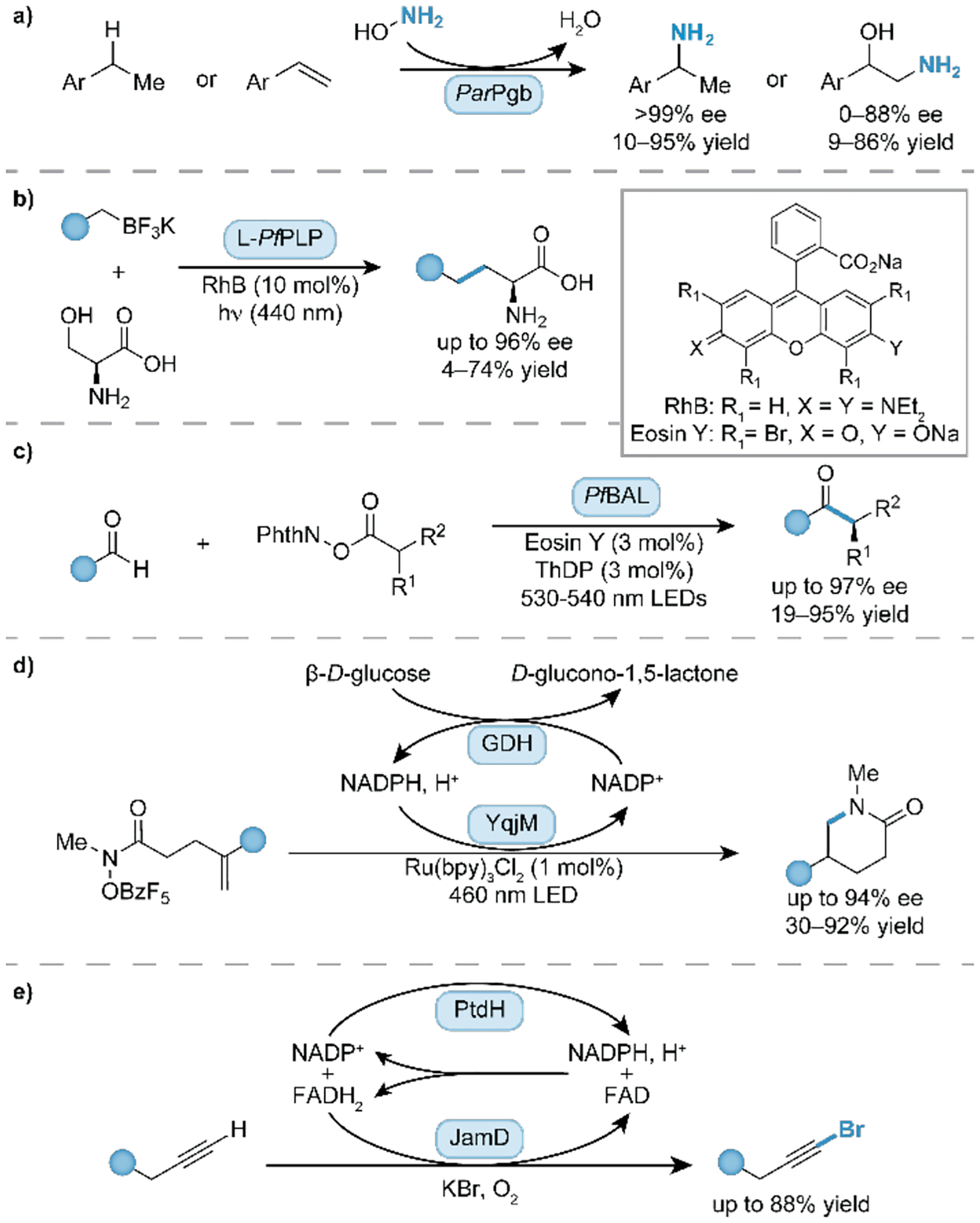
Biocatalytic transformations for the construction of small, versatile building blocks. (a) Pyrobaculum arsenaticum protoglobin (ParPgb) catalyzed amination of benzylic C-H bonds and alkenes using hydroxylamine as aminating reagent [15]. (b) A combined photoredox-PLP catalytic system for the construction of ncAAs relying on Pyrococcus furiosus tryptophan synthase β subunit (L-PfPLP) [22]. (c) Synthesis of α-chiral ketones relying on a dual organophotoredox and ThDP-dependent Pseudomonas fluorescens benzaldehyde lyase (PfBAL) [30]. (d) Intramolecular hydroamination for the formation of lactams using Bacillus subtilis flavin-dependent ene-reductase (YqjM) [34]. (e) Bromination of terminal alkynes using Moorena producens flavin-dependent halogenase (JamD) [37]. GDH = glucose dehydrogenase, PtdH = phosphite dehydrogenase.
Gathering mechanistic insights can help address the need for robust and novel biocatalytic processes. Hilvert and coworkers investigated a myoglobin model system to uncover the cause for the unproductive reduction of nitrenes generated from azide precursors [18]. Based on their findings, more competent heme-based nitrene transfer biocatalysts could be developed by fine-tuning the reduction potential of the enzyme, which in turn suppressed unproductive reduction pathways.
The selective introduction of azide groups as masked primary amine precursors presents another strategy to access nitrogen-decorated scaffolds. In this context, Huang and coworkers utilized a non-heme iron enzyme for benzylic azidation implementing sodium azide as the external azide source [19]. While all these examples showcase interesting directions for the design of novel synthetic routes, further protein engineering efforts to improve biocatalyst properties, including TTN and stability under process conditions, are necessary to facilitate their use at scale.
Emerging strategies for the efficient synthesis of non-canonical amino acids.
Key pillars in modern small-molecule pharmaceuticals and peptidomimetics are finding and improving therapeutic targets by the incorporation of noncanonical amino acids (ncAAs) or isotopically labeled derivatives [20]. Selective access to Cα and/or Cβ deuterated amino acids was achieved with a pyridoxal 5’-phosphate (PLP)-dependent two-enzyme system (DasD, DasE), providing labeled enantiopure amino acids on analytic and semi-preparative scales (>600 mg deuterated Ile, 0.5 mmol) [21]. Relying also on PLP-dependent enzymes, a synergistic photoredox-PLP biocatalytic approach was introduced by Yang and coworkers to facilitate the construction of ncAAs (Fig. 3b) [*22]. In this reaction, photocatalytically generated radicals engage in a stereoselective C–C bond forming reaction with a covalently bound substrate-enzyme intermediate. Extension of their work to glycine and α-branched amino acids substrates enabled the enantioselective synthesis of α-tri and tetrasubstituted ncAAs exhibiting up to two contiguous stereocenters [23]. Related work by Hyster and coworkers also demonstrated the utility of engineered threonine aldolases as a platform to synthetically access α-tertiary amino acids using pyridinium salts as alkylating agents [24]. In a different study, Arnold and coworkers achieved enantioselective synthesis of azetidine products, bioisosteres of proline, using an engineered P450 variant with an axial serine substitution, classified as P411 enzymes, to mediate a one-carbon ring expansion of aziridines [25]. To further expand the set of chemically available proline bioisosteres, Renata and coworkers reported the construction of five- to eight-membered cyclic ncAAs based on a two-step chemoenzymatic cascade relying on a transaminase-mediated cyclization followed by a stereocontrolled PtO2 hydrogenation [26]. Again, using these catalysts to make other ncAAs for industrial purposes will require further enzyme engineering.
Synthesis and functionalization of carbonyl intermediates.
Several enzyme classes have been explored to expand the toolkit of organic transformations that can access and functionalize carbonyl-containing substrates and products. Flavin-dependent ene-reductases could be harnessed for the challenging chemo- and enantioselective C(sp3)–C(sp3) cross coupling of alkyl halides and nitroalkenes to access derivatized ketone products [27]. This reaction is proposed to proceed via a quaternary charge transfer complex in the presence of all reaction partners in the FMN active site. The same enzyme class was also utilized in the desymmetrization of enones to access enantioenriched cyclohexanones from either cyclohexanone or cyclohexadienone substrates [28]. Recently, engineered cytochrome P450s were further improved to enable α-C–H functionalization of cyclic amines via a carbene transfer reaction using diazo precursors on gram scales to attach carbonyl functional handles [29]. Also, a dual system comprised of a thiamine diphosphate (ThDP)-dependent radical acyl transferase and an organophotoredox catalyst facilitated the synthesis of α-chiral ketones (Fig. 3c) [*30]. The reaction is proposed to proceed via the formation of a Breslow intermediate by the condensation of the aldehyde with the ThDP cofactor. This intermediate is prone to oxidation and subsequent radical rebound with the alkyl radical to generate the carbonyl product. While these examples represent exciting new directions of implementing new-to-nature enzyme-catalyzed transformations, scale-up challenges associated with photochemical processes must be overcome to enable efficient application at scale.
Construction of ring systems through C–C bond formation.
The synthesis of cyclic compounds via carbon-carbon bond formation represents a fundamental strategy to introduce complexity to molecular scaffolds. Lactones and lactams were accessed by P411 enzymes via an intramolecular carbene insertion strategy and by B12- and heme-dependent enzymes via a radical cyclization mechanism of tertiary alkyl halides with alkenes or arenes [31–33]. Hyster and coworkers reported a hydroamination reaction using ene-reductases to access a variety of enantiopure lactams and amides via a synergistic photo- and biocatalytic strategy (Fig. 3d) [*34]. High enantioselectivities are obtained by generation and subsequent coupling of the reactive amidyl radical with the alkene in the protein active site. Besides lactones and lactams, the efficient and stereoselective construction of small carbo-cyclic scaffolds is also synthetically relevant. In most cases, relatively reactive carbene precursors (e.g. diazoacetate) are necessary to enable efficient construction of cyclopropane rings from alkenes [35]. On larger scales, the implementation of more stable carbene precursors for transfer reactions may be attractive due to their improved safety profiles. Arnold and coworkers reported the use of diazirine reagents in biocatalytic cyclopropanations as well as N–H, B–H, and Si–H insertion reactions [36]. Together with further improvement of enzyme stability and activities at high substrate and product concentrations, these developments increase the potential for ‘carbene transferases’ to be established in safe and sustainable industrial processes.
Halogenations for the installation of functional handles.
Derivatization of molecular scaffolds by the installation of halogen atoms is an important strategy to increase complexity and introduce functional groups. Lewis and coworkers demonstrated that the bromination and iodination of several arenes and an alkene can be catalyzed by a flavin-dependent halogenase AetF [38]. In a study by Lukowski and coworkers, another flavin-dependent halogenase, JamD, was identified to catalyze chemoselective bromination and iodination of terminal alkynes over electron-rich arenes (Fig. 3e) [37]. Impressively, the naturally occurring enzyme was shown to exhibit a broad substrate scope, affording simple and late-stage haloalkynes. The intramolecular construction of benzylic C(sp3)–F bonds using N-fluoroamide substrates was enabled by repurposing a non-heme iron epoxidase from Streptomyces viridochromogenes (SvHppE) [39]. In a different study, a native α-KGD hydroxylase was engineered to perform selective halogenation over hydroxylation of C(sp3)–H bonds in lysine [40]. Even though a variety of naturally occurring and engineered halogenases have been identified to perform useful transformations on aromatic scaffolds, enzymatic halogenation of unactivated C–H bonds is still in its infancy, with further biocatalyst engineering required in many cases.
Biocatalysts for Streamlined Synthesis of Complex Biomolecules
Synthesizing complex biomolecules often requires preactivated substrates and orthogonal protecting groups. These approaches generate waste and necessitate chromatographic purification of target compounds. Enzymes can circumvent these disadvantages by specific substrate orientation and thus selectively reacting with a single functional group among others. This molecular precision results in fewer synthetic steps, increased atom efficiency, and more sustainable processes. Below we highlight strategies that use enzymes for the synthesis of complex biomolecules such as natural products, peptides, and oligonucleotides.
Late-stage oxidation of natural products.
Natural product synthesis is a complex, multistep process usually requiring the manipulation of protecting groups. Biocatalysts provide a useful alternative to enable regio- and stereoselective control in the presence of bare functional groups (Fig. 1, Natural Products). Examples of late-stage modifications of natural products are inspired by native pathways, utilizing P450s or other oxygenases for oxidation of steroids and fatty acids. Alcalde and coworkers evolved a fungal peroxygenase to selectively hydroxylate fatty acids at the ω-1 position [41]. A single mutation caused a narrowing of the active site, resulting in tighter binding of the fatty acid substrate and allowing for targeted hydroxylation. Similarly, a steroid hydroxylase, CYP109B4, was discovered through genome mining to oxidize steroids at the C16 position. This regioselectivity was then altered using directed evolution to instead precisely hydroxylate at C15 [42]. In another example, Renata and coworkers streamlined the total synthesis of ansellone B by discovering and engineering a P450 monooxygenase capable of hydroxylating the C6 position of sclareol with six-fold improved catalytic activity compared to their initial variant [43].
Cyclization of peptides.
Cyclization of peptides is synthetically challenging because intramolecular reactions must compete with intermolecular couplings, and unprotected amino acid side chains can lead to undesired side reactions (Fig. 1, Peptides). In native systems, nonribosomal peptide (NRP) synthetases utilize thioester-linked peptides for cyclization. Wakimoto and coworkers designed an alternative leaving group, ethylene glycol, which could be easily installed on the resin support during solid-phase peptide synthesis [44]. Using this strategy, they discovered a wild type NRP cyclase (SurE) capable of selective head-to-tail cyclization of peptides containing N-terminal L-isoleucine and C-terminal D-leucine. After protein engineering, a SurE variant cyclized peptides without the terminal amino acid requirements, illustrating the evolvability of SurE. Another cyclization strategy, biaryl cross coupling with tyrosine side chains, was investigated by a group at Genentech [45] inspired by a complementary example for naphthol cross coupling by the Na rayan lab [46]. These few examples begin to set the stage for new and selective late-stage peptide modifications without the need for noncanonical amino acid incorporation.
Chemoenzymatic oligonucleotide synthesis.
Modified nucleic acid therapeutics are a class of molecules traditionally synthesized by solid-phase phos-phoramidite-based synthesis. This process requires densely protected monomers, generates significant waste during synthesis and purification, and yield deteriorates as oligo length increases. The Lovelock lab discovered a cascade of native enzymes that allows for polymerization of oligonucleotides and incorporation of unnatural monomers [*47]. Even without protein engineering, the scope of the native polymerases supports many noncanonical oligonucleotide building blocks (thiophosphate, locked, and 2’-modified nucleotides) and could serve as starting points for future directed evolution campaigns (Fig. 1, Oligonucleotides), similar to what was achieved for incorporating 2’-O-(2-methoxyethyl) nucleic acids in oligo synthesis [48]. To date, most oligonucleotide syntheses require a template strand for polymerization, and ways to incorporate unnatural nucleotide monomers remain to be explored.
Pollutant Degradation and Biomass Valorization Using Biocatalysts
Advances in biocatalysis also include using enzymes to break down environmentally persistent compounds and polymers. Man-made volatile methyl siloxanes (VMS) (Fig. 1, Persistent Pollutants) used in consumer products such as detergents and cosmetics are produced on megaton scale and are not naturally degraded by microorganisms. Arnold and coworkers engineered a cytochrome P450 to oxidize terminal carbons in selected VMS, leading to Si–C bond cleavage via the carbinol intermediates. Enzymatic cleavage of this critical bond hints that biodegradation of siloxanes may one day be possible [*49]. Depolymerization of polyethylene terephthalate (PET) has been achieved using an engineered thermostable hydrolase capable of catalysis at the glass transition temperature of PET [50] (Fig. 1, Plastics). A major component of biomass, lignin can be depolymerized by chemical strategies, but feasible methods to convert those degradation products into valuable chemicals are needed. An engineered eugenol oxidase (EUGO) converts the most abundant lignin monomer, 4-n-propylguaiacol, into isoeugenol, a commodity chemical used for industrial production of fragrances, flavors, and polymers [51] (Fig. 1, Biomass).
Outlook
Biocatalytic strategies are increasingly recognized as attractive, sustainable alternatives to traditional organic chemistry approaches. Enzymes offer new solutions to synthetic challenges, enabling access to small, enantioenriched intermediates and complex biomolecules. Protein engineering efforts have facilitated the development of simplified synthetic routes that implement resilient, highly selective enzymes in cascade reactions. Beyond transforming the organic chemistry landscape, degradation of persistent pollutants and polymers have also been addressed with enzymes. Most of the biocatalysts described in recent reports, however, are of limited applicability. General shortcomings such as enzyme robustness, substrate scope, ability to work with high concentrations of substrate and product, and reaction yield need to be improved to successfully implement biocatalysis in industrial manufacturing campaigns [52]. The feasibility of this approach is amply illustrated by the examples reviewed here. Performing directed evolution more efficiently in combination with process engineering to streamline scale up will accelerate the implementation of biocatalysis. In general, the field will advance by exploiting new technologies, such as assay automation and machine learning-assisted directed evolution [53], all pointing toward the goal of making these processes more accessible, cost-effective, and easier to implement.
Highlights:
Engineered biocatalysts that access pharmaceutical products through more sustainable synthetic routes.
Novel biocatalysts that construct enantiomerically enriched small-molecule intermediates.
Complex biomolecules synthesized using enzymatic cascade reactions.
Biocatalytic degradation of persistent pollutants and valorization of biomass to afford valuable compounds.
Acknowledgments
This work was supported by the National Science Foundation Division of Molecular and Cellular Biosciences (MCB-2016137 to F.H.A.) and the U.S. Army Research Office cooperative agreement (W911NF-19-2-0026 to F.H.A.). This material is based upon work supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC0021141. J.C.R. acknowledges support from the Swiss National Science Foundation (SNSF) Postdoc.Mobility (P500PN_214290). K.M.S. acknowledges support from NIH Ruth L. Kirschstein National Research Service Award (1F32GM145123-01A1). The authors thank Sabine Brinkmann-Chen, Jennifer L. Kennemur, Edwin Alfonzo, Anuvab Das, and Ravi G. Lal for critical proof reading and helpful discussion.
Footnotes
Conflict of Interest Statement
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
References
- 1.Anastas P, Eghbali N: Green chemistry: principles and practice. Chem Soc Rev 2010, 39:301–312, doi: 10.1039/B918763B. [DOI] [PubMed] [Google Scholar]
- 2.Sanderson K: Chemistry: it’s not easy being green. Nature 2011, 469:18–20, doi: 10.1038/469018a. [DOI] [PubMed] [Google Scholar]
- 3.Arnold FH: Directed evolution: bringing new chemistry to life. Angew Chem Int Ed 2018, 57:4143–4148, doi: 10.1002/anie.201708408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reetz MT, Directed Evolution of Selective Enzymes: Catalysts for Organic Chemistry and Biotechnology, first ed., Wiley-VCH, Germany, 2016. [Google Scholar]
- 5.*.Cheung-Lee WL, Kolev JN, McIntosh JA, Gil AA, Pan W, Xiao L, Velásquez JE, Gangam R, Winston MS, Li S, et al. : Engineering hydroxylase activity, selectivity, and stability for a scalable concise synthesis of a key intermediate to belzutifan. Angew Chem Int Ed 2024, 63:e202316133, doi: 10.1002/anie.202316133. [DOI] [PubMed] [Google Scholar]; Merck scientists replaced five synthetic steps with a single enantioselective hydroxylation using an engineered α-KGD en route to the synthesis of belzutifan.
- 6.Fryszkowska A, An C, Alvizo O, Banerjee G, Canada KA, Cao Y, DeMong D, Devine PN, Duan D, Elgart DM, et al. : A chemoenzymatic strategy for site-selective functionalization of native peptides and proteins. Science 2022, 376:1321–1327, doi: 10.1126/science.abn2009. [DOI] [PubMed] [Google Scholar]
- 7.Gilio AK, Thorpe TW, Turner N, Grogan G: Reductive aminations by imine reductases: from milligrams to tons. Chem Sci 2022, 13:4697–4713, doi: 10.1039/D2SC00124A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schober M, MacDermaid C, Ollis AA, Chang S, Khan D, Hosford J, Latham J, Ihnken LAF, Brown MJB, Fuerst D, et al. : Chiral synthesis of LSD1 inhibitor GSK2879552 enabled by directed evolution of an imine reductase. Nat Catal 2019, 2:909–915, doi: 10.1038/s41929-019-0341-4. [DOI] [Google Scholar]
- 9.*.Kumar R, Karmilowicz MJ, Burke D, Burns MP, Clark LA, Connor CG, Cordi E, Do NM, Doyle KM, Hoagland S, et al. : Biocatalytic reductive amination from discovery to commercial manufacturing applied to abrocitinib JAK1 inhibitor. Nat Catal 2021, 4:775–782, doi: 10.1038/s41929-021-00671-5. [DOI] [Google Scholar]; Stereoselective reductive amination to generate a key chiral amine intermediate for the synthesis of abrocitinib on a 100 kg scale.
- 10.Duan S, Widlicka DW, Burns MP, Kumar R, Hotham I, Desrosiers J-N, Bowles P, Jones KN, Nicholson LD, Buetti-Weekly MT, et al. : Application of biocatalytic reductive amination for the synthesis of a key intermediate to a CDK 2/4/6 inhibitor. Org Process Res Dev 2022, 26:879–890, doi: 10.1021/acs.oprd.1c00255. [DOI] [Google Scholar]
- 11.Steflik J, Gilio A, Burns M, Grogan G, Kumar R, Lewis R, Martinez C: Engineering of a reductive aminase to enable the synthesis of a key intermediate to a CDK 2/4/6 inhibitor. ACS Catal 2023, 13:10065–10075, doi: 10.1021/acscatal.3c01534. [DOI] [Google Scholar]
- 12.Huffman MA, Fryszkowska A, Alvizo O, Borra-Garske M, Campos KR, Canada KA, Devine PN, Duan D, Forstater JH, Grosser ST, et al. : Design of an in vitro biocatalytic cascade for the manufacture of islatravir. Science 2019, 366:1255–1259, doi: 10.1126/science.aay8484. [DOI] [PubMed] [Google Scholar]
- 13.McIntosh JA, Benkovics T, Silverman SM, Huffman MA, Kong J, Maligres PE, Itoh T, Yang H, Verma D, Pan W, et al. : Engineered ribosyl-1-kinase enables concise synthesis of molnupiravir, an antiviral for COVID-19. ACS Cent Sci 2021, 7:1980–1985, doi: 10.1021/acscentsci.1c00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.*.McIntosh JA, Liu Z, Andresen BM, Marzijarani NS, Moore JC, Marshall NM, Borra-Garske M, Obligacion JV, Fier PS, Peng F, et al. : A kinase-cGAS cascade to synthesize a therapeutic STING activator. Nature 2022, 603:439–444, doi: 10.1038/s41586-022-04422-9. [DOI] [PubMed] [Google Scholar]; Enzymatic convergent cascade for enantioselective synthesis of a cyclic dinucleotide therapeutic without protecting groups or chiral auxiliaries.
- 15.*.Gao S, Das A, Alfonzo E, Sicinski KM, Rieger D, Arnold FH: Enzymatic nitrogen incorporation using hydroxylamine. J Am Chem Soc 2023, 145:20196–20201, doi: 10.1021/jacs.3c08053. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nitrene chemistry, including alkene and C–H bond functionalizations, was accomplished with inexpensive hydroxylamine hydrochloride as nitrene precursor, generating water as the sole byproduct.
- 16.Hanley D, Li Z-Q, Gao S, Virgil SC, Arnold FH, AIfonzo E: Stereospecific enzymatic conversion of boronic acids to amines. J Am Chem Soc 2024, doi: 10.1021/jacs.4c04190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Feng J, Yang C, Cui H, Harrison W, Zhong D, Wang B, Zhao H: Photoenzymatic enantioselective intermolecular radical hydroamination. Nat Catal 2023, 6:687–694, doi: 10.1038/s41929-023-00994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tinzl M, Diedrich JV, Mittl PRE, Clémancey M, Reiher M, Proppe J, Latour J-M, Hilvert D: Myo-globin-catalyzed azide reduction proceeds via an anionic metal amide intermediate. J Am Chem Soc 2024, 146:1957–1966, doi: 10.1021/jacs.3c09279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rui J, Zhao Q, Huls AJ, Soler J, Paris JC, Chen Z, Reshetnikov V, Yang Y, Guo Y, Garcia-Borràs M, et al. : Directed evolution of nonheme iron enzymes to access abiological radical-relay C(sp3)-H azidation. Science 2022, 376:869–874, doi: 10.1126/science.abj2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alfonzo E, Das A, Arnold FH: New additions to the arsenal of biocatalysts for noncanonical amino acid synthesis. Curr Opin Green Sustain Chem 2022, 38:100701, doi: 10.1016/j.cogsc.2022.100701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doyon TJ, Buller AR: Site-selective deuteration of amino acids through dual-protein catalysis. J Am Chem Soc 2022, 144:7327–7336, doi: 10.1021/jacs.2c00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.*.Cheng L, Li D, Mai BK, Bo Z, Cheng L, Liu P, Yang Y: Stereoselective amino acid synthesis by synergistic photoredox-pyridoxal radical biocatalysis. Science 2023, 381:444–451, doi: 10.1126/science.adg2420. [DOI] [PMC free article] [PubMed] [Google Scholar]; Novel strategy based on cooperative photoredox catalysis and PLP-dependent biocatalysis for stereoselective synthesis of noncanonical amino acids.
- 23.Wang T-C, Mai BK, Zhang Z, Bo Z, Li J, Liu P, Yang Y: Stereoselective amino acid synthesis by photobiocatalytic oxidative coupling. Nature 2024, 629:98–104, doi: 10.1038/s41586-024-07284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouyang Y, Page CG, Bilodeau C, Hyster TK: Synergistic photoenzymatic catalysis enables synthesis of α-tertiary amino acids using threonine aldolases. J Am Chem Soc 2024, 146:13754–13759, doi: 10.1021/jacs.4c04661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller DC, Lal RG, Marchetti LA, Arnold FH: Biocatalytic one-carbon ring expansion of aziridines to azetidines via a highly enantioselective [1,2]-Stevens rearrangement. J Am Chem Soc 2022, 144:4739–4745, doi: 10.1021/jacs.2c00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chao T-H, Wu X, Fu Y, Yang L, Renata H: Harnessing transaminases to construct azacyclic non-canonical amino acids. Nat Synth 2024, 3:662–669, doi: 10.1038/s44160-024-00514-8. [DOI] [Google Scholar]
- 27.Fu H, Cao J, Qiao T, Qi Y, Charnock SJ, Garfinkle S, Hyster TK: An asymmetric sp3–sp3 cross-electrophile coupling using ‘ene’-reductases. Nature 2022, 610:302–307, doi: 10.1038/s41586-022-05167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng Q-Q, Zhou Q-Y, Calvó-Tusell C, Dai S-Y, Zhao X, Carcia-Borràs M, Liu Z: Biocatalytic desymmetrization for synthesis of chiral enones using flavoenzymes. Nat Synth 2024, doi: 10.1038/s44160-024-00596-4. [DOI] [Google Scholar]
- 29.Ren X, Couture BM, Liu N, Lall MS, Kohrt JT, Fasan R: Enantioselective single and dual α-C–H bond functionalization of cyclic amines via enzymatic carbene transfer. J Am Chem Soc 2023, 145:537–550, doi: 10.1021/jacs.2c10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.*.Xu Y, Chen H, Yu L, Peng X, Zhang J, Xing Z, Bao Y, Liu A, Zhao Y, Tian C, et al. : A light-driven enzymatic enantioselective radical acylation. Na ture 2024, 625:74–78, doi: 10.1038/s41586-023-06822-x. [DOI] [PubMed] [Google Scholar]; A dual catalytic system comprised of a thiamine diphosphate (ThDP)-dependent radical acyl transferase and an organophotoredox catalyst was developed for the synthesis of α-chiral ketones.
- 31.Wackelin DJ, Mao R, Sicinski KM, Zhao Y, Das A, Chen K, Arnold FH: Enzymatic assembly of diverse lactone structures: an intramolecular C–H functionalization strategy. J Am Chem Soc 2024, 146:1580–1587, doi: 10.1021/jacs.3c11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Kumar A, Lewis JC: Non-native intramolecular radical cyclization catalyzed by a B12-dependent enzyme. Angew Chem Int Ed 2023, 62:e202312893, doi: 10.1002/anie.202312893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Q, Chin M, Fu Y, Liu P, Yang Y: Stereodivergent atom-transfer radical cyclization by engineered cytochromes P450. Science 2021, 374:1612–1616, doi: 10.1126/science.abk1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.*.Ye Y, Cao J, Oblinsky DG, Verma D, Prier CK, Scholes GD, Hyster TK: Using enzymes to tame nitrogen-centred radicals for enantioselective hydroamination. Nat Chem 2023, 15:206–212, doi: 10.1038/s41557-022-01083-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; A synergistic photo- and biocatalytic strategy based on an ‘ene’ reductase was implemented to facilitate intra- and intermolecular hydroamination reactions to access a variety of enantioenriched lactams and amides.
- 35.Siriboe MG, Vargas DA, Fasan R: Dehaloperoxidase catalyzed stereoselective synthesis of cyclopropanol esters. J Org Chem 2023, 88:7630–7640, doi: 10.1021/acs.joc.2c02030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porter NJ, Danelius E, Gonen T, Arnold FH: Biocatalytic carbene transfer using diazirines. J Am Chem Soc 2022, 144:8892–8896, doi: 10.1021/jacs.2c02723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lukowski AL, Hubert FM, Ngo T-E, Avalon NE, Gerwick WH, Moore BS: Enzymatic halogenation of terminal alkynes. J Am Chem Soc 2023, 145:18716–18721, doi: 10.1021/jacs.3c05750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang Y, Snodgrass HM, Zubi YS, Roof CV, Guan Y, Mondal D, Honeycutt NH, Lee JW, Lewis RD, Martinez CA, et al. : The single-component flavin reductase/flavin-dependent halogenase AetF is a versatile catalyst for selective bromination and iodination of arenes and olefins. Angew Chem Int Ed 2022, 61:e202214610, doi: 10.1002/anie.202214610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Q, Chen Z, Soler J, Chen X, Rui J, Ji NT, Yu QE, Yang Y, Garcia-Borràs M, Huang X: Engineering non-haem iron enzymes for enantioselective C(sp3)-F bond formation via radical fluorine transfer. Nat Synth 2024, doi: 10.1038/s44160-024-00507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neugebauer ME, Kissman EN, Marchand JA, Pelton JG, Sambold NA, Millar DC, Chang MCY: Reaction pathway engineering converts a radical hydroxylase into a halogenase. Nat Chem Biol 2022, 18:171–179, doi: 10.1038/s41589-021-00944-x. [DOI] [PubMed] [Google Scholar]
- 41.Gomez de Santos P, González-Benjumea A, Fer-nandez-Garcia A, Aranda C, Wu Y, But A, Mo-lina-Espeja P, Maté DM, Gonzalez-Perez D, Zhang W, et al. : Engineering a highly regioselective fungal peroxygenase for the synthesis of hydroxy fatty acids. Angew Chem Int Ed 2023, 62:e202217372, doi: 10.1002/anie.202217372. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Shen P, Zhao J, Chen Y, Li X, Huang J-W, Zhang L, Li Q, Gao C, Xing Q, et al. : Rationally controlling selective steroid hydroxylation via scaffold sampling of a P450 family. ACS Catal 2023, 13:1280–1289, doi: 10.1021/acscatal.2c04906. [DOI] [Google Scholar]
- 43.Li F, Deng H, Renata H: Remote B-ring oxidation of sclareol with an engineered P450 facilitates divergent access to complex terpenoids. J Am Chem Soc 2022, 144:7616–7621, doi: 10.1021/jacs.2c02958. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi M, Fujita K, Matsuda K, Wakimoto T: Streamlined chemoenzymatic synthesis of cyclic peptides by non-ribosomal peptide cyclases. J Am Chem Soc 2023, 145:3270–3275, doi: 10.1021/jacs.2c11082. [DOI] [PubMed] [Google Scholar]
- 45.Molinaro C, Kawasaki Y, Wanyoike G, Nishioka T, Yamamoto T, Snedecor B, Robinson SJ, Gosselin F: Engineered cytochrome P450-catalyzed oxidative biaryl coupling reaction provides a scalable entry into arylomycin antibiotics. J Am Chem Soc 2022, 144:14838–14845, doi: 10.1021/jacs.2c06019. [DOI] [PubMed] [Google Scholar]
- 46.Zetzsche LE, Yazarians JA, Chakrabarty S, Hinze ME, Murray LAM, Lukowski AL, Joyce LA, Narayan ARH: Biocatalytic oxidative cross-coupling reactions for biaryl bond formation. Nature 2022, 603:79–85, doi: 10.1038/s41586-021-04365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.*.Moody ER, Obexer R, NickI F, Spiess R, Lovelock SL : An enzyme cascade enables production of therapeutic oligonucleotides in a single operation. Science 2023, 380:1150–1154, doi: 10.1126/science.add5892. [DOI] [PubMed] [Google Scholar]; An enzyme cascade enabled production of the therapeutic oligonucleotides in a single operation.
- 48.Freund N, Taylor AI, Arangundy-Franklin S, Subramanian N, Peak-Chew S-Y, Whitaker AM, Freudenthal BD, Abramov M, Herdewijn P, Holliger P:A two-residue nascent-strand steric gate controls synthesis of 2’-O-methyl- and 2’-O-(2-methoxyethyl)-RNA. Nat Chem 2023, 15:91100, doi: 10.1038/s41557-022-01050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.*.Sarai NS, Fulton TJ, O’Meara RL, Johnston KE, Brinkmann-Chen S, Maar RR, Tecklenburg RE, Roberts JM, Reddel JCT, Katsoulis DE, et al. : Directed evolution of enzymatic silicon-carbon bond cleavage in siloxanes. Science 2024, 383:438–443, doi: 10.1126/science.adi5554. [DOI] [PubMed] [Google Scholar]; The first enzyme shown to cleave Si-C bonds opens the possibility that volatile methyl siloxanes can be degraded biologically
- 50.Bell EL, Smithson R, Kilbride S, Foster J, Hardy FJ, Ramachandran S, Tedstone AA, Haigh SJ, Garforth AA, Day PJR, et al. : Directed evolution of an efficient and thermostable PET depolymerase. Nat Catal 2022, 5:673–681, doi: 10.1038/s41929-022-00821-3. [DOI] [Google Scholar]
- 51.Guo Y, Alvigini L, Trajkovic M, Alonso-Cotchico L, Monza E, Savino S, Marić I, Mattevi A, Fraaije MW: Structure- and computational-aided engineering of an oxidase to produce isoeugenol from a lignin-derived compound. Nat Commun 2022, 13:7195, doi: 10.1038/s41467-022-34912-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woodley JM: Integrating protein engineering into biocatalytic process scale-up. Trends Chem 2022, 4:371–373, doi: 10.1016/j.trechm.2022.02.007. [DOI] [Google Scholar]
- 53.Rapp JT, Bremer BJ, Romero PA: Self-driving laboratories to autonomously navigate the protein fitness landscape. Nat Chem Eng 2024, 1:97–107, doi: 10.1038/s44286-023-00002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


