Abstract
1. Subcellularly localized Ca2+ signals have been proposed to represent elementary events of cardiac Ca2+ signalling (Ca2+ sparks), whereby an individual sarcolemmal L-type Ca2+ channel locally controls opening of a single (or a few) Ca2+ release channels in the sarcoplasmic reticulum (SR). 2. To investigate directly the elementary nature of this Ca(2+)-induced Ca2+ release mechanism we used flash photolysis of caged Ca2+ while simultaneously measuring the intracellular Ca2+ concentration ([Ca2+]i) with a laser-scanning confocal microscope. 3. Power spectral analysis of the confocal images performed in the spatial domain revealed that only Ca2+ signalling events involving the L-type Ca2+ channel pathway gave rise to Ca2+ sparks. In contrast, SR Ca2+ release triggered by photolytic [Ca2+]i jumps resulted in Ca2+ transients that were always spatially homogeneous. 4. From these findings we conclude that the fundamental event of Ca2+ signalling in cardiac muscle may be smaller in size or amplitude than a Ca2+ spark. 5. We term this event a 'Ca2+ quark' possibly resulting from gating of a single SR Ca2+ release channel. It is proposed that concerted activation of several 'Ca2+ quarks' may be required for a Ca2+ spark. The 'Ca2+ quark' could also be the fundamental event in other cell types implementing a hierarchical Ca2+ signalling concept.
Full text
PDF

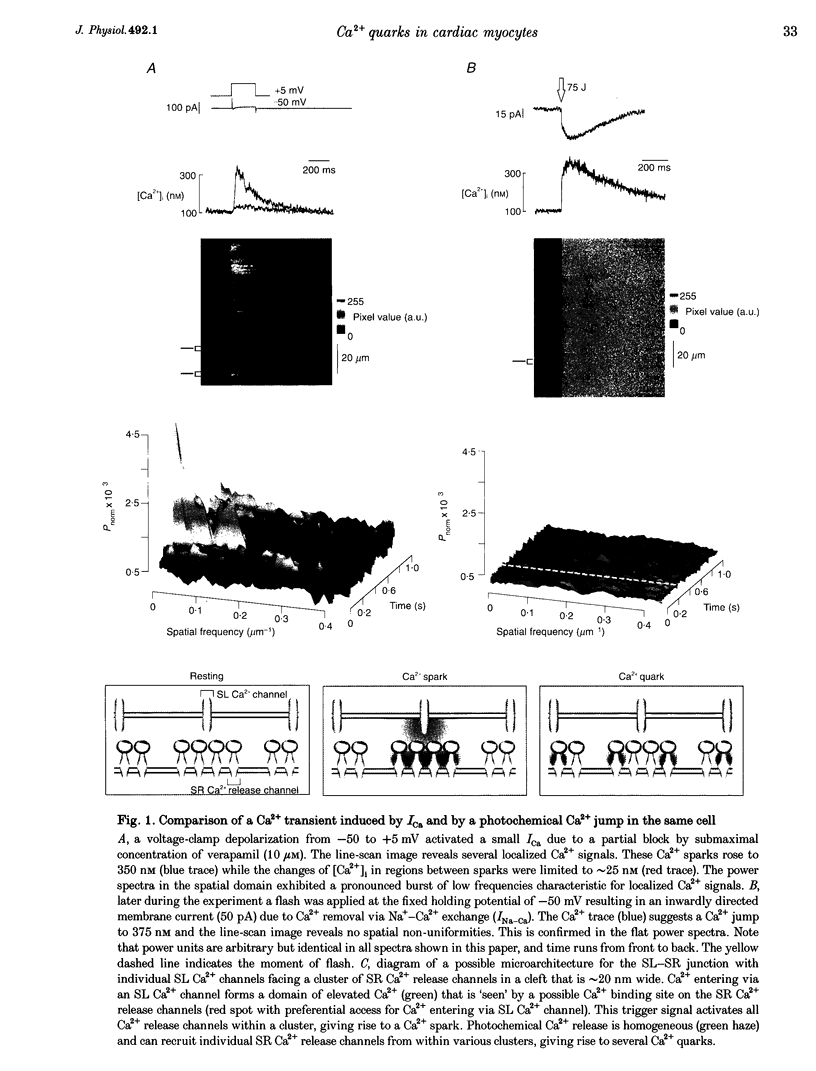
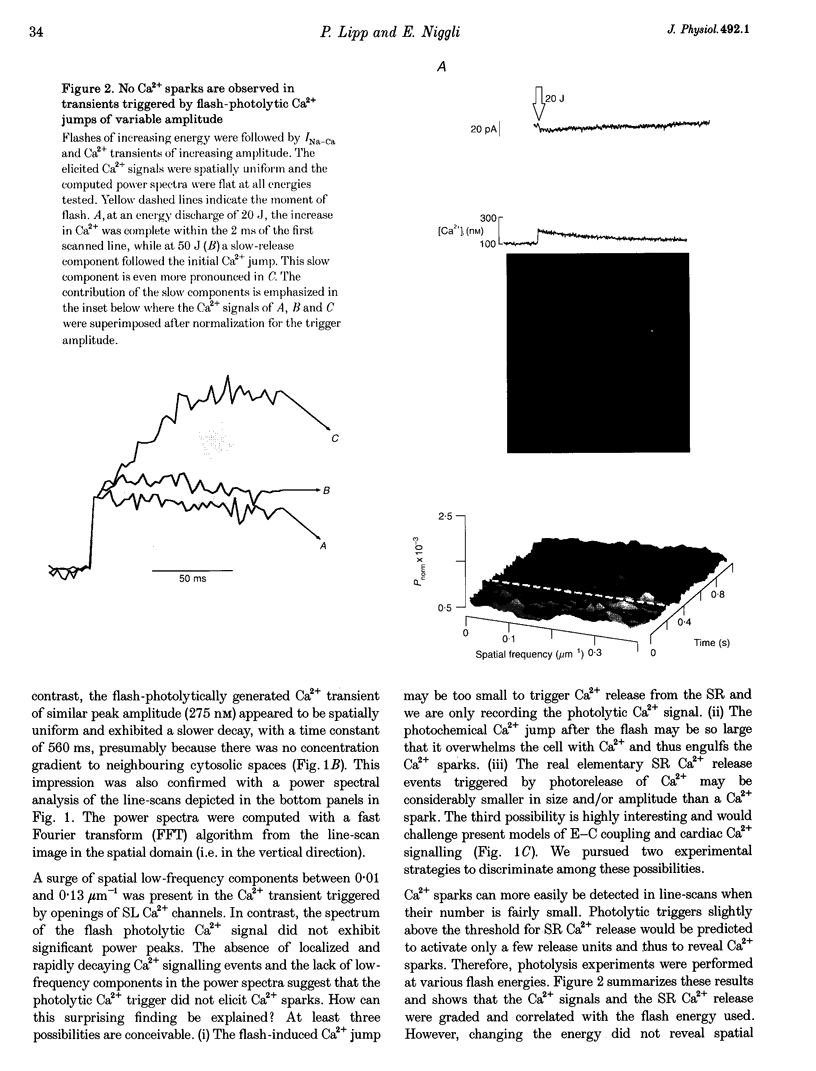
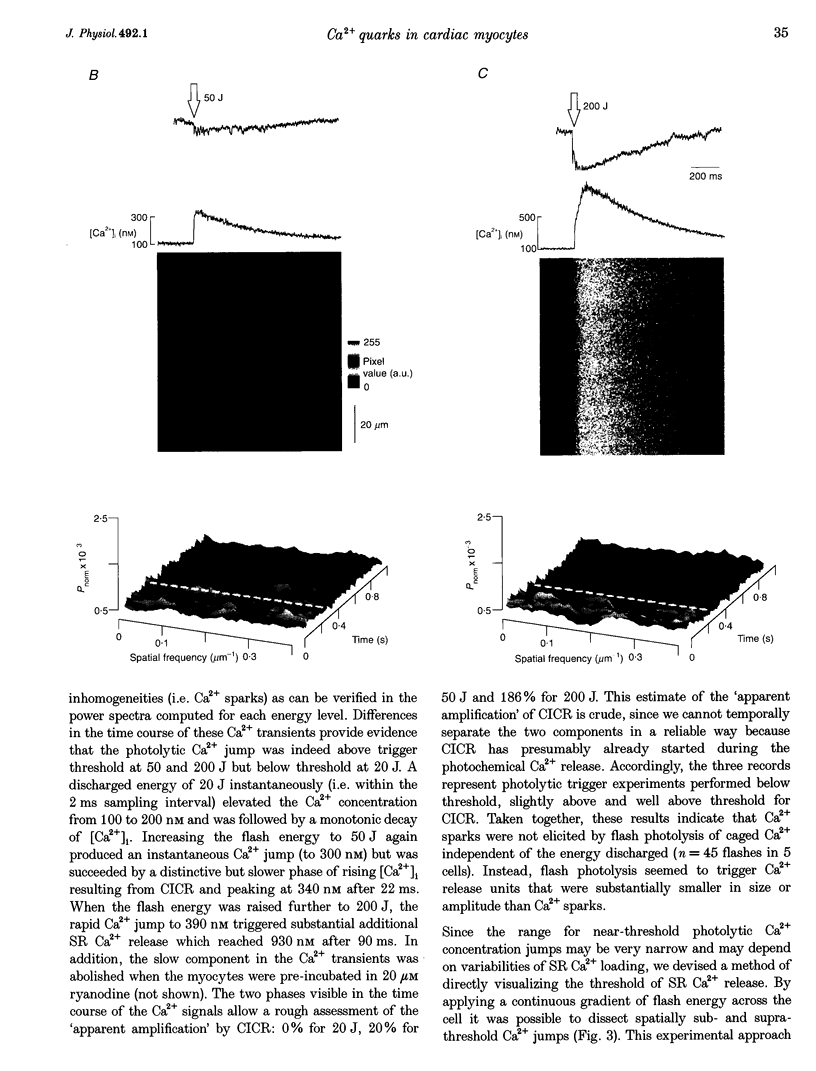
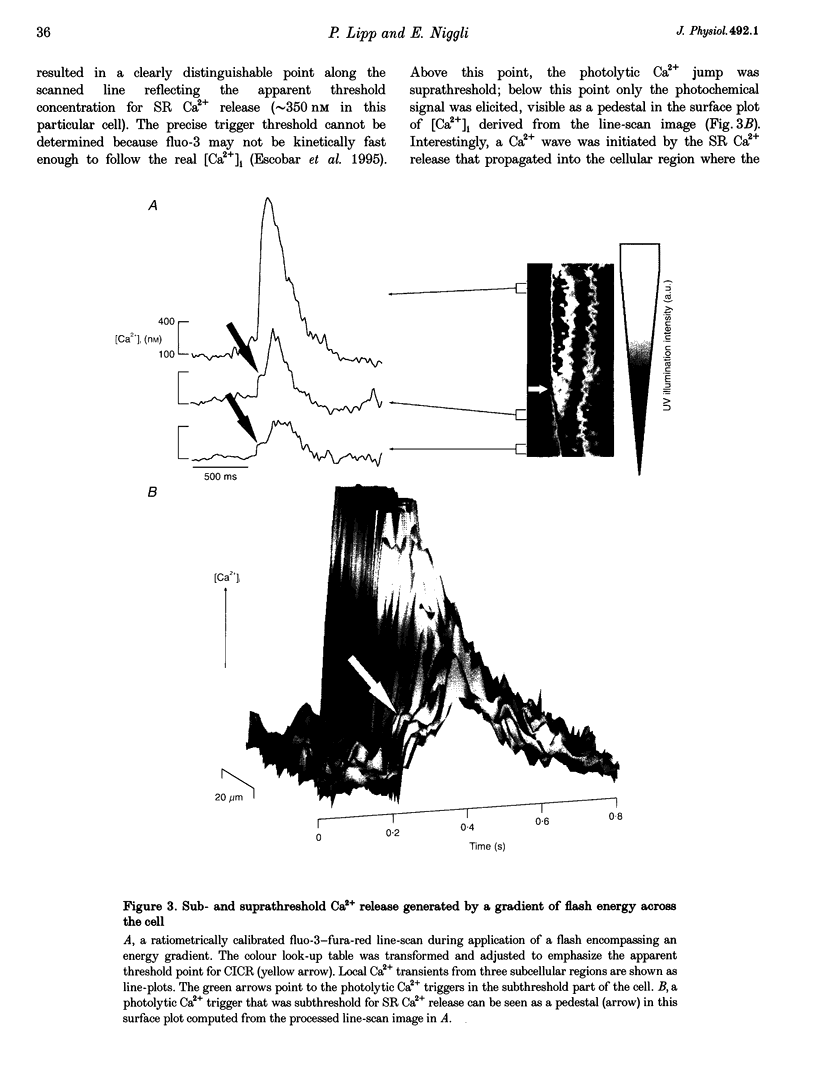


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Block B. A., Imagawa T., Campbell K. P., Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988 Dec;107(6 Pt 2):2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Cheng H., Lederer W. J. Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophys J. 1994 Nov;67(5):1942–1956. doi: 10.1016/S0006-3495(94)80677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar A. L., Cifuentes F., Vergara J. L. Detection of Ca(2+)-transients elicited by flash photolysis of DM-nitrophen with a fast calcium indicator. FEBS Lett. 1995 May 15;364(3):335–338. doi: 10.1016/0014-5793(95)00425-9. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Modulation of Ca2+ release in cultured neonatal rat cardiac myocytes. Insight from subcellular release patterns revealed by confocal microscopy. Circ Res. 1994 May;74(5):979–990. doi: 10.1161/01.res.74.5.979. [DOI] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Sodium current-induced calcium signals in isolated guinea-pig ventricular myocytes. J Physiol. 1994 Feb 1;474(3):439–446. doi: 10.1113/jphysiol.1994.sp020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-López J. R., Shacklock P. S., Balke C. W., Wier W. G. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 1995 May 19;268(5213):1042–1045. doi: 10.1126/science.7754383. [DOI] [PubMed] [Google Scholar]
- Niggli E., Lederer W. J. Voltage-independent calcium release in heart muscle. Science. 1990 Oct 26;250(4980):565–568. doi: 10.1126/science.2173135. [DOI] [PubMed] [Google Scholar]
- Niggli E., Lipp P. Subcellular features of calcium signalling in heart muscle: what do we learn? Cardiovasc Res. 1995 Apr;29(4):441–448. [PubMed] [Google Scholar]
- Näbauer M., Callewaert G., Cleemann L., Morad M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989 May 19;244(4906):800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- Stern M. D. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992 Aug;63(2):497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafford A. W., O'Neill S. C., Eisner D. A. Factors affecting the propagation of locally activated systolic Ca transients in rat ventricular myocytes. Pflugers Arch. 1993 Oct;425(1-2):181–183. doi: 10.1007/BF00374521. [DOI] [PubMed] [Google Scholar]
- Tsugorka A., Ríos E., Blatter L. A. Imaging elementary events of calcium release in skeletal muscle cells. Science. 1995 Sep 22;269(5231):1723–1726. doi: 10.1126/science.7569901. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Cannell M. B., Berlin J. R., Marban E., Lederer W. J. Cellular and subcellular heterogeneity of [Ca2+]i in single heart cells revealed by fura-2. Science. 1987 Jan 16;235(4786):325–328. doi: 10.1126/science.3798114. [DOI] [PubMed] [Google Scholar]