Abstract
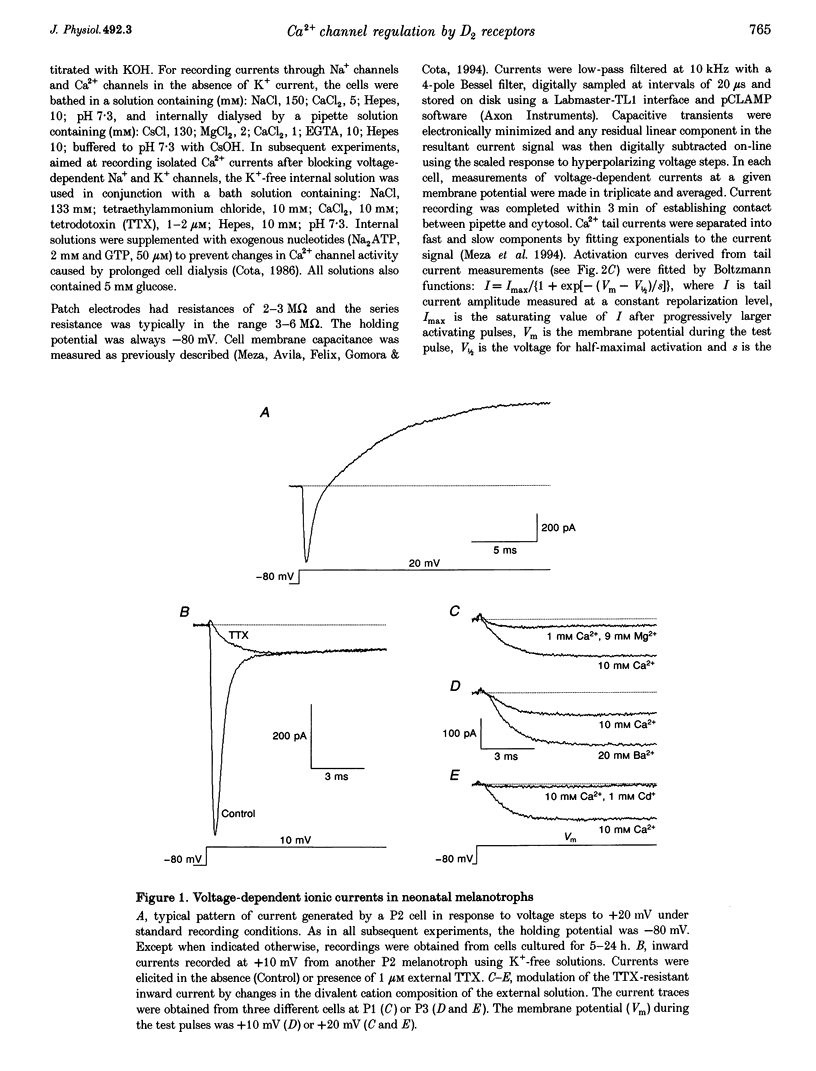
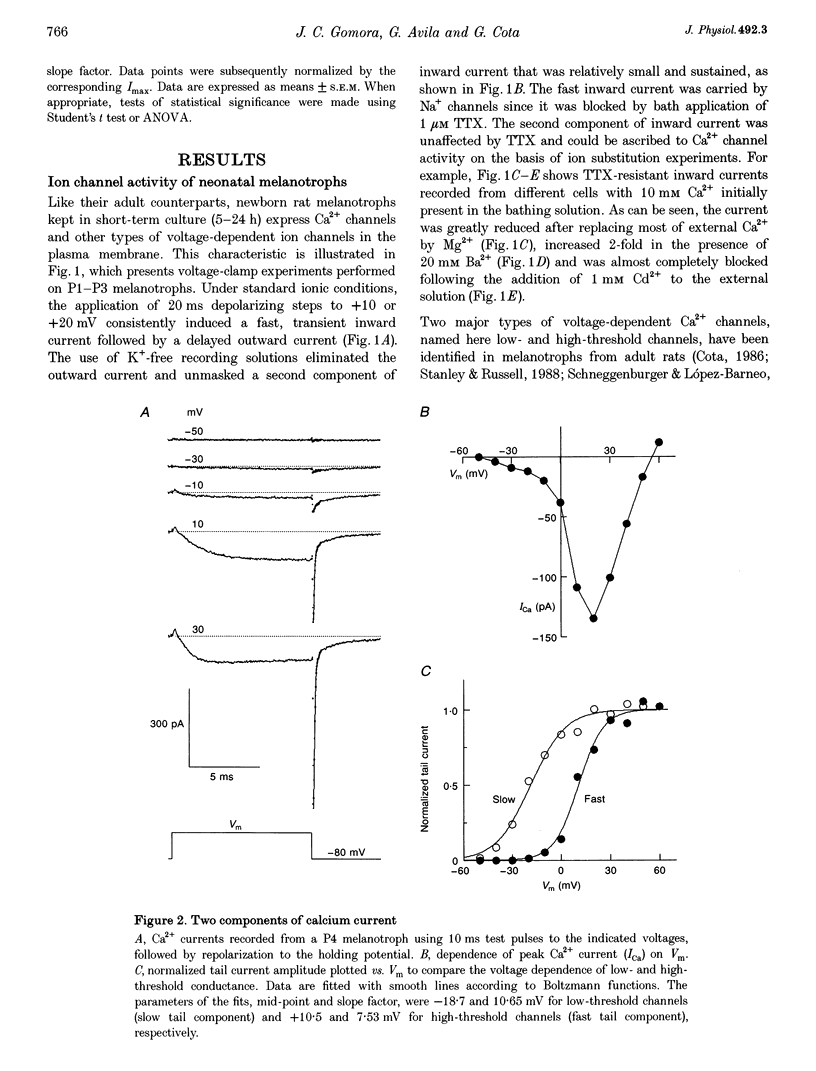
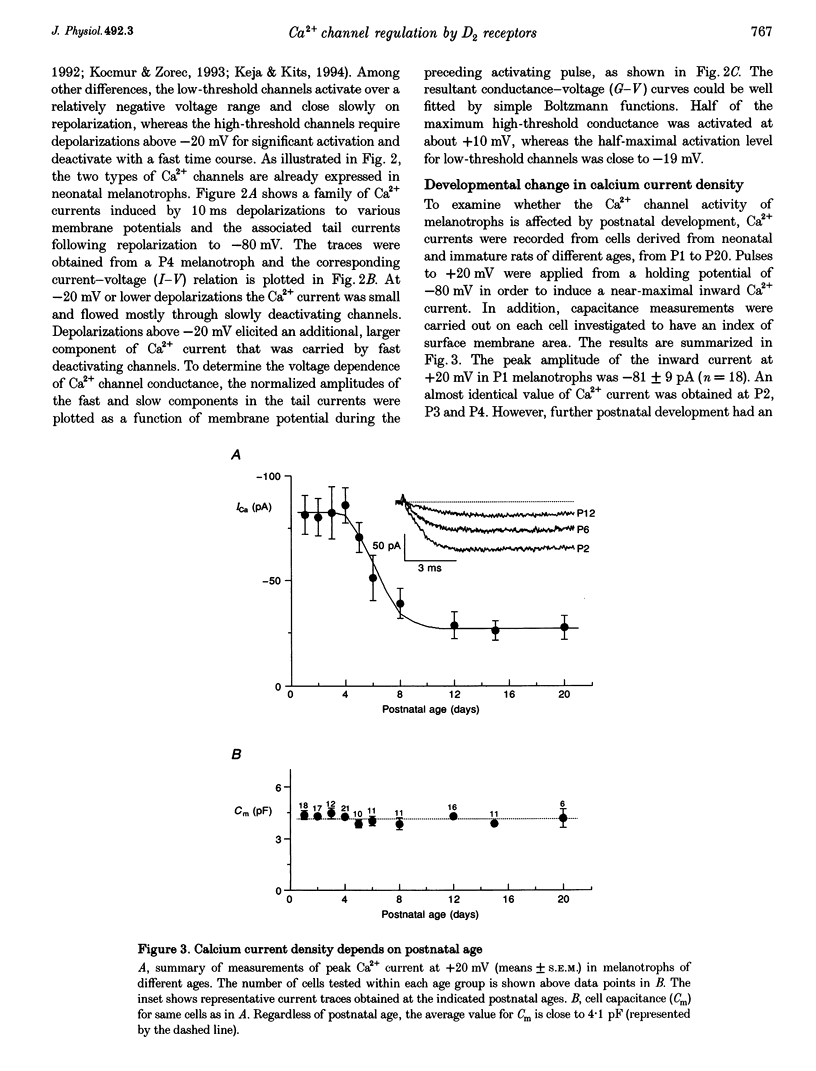
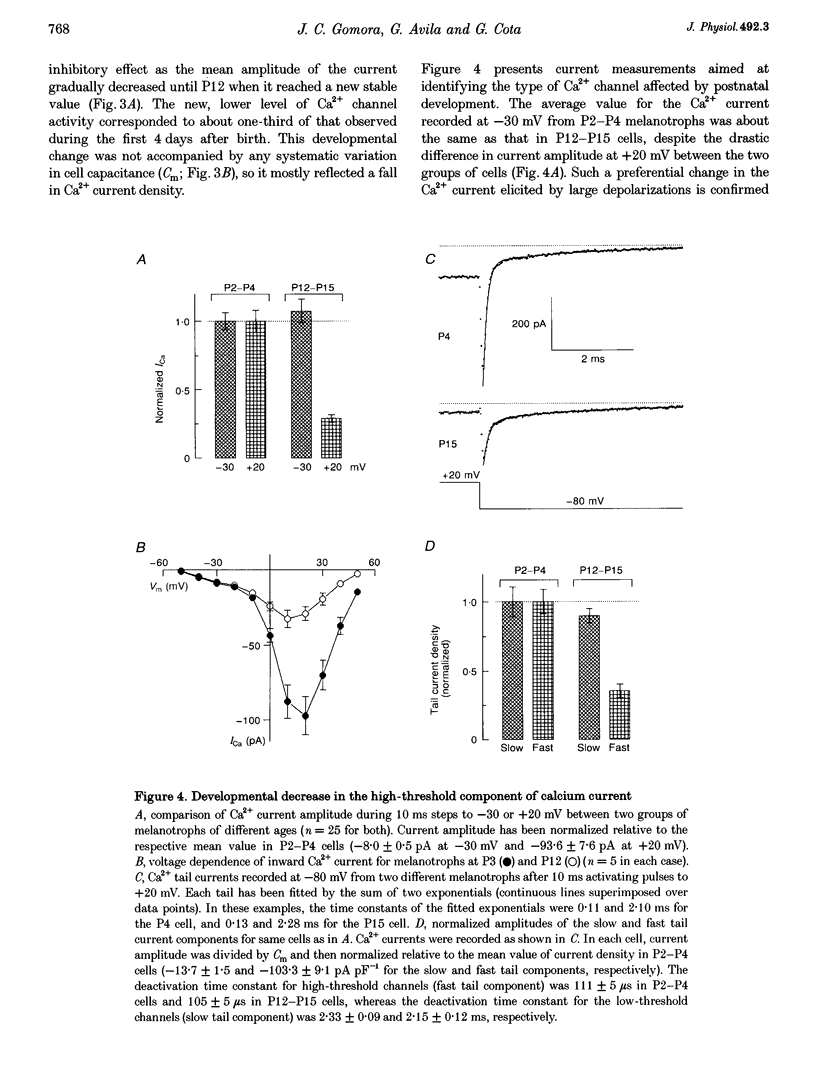
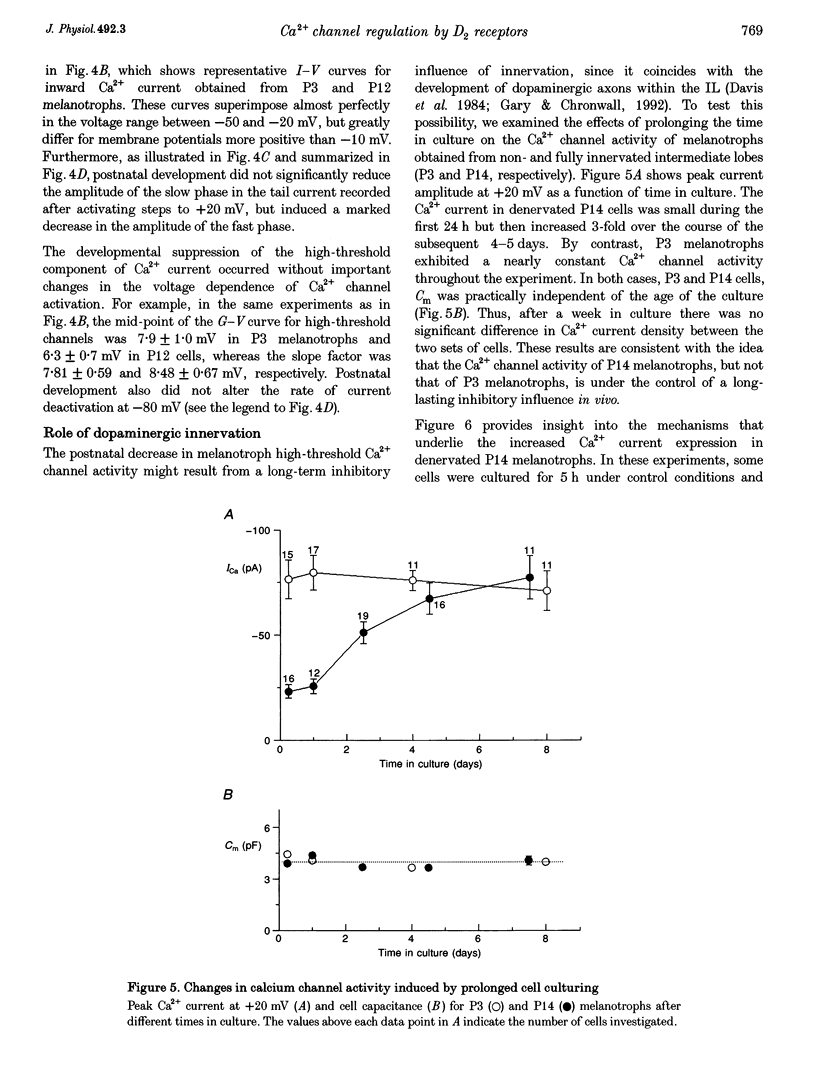
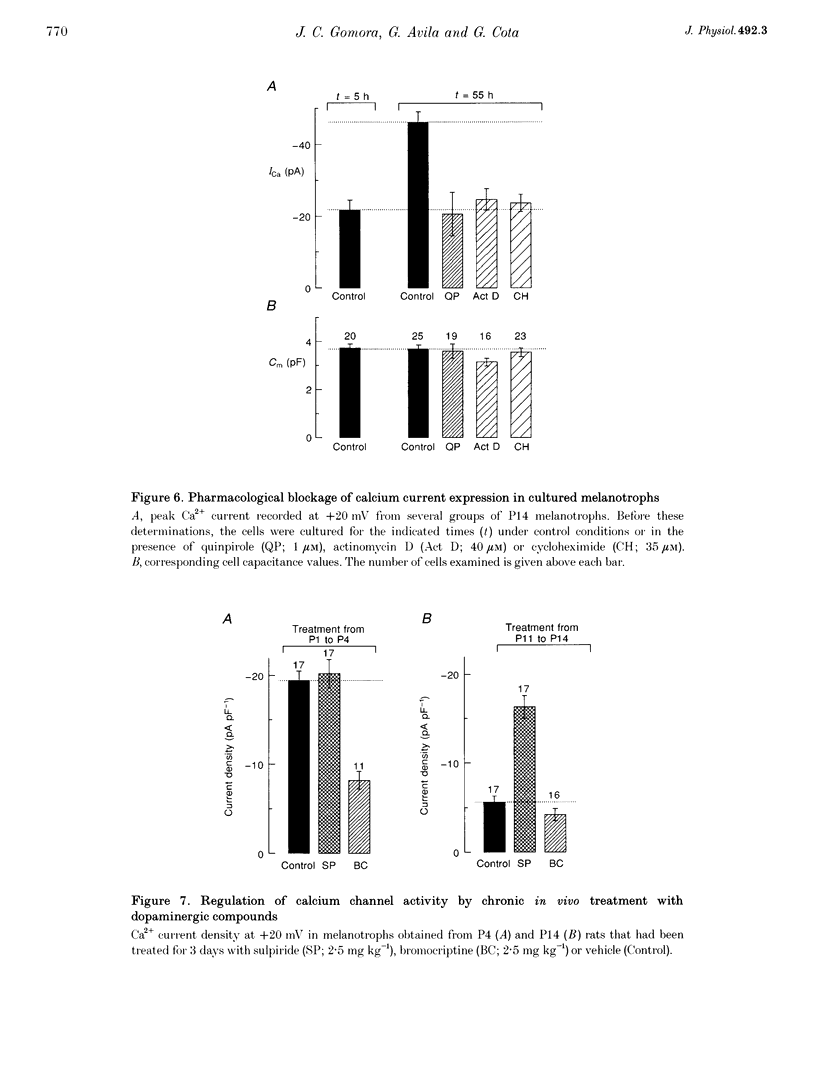
1. We have examined the voltage-dependent Ca2+ channel activity of rat melanotrophs during the early postnatal period. The cells were dissociated from pituitary intermediate lobes, kept in culture for 5-24 h and then subjected to whole-cell patch-clamp experiments. 2. Like their adult counterparts, neonatal melanotrophs were able to generate Na+ currents, K+ currents and Ca2+ currents in response to membrane depolarization. Ca2+ currents were carried by both low- and high-threshold Ca2+ channels. 3. High-threshold Ca2+ current density decreased sharply between postnatal day 4 (P4) and P12. This period coincides with the onset of dopaminergic innervation within the intermediate lobe. Accordingly, the developmental decrease in Ca2+ current density was largely reversed by chronic in vivo treatment with sulpiride, a dopamine D2 receptor antagonist. 4. Prolonging the time in culture from 5 h to 8 days did not significantly alter the Ca2+ channel activity of P3 melanotrophs, whereas the high-threshold Ca2+ current in previously innervated (P14) melanotrophs stayed small for the first 24 h and then increased 3-fold during the subsequent 4-5 days. This increase required RNA and protein synthesis and was prevented by adding D2 agonists to the culture medium. 5. These results provide evidence for a postnatal suppression of high-threshold Ca2+ current expression in pituitary melanotrophs mediated by presynaptic dopamine neurons through D2 dopamine receptors.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Childs G., Unabia G. Application of the avidin-biotin-peroxidase complex (ABC) method to the light microscopic localization of pituitary hormones. J Histochem Cytochem. 1982 Jul;30(7):713–716. doi: 10.1177/30.7.6286753. [DOI] [PubMed] [Google Scholar]
- Chronwall B. M., Beatty D. M., Sharma P., Morris S. J. Dopamine D2 receptors regulate in vitro melanotrope L-type Ca2+ channel activity via c-fos. Endocrinology. 1995 Feb;136(2):614–621. doi: 10.1210/endo.136.2.7835295. [DOI] [PubMed] [Google Scholar]
- Cota G. Calcium channel currents in pars intermedia cells of the rat pituitary gland. Kinetic properties and washout during intracellular dialysis. J Gen Physiol. 1986 Jul;88(1):83–105. doi: 10.1085/jgp.88.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota G., Hiriart M. Hormonal and neurotransmitter regulation of Ca channel activity in cultured adenohypophyseal cells. Soc Gen Physiol Ser. 1989;44:143–165. [PubMed] [Google Scholar]
- Cote T. E., Grewe C. W., Tsuruta K., Stoof J. C., Eskay R. L., Kebabian J. W. D-2 dopamine receptor-mediated inhibition of adenylate cyclase activity in the intermediate lobe of the rat pituitary gland requires guanosine 5'-triphosphate. Endocrinology. 1982 Mar;110(3):812–819. doi: 10.1210/endo-110-3-812. [DOI] [PubMed] [Google Scholar]
- Davis M. D., Lichtensteiger W., Schlumpf M., Bruinink A. Early postnatal development of pituitary intermediate lobe control in the rat by dopamine neurons. Neuroendocrinology. 1984 Jul;39(1):1–12. doi: 10.1159/000123947. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Taraskevich P. S. Action potentials in gland cells of rat pituitary pars intermedia: inhibition by dopamine, an inhibitor of MSH secretion. J Physiol. 1978 Dec;285:171–184. doi: 10.1113/jphysiol.1978.sp012565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary K. A., Chronwall B. M. The onset of dopaminergic innervation during ontogeny decreases melanotrope proliferation in the intermediate lobe of the rat pituitary. Int J Dev Neurosci. 1992 Apr;10(2):131–142. doi: 10.1016/0736-5748(92)90041-w. [DOI] [PubMed] [Google Scholar]
- Gingrich J. A., Caron M. G. Recent advances in the molecular biology of dopamine receptors. Annu Rev Neurosci. 1993;16:299–321. doi: 10.1146/annurev.ne.16.030193.001503. [DOI] [PubMed] [Google Scholar]
- Gonoi T., Hagihara Y., Kobayashi J., Nakamura H., Ohizumi Y. Geographutoxin-sensitive and insensitive sodium currents in mouse skeletal muscle developing in situ. J Physiol. 1989 Jul;414:159–177. doi: 10.1113/jphysiol.1989.sp017682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonoi T., Hasegawa S. Post-natal disappearance of transient calcium channels in mouse skeletal muscle: effects of denervation and culture. J Physiol. 1988 Jul;401:617–637. doi: 10.1113/jphysiol.1988.sp017183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Hall Z. W. Immunological evidence for a change in subunits of the acetylcholine receptor in developing and denervated rat muscle. Neuron. 1988 Apr;1(2):117–125. doi: 10.1016/0896-6273(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Hindelang C., Félix J. M., Laurent F. M., Klein M. J., Stoeckel M. E. Ontogenesis of proopiomelanocortin gene expression and regulation in the rat pituitary intermediate lobe. Mol Cell Endocrinol. 1990 May 7;70(3):225–235. doi: 10.1016/0303-7207(90)90213-r. [DOI] [PubMed] [Google Scholar]
- Kehl S. J., McBurney R. N. The firing patterns of rat melanotrophs recorded using the patch clamp technique. Neuroscience. 1989;33(3):579–586. doi: 10.1016/0306-4522(89)90410-7. [DOI] [PubMed] [Google Scholar]
- Keja J. A., Kits K. S. Single-channel properties of high- and low-voltage-activated calcium channels in rat pituitary melanotropic cells. J Neurophysiol. 1994 Mar;71(3):840–855. doi: 10.1152/jn.1994.71.3.840. [DOI] [PubMed] [Google Scholar]
- Keja J. A., Stoof J. C., Kits K. S. Dopamine D2 receptor stimulation differentially affects voltage-activated calcium channels in rat pituitary melanotropic cells. J Physiol. 1992 May;450:409–435. doi: 10.1113/jphysiol.1992.sp019134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keja J. A., Stoof J. C., Kits K. S. Voltage-activated currents through calcium channels in rat pituitary melanotrophic cells. Neuroendocrinology. 1991 Apr;53(4):349–359. doi: 10.1159/000125741. [DOI] [PubMed] [Google Scholar]
- Kocmur L., Zorec R. A new approach to separation of voltage-activated Ca currents in rat melanotrophs. Pflugers Arch. 1993 Oct;425(1-2):172–174. doi: 10.1007/BF00374518. [DOI] [PubMed] [Google Scholar]
- Loeffler J. P., Kley N., Pittius C. W., Höllt V. Calcium ion and cyclic adenosine 3',5'-monophosphate regulate proopiomelanocortin messenger ribonucleic acid levels in rat intermediate and anterior pituitary lobes. Endocrinology. 1986 Dec;119(6):2840–2847. doi: 10.1210/endo-119-6-2840. [DOI] [PubMed] [Google Scholar]
- Mansour A., Meador-Woodruff J. H., Bunzow J. R., Civelli O., Akil H., Watson S. J. Localization of dopamine D2 receptor mRNA and D1 and D2 receptor binding in the rat brain and pituitary: an in situ hybridization-receptor autoradiographic analysis. J Neurosci. 1990 Aug;10(8):2587–2600. doi: 10.1523/JNEUROSCI.10-08-02587.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza U., Avila G., Felix R., Gomora J. C., Cota G. Long-term regulation of calcium channels in clonal pituitary cells by epidermal growth factor, insulin, and glucocorticoids. J Gen Physiol. 1994 Dec;104(6):1019–1038. doi: 10.1085/jgp.104.6.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemura M., Cote T. E., Tsuruta K., Eskay R. L., Kebabian J. W. The dopamine receptor in the intermediate lobe of the rat pituitary gland: pharmacological characterization. Endocrinology. 1980 Dec;107(6):1676–1683. doi: 10.1210/endo-107-6-1676. [DOI] [PubMed] [Google Scholar]
- Offord J., Catterall W. A. Electrical activity, cAMP, and cytosolic calcium regulate mRNA encoding sodium channel alpha subunits in rat muscle cells. Neuron. 1989 May;2(5):1447–1452. doi: 10.1016/0896-6273(89)90190-6. [DOI] [PubMed] [Google Scholar]
- Ramsdell J. S. Voltage-dependent calcium channels regulate GH4 pituitary cell proliferation at two stages of the cell cycle. J Cell Physiol. 1991 Feb;146(2):197–206. doi: 10.1002/jcp.1041460203. [DOI] [PubMed] [Google Scholar]
- Ritchie A. K. Estrogen increases low voltage-activated calcium current density in GH3 anterior pituitary cells. Endocrinology. 1993 Apr;132(4):1621–1629. doi: 10.1210/endo.132.4.8462461. [DOI] [PubMed] [Google Scholar]
- Robertson G. S., Vincent S. R., Fibiger H. C. D1 and D2 dopamine receptors differentially regulate c-fos expression in striatonigral and striatopallidal neurons. Neuroscience. 1992 Jul;49(2):285–296. doi: 10.1016/0306-4522(92)90096-k. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R., López-Barneo J. Patch-clamp analysis of voltage-gated currents in intermediate lobe cells from rat pituitary thin slices. Pflugers Arch. 1992 Mar;420(3-4):302–312. doi: 10.1007/BF00374463. [DOI] [PubMed] [Google Scholar]
- Sherman S. J., Catterall W. A. Biphasic regulation of development of the high-affinity saxitoxin receptor by innervation in rat skeletal muscle. J Gen Physiol. 1982 Nov;80(5):753–768. doi: 10.1085/jgp.80.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack J., Surprenant A. Dopamine actions on calcium currents, potassium currents and hormone release in rat melanotrophs. J Physiol. 1991 Aug;439:37–58. doi: 10.1113/jphysiol.1991.sp018655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. F., Russell J. T. Inactivation of calcium channels in rat pituitary intermediate lobe cells. Brain Res. 1988 Dec 13;475(1):64–72. doi: 10.1016/0006-8993(88)90199-0. [DOI] [PubMed] [Google Scholar]
- Thomas P., Surprenant A., Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990 Nov;5(5):723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]
- Valentijn J. A., Vaudry H., Cazin L. Multiple control of calcium channel gating by dopamine D2 receptors in frog pituitary melanotrophs. Ann N Y Acad Sci. 1993 May 31;680:211–228. doi: 10.1111/j.1749-6632.1993.tb19686.x. [DOI] [PubMed] [Google Scholar]
- Williams P. J., MacVicar B. A., Pittman Q. J. Synaptic modulation by dopamine of calcium currents in rat pars intermedia. J Neurosci. 1990 Mar;10(3):757–763. doi: 10.1523/JNEUROSCI.10-03-00757.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. F., Robinson R. B., Siegelbaum S. A. Sympathetic neurons mediate developmental change in cardiac sodium channel gating through long-term neurotransmitter action. Neuron. 1992 Jul;9(1):97–103. doi: 10.1016/0896-6273(92)90224-2. [DOI] [PubMed] [Google Scholar]


