Abstract
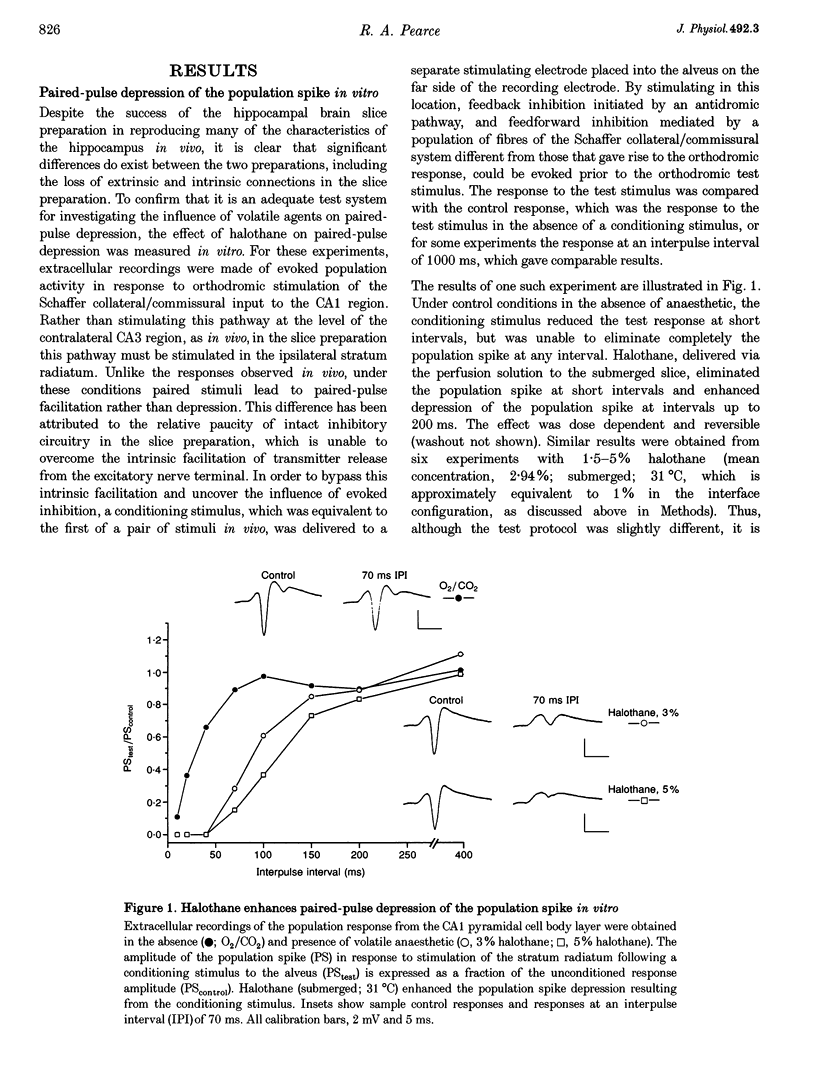
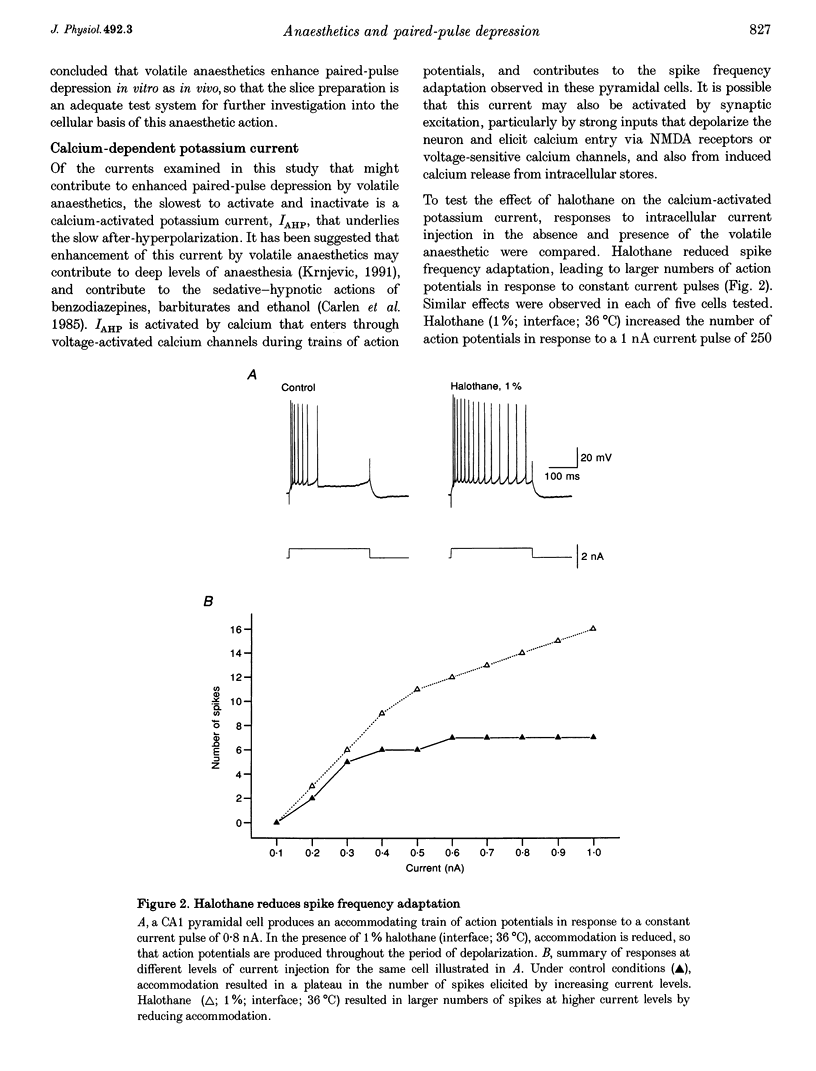
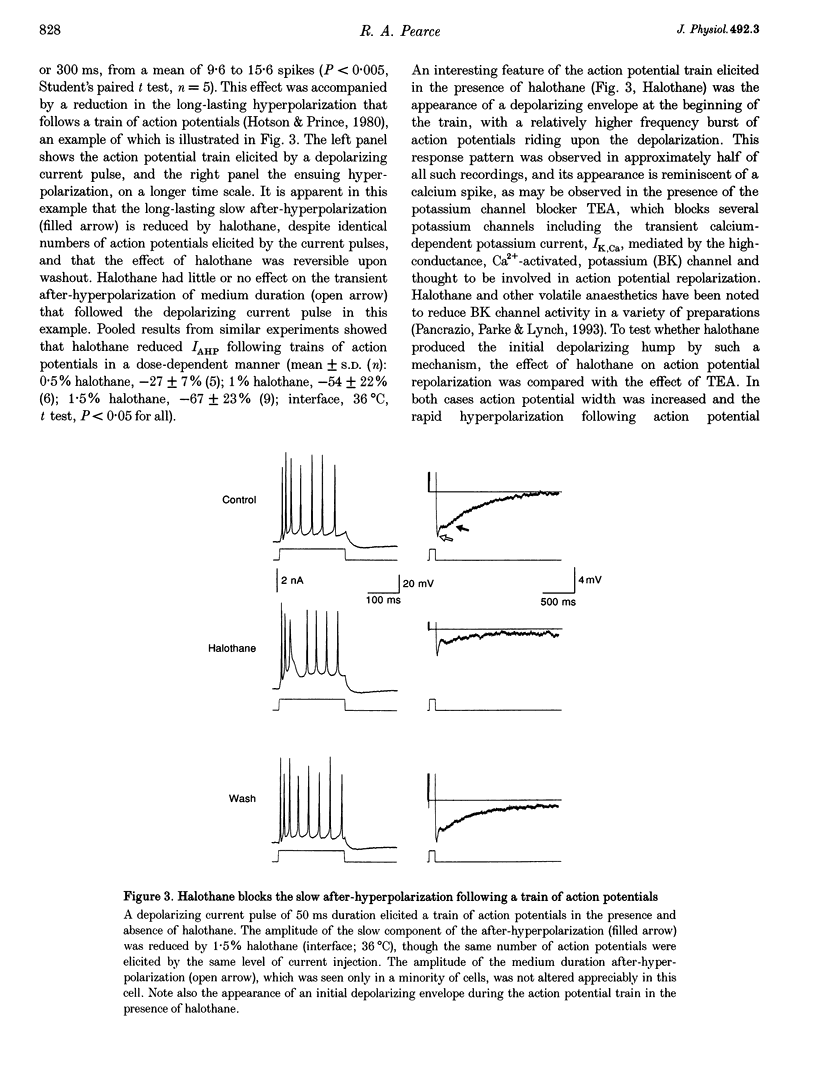
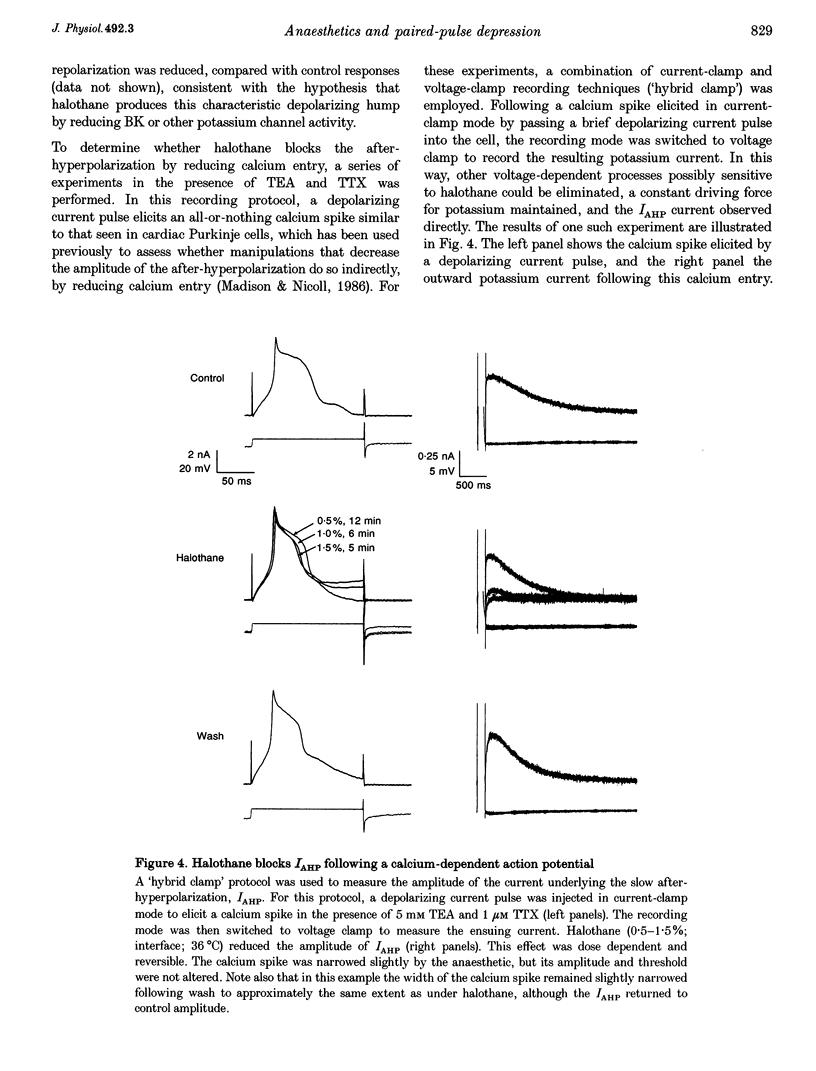
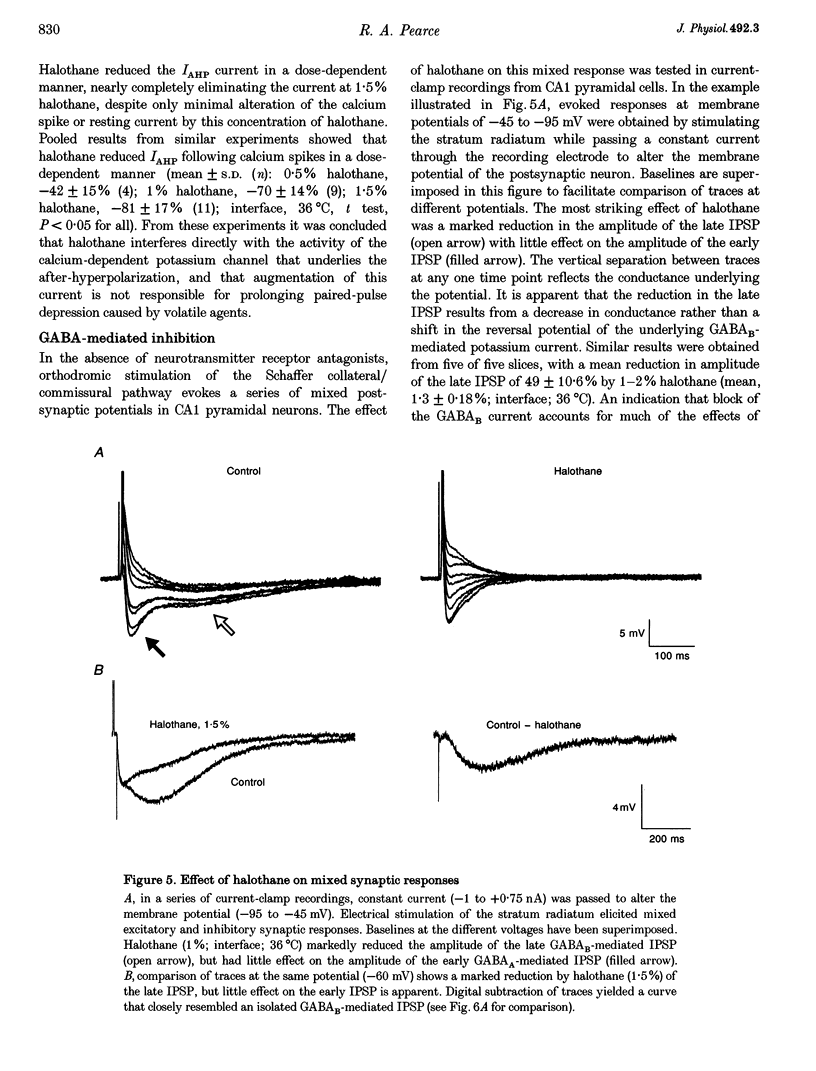
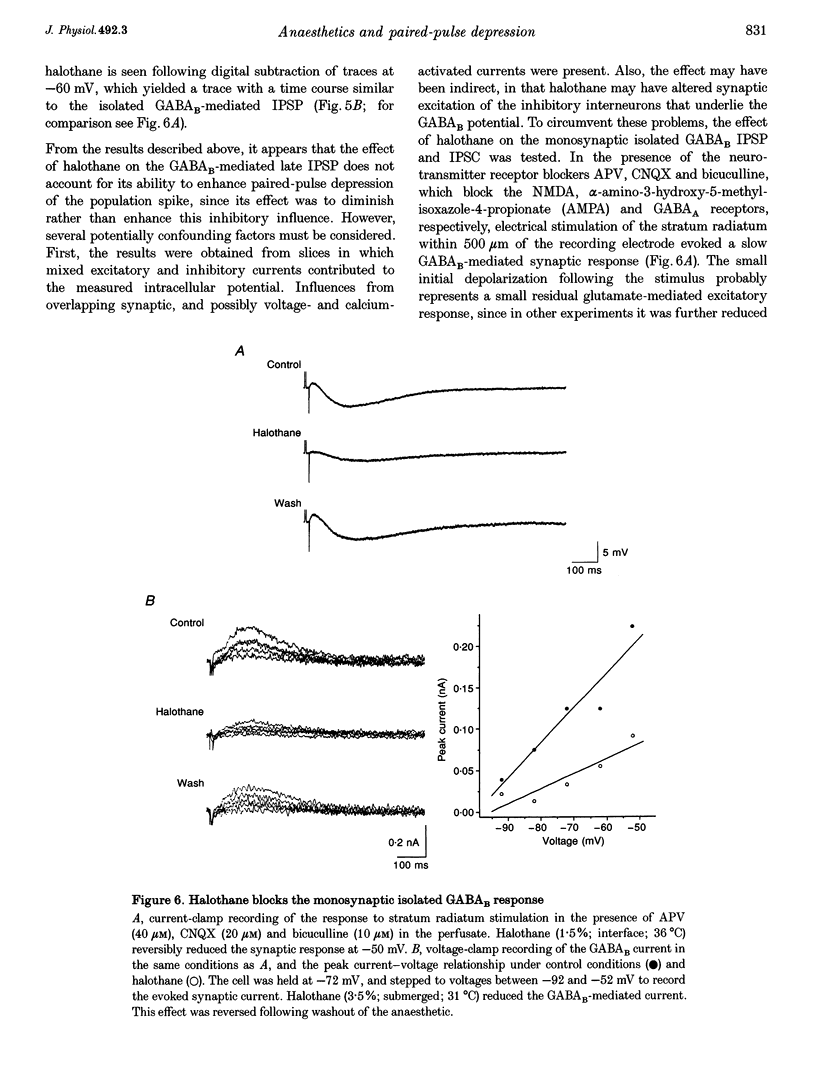
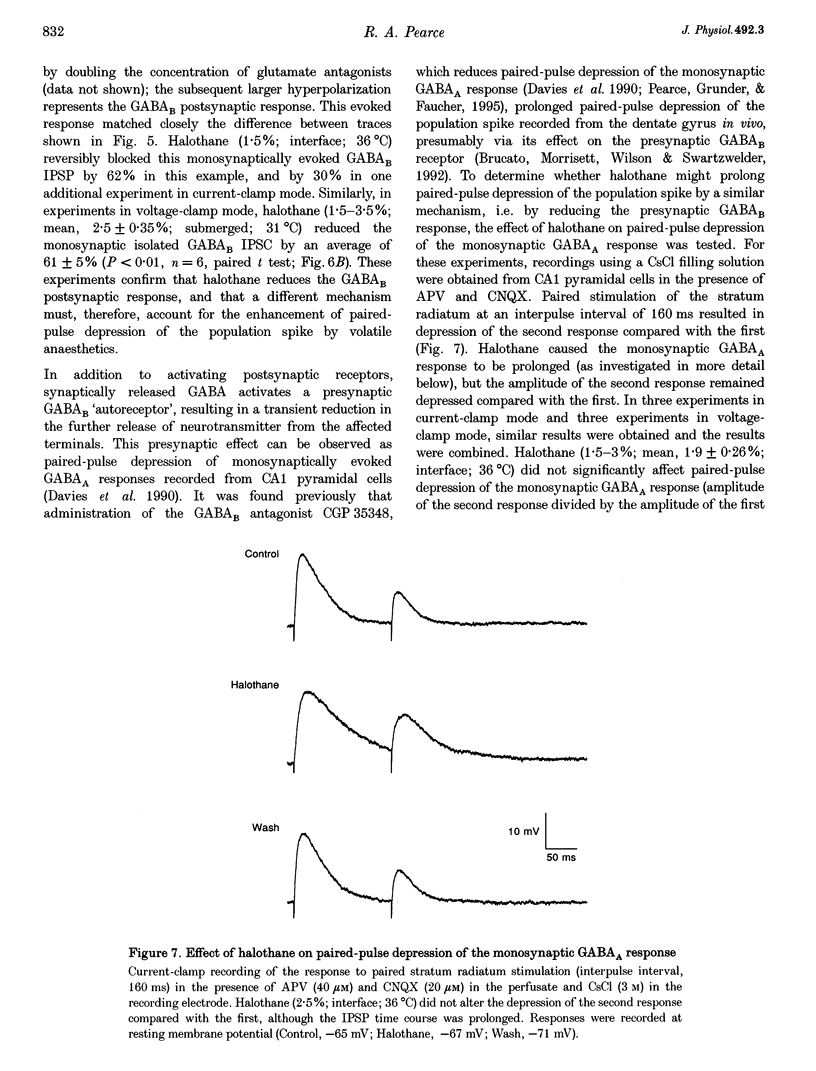
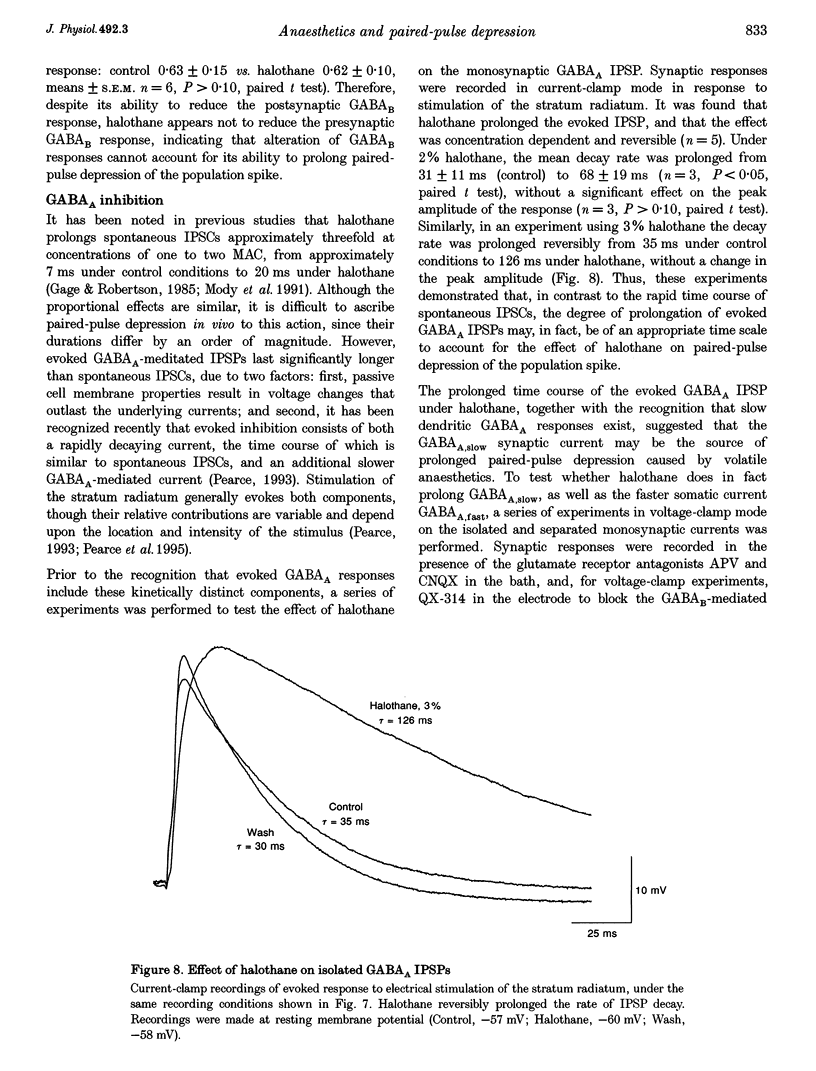
1. A prominent in vivo effect of general anaesthetics, including volatile anaesthetics such as halothane, is the prolonging of paired-pulse depression of the hippocampal CA1 population spike. The mechanisms by which volatile anaesthetics produce this effect were investigated in the hippocampal brain slice preparation by testing the effect of halothane on several long-lasting inhibitory processes, including the calcium-activated potassium current that underlies the slow after-hyperpolarization (IAHP), the GABAB-mediated potassium current that underlies the late IPSP, and the fast and slow components of the early GABAA-mediated IPSP. 2. Halothane produced a dose-dependent block of IAHP at concentrations between 0.5 and 1.5%. This block was manifested as a reduction in spike frequency adaptation, a reduction in the amplitude of the slow after-hyperpolarization following a train of action potentials, and a reduction in the amplitude of the voltage-clamped current following a calcium spike elicited in the presence of tetraethylammonium and tetrodotoxin. The effect did not appear to be caused by blockade of voltage-sensitive calcium channels, since halothane markedly reduced IAHP at a concentration (1.5%) that had little effect on the depolarization-evoked calcium spike. 3. Halothane reduced the amplitude of the late GABAB-mediated IPSP by approximately 50% at concentrations between 1 and 2%. Similar results were obtained for polysynaptic and monosynaptic responses, and with current-clamp and voltage-clamp recordings. However, halothane, at concentrations up to 3%, had no effect on the presynaptic GABAB response, as indicated by no reduction in paired-pulse depression of the monosynaptic GABAA response. 4. Halothane (2%) and enflurane (4%) prolonged the decay phase of the slow component of the monosynaptic GABAA-mediated IPSC approximately twofold, but did not alter the amplitude of the response. Halothane also prolonged the decay phase of the fast component of the GABAA-mediated IPSC, with no effect on the amplitude. However, enflurane markedly reduced the amplitude of the fast component of the GABAA IPSC, so that only a small slow current remained in response to a selective stimulus. 5. It is concluded that the effects of halothane on IAHP and on GABAB responses cannot account for its effects on paired-pulse depression, but that volatile anaesthetics enhance paired-pulse depression by prolonging the decay of the slow dendritic GABAA response. Furthermore, it is speculated that the proconvulsant property of enflurane is related to its depression of the fast somatic component of GABAA inhibition.
Full text
PDF


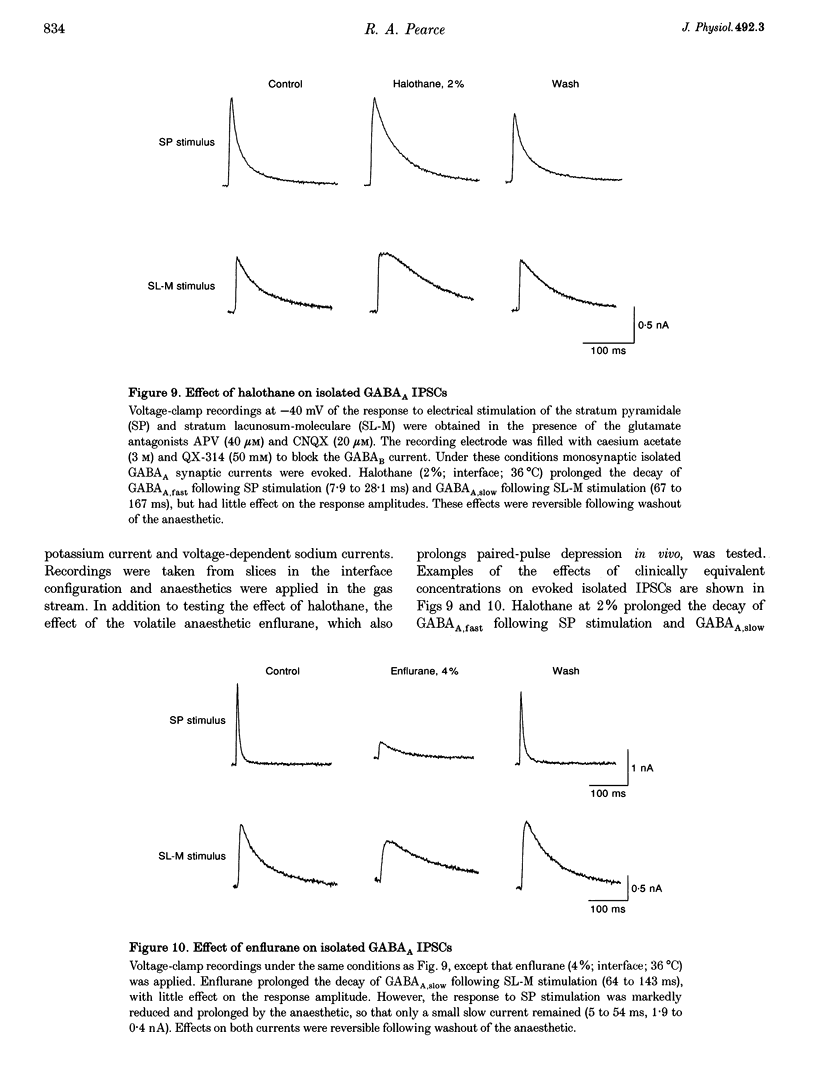
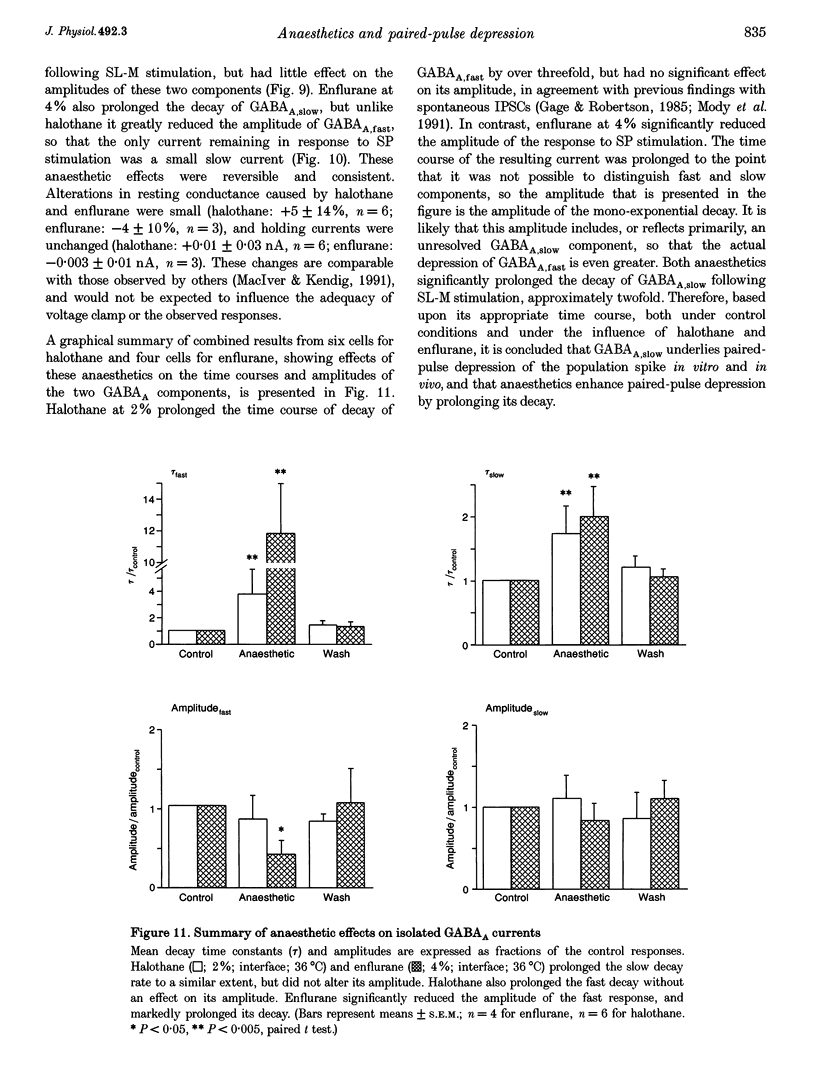





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton D., Wauquier A. Modulation of a GABA-ergic inhibitory circuit in the in vitro hippocampus by etomidate isomers. Anesth Analg. 1985 Oct;64(10):975–980. [PubMed] [Google Scholar]
- Black G. W. Enflurane. Br J Anaesth. 1979 Jul;51(7):627–640. doi: 10.1093/bja/51.7.627. [DOI] [PubMed] [Google Scholar]
- Brucato F. H., Morrisett R. A., Wilson W. A., Swartzwelder H. S. The GABAB receptor antagonist, CGP-35348, inhibits paired-pulse disinhibition in the rat dentate gyrus in vivo. Brain Res. 1992 Aug 14;588(1):150–153. doi: 10.1016/0006-8993(92)91355-i. [DOI] [PubMed] [Google Scholar]
- Carlen P. L., Gurevich N., Davies M. F., Blaxter T. J., O'Beirne M. Enhanced neuronal K+ conductance: a possible common mechanism for sedative-hypnotic drug action. Can J Physiol Pharmacol. 1985 Jul;63(7):831–837. doi: 10.1139/y85-137. [DOI] [PubMed] [Google Scholar]
- Davies C. H., Davies S. N., Collingridge G. L. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990 May;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T. V., Worth T. S., Olsen R. W. Facilitation of recurrent inhibition in rat hippocampus by barbiturate and related nonbarbiturate depressant drugs. J Pharmacol Exp Ther. 1986 Aug;238(2):564–575. [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. A physiological role for GABAB receptors in the central nervous system. Nature. 1988 Mar 10;332(6160):156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994 Feb 17;367(6464):607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- Fujiwara N., Higashi H., Nishi S., Shimoji K., Sugita S., Yoshimura M. Changes in spontaneous firing patterns of rat hippocampal neurones induced by volatile anaesthetics. J Physiol. 1988 Aug;402:155–175. doi: 10.1113/jphysiol.1988.sp017198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Robertson B. Prolongation of inhibitory postsynaptic currents by pentobarbitone, halothane and ketamine in CA1 pyramidal cells in rat hippocampus. Br J Pharmacol. 1985 Jul;85(3):675–681. doi: 10.1111/j.1476-5381.1985.tb10563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotson J. R., Prince D. A. A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. J Neurophysiol. 1980 Feb;43(2):409–419. doi: 10.1152/jn.1980.43.2.409. [DOI] [PubMed] [Google Scholar]
- Jones M. V., Harrison N. L. Effects of volatile anesthetics on the kinetics of inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurophysiol. 1993 Oct;70(4):1339–1349. doi: 10.1152/jn.1993.70.4.1339. [DOI] [PubMed] [Google Scholar]
- Kapur J., Stringer J. L., Lothman E. W. Evidence that repetitive seizures in the hippocampus cause a lasting reduction of GABAergic inhibition. J Neurophysiol. 1989 Feb;61(2):417–426. doi: 10.1152/jn.1989.61.2.417. [DOI] [PubMed] [Google Scholar]
- MacIver M. B., Kendig J. J. Anesthetic effects on resting membrane potential are voltage-dependent and agent-specific. Anesthesiology. 1991 Jan;74(1):83–88. doi: 10.1097/00000542-199101000-00014. [DOI] [PubMed] [Google Scholar]
- MacIver M. B., Roth S. H. Enflurane-induced burst firing of hippocampal CA 1 neurones. In vitro studies using a brain slice preparation. Br J Anaesth. 1987 Mar;59(3):369–378. doi: 10.1093/bja/59.3.369. [DOI] [PubMed] [Google Scholar]
- MacIver M. B., Tauck D. L., Kendig J. J. General anaesthetic modification of synaptic facilitation and long-term potentiation in hippocampus. Br J Anaesth. 1989 Mar;62(3):301–310. doi: 10.1093/bja/62.3.301. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Cyclic adenosine 3',5'-monophosphate mediates beta-receptor actions of noradrenaline in rat hippocampal pyramidal cells. J Physiol. 1986 Mar;372:245–259. doi: 10.1113/jphysiol.1986.sp016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miu P., Puil E. Isoflurane-induced impairment of synaptic transmission in hippocampal neurons. Exp Brain Res. 1989;75(2):354–360. doi: 10.1007/BF00247941. [DOI] [PubMed] [Google Scholar]
- Mody I., Tanelian D. L., MacIver M. B. Halothane enhances tonic neuronal inhibition by elevating intracellular calcium. Brain Res. 1991 Jan 11;538(2):319–323. doi: 10.1016/0006-8993(91)90447-4. [DOI] [PubMed] [Google Scholar]
- Nakahiro M., Yeh J. Z., Brunner E., Narahashi T. General anesthetics modulate GABA receptor channel complex in rat dorsal root ganglion neurons. FASEB J. 1989 May;3(7):1850–1854. doi: 10.1096/fasebj.3.7.2541038. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Madison D. V. General anesthetics hyperpolarize neurons in the vertebrate central nervous system. Science. 1982 Sep 10;217(4564):1055–1057. doi: 10.1126/science.7112112. [DOI] [PubMed] [Google Scholar]
- Pancrazio J. J., Park W. K., Lynch C., 3rd Inhalational anesthetic actions on voltage-gated ion currents of bovine adrenal chromaffin cells. Mol Pharmacol. 1993 May;43(5):783–794. [PubMed] [Google Scholar]
- Pearce R. A., Grunder S. D., Faucher L. D. Different mechanisms for use-dependent depression of two GABAA-mediated IPSCs in rat hippocampus. J Physiol. 1995 Apr 15;484(Pt 2):425–435. doi: 10.1113/jphysiol.1995.sp020675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce R. A. Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron. 1993 Feb;10(2):189–200. doi: 10.1016/0896-6273(93)90310-n. [DOI] [PubMed] [Google Scholar]
- Pearce R. A., Stringer J. L., Lothman E. W. Effect of volatile anesthetics on synaptic transmission in the rat hippocampus. Anesthesiology. 1989 Oct;71(4):591–598. doi: 10.1097/00000542-198910000-00019. [DOI] [PubMed] [Google Scholar]
- Proctor W. R., Mynlieff M., Dunwiddie T. V. Facilitatory action of etomidate and pentobarbital on recurrent inhibition in rat hippocampal pyramidal neurons. J Neurosci. 1986 Nov;6(11):3161–3168. doi: 10.1523/JNEUROSCI.06-11-03161.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia G., Costa E., Vicini S. Functional diversity of GABA-activated Cl- currents in Purkinje versus granule neurons in rat cerebellar slices. Neuron. 1994 Jan;12(1):117–126. doi: 10.1016/0896-6273(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Rock D. M., Taylor C. P. Effects of diazepam, pentobarbital, phenytoin and pentylenetetrazol on hippocampal paired-pulse inhibition in vivo. Neurosci Lett. 1986 Apr 24;65(3):265–270. doi: 10.1016/0304-3940(86)90272-7. [DOI] [PubMed] [Google Scholar]
- Southan A. P., Wann K. T. Inhalation anaesthetics block accommodation of pyramidal cell discharge in the rat hippocampus. Br J Anaesth. 1989 Nov;63(5):581–586. [PubMed] [Google Scholar]
- Takenoshita M., Steinbach J. H. Halothane blocks low-voltage-activated calcium current in rat sensory neurons. J Neurosci. 1991 May;11(5):1404–1412. doi: 10.1523/JNEUROSCI.11-05-01404.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenoshita M., Takahashi T. Mechanisms of halothane action on synaptic transmission in motoneurons of the newborn rat spinal cord in vitro. Brain Res. 1987 Feb 3;402(2):303–310. doi: 10.1016/0006-8993(87)90037-0. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A. Formation of heteromeric gamma-aminobutyric acid type A receptors containing two different alpha subunits. Mol Pharmacol. 1994 Mar;45(3):475–480. [PubMed] [Google Scholar]


