Abstract
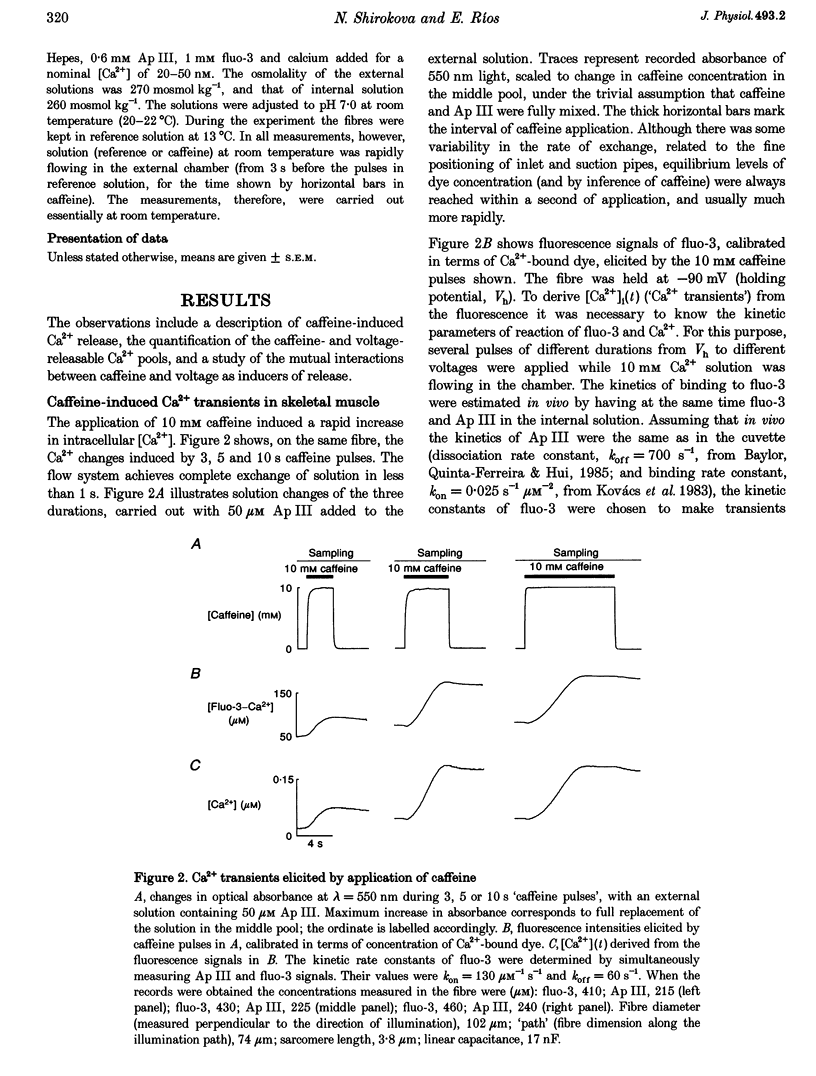
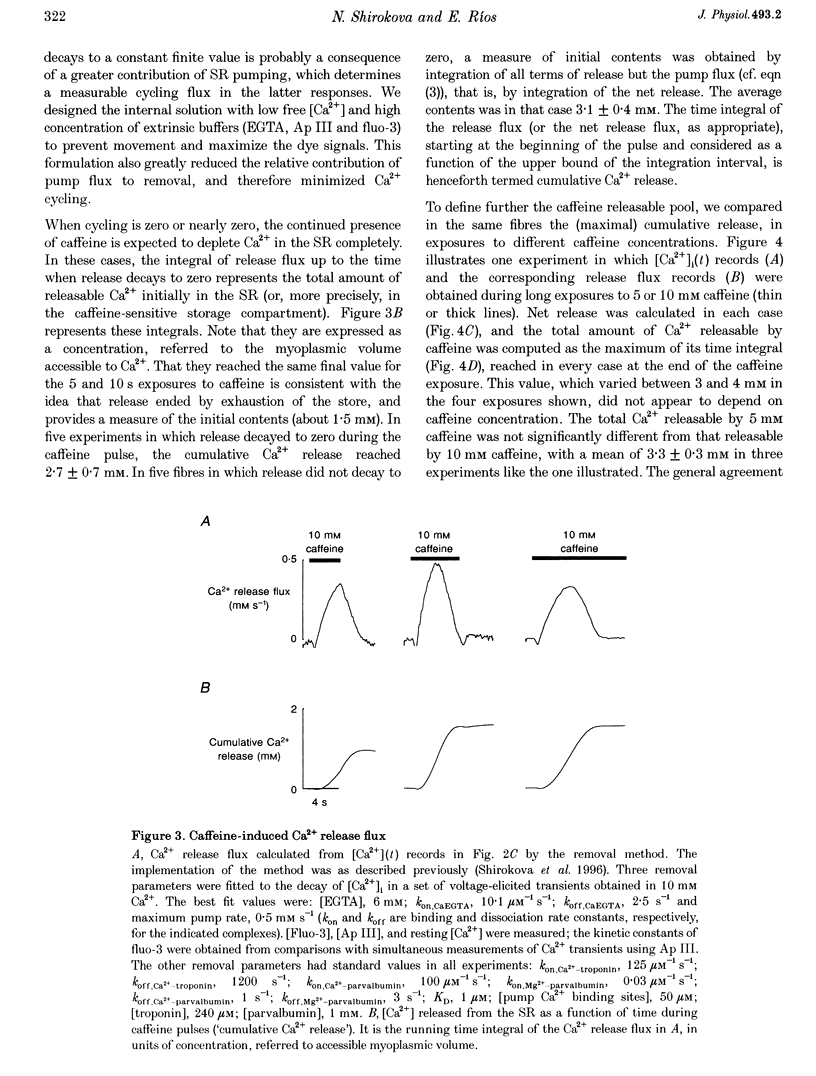
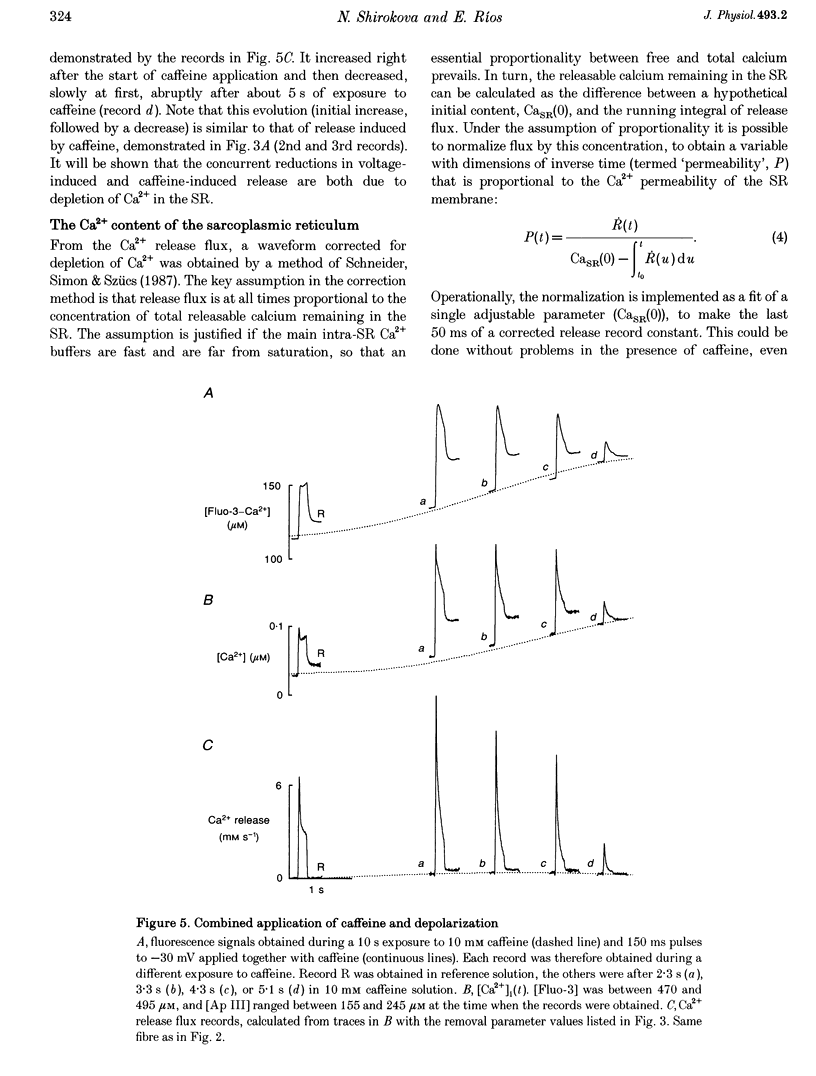
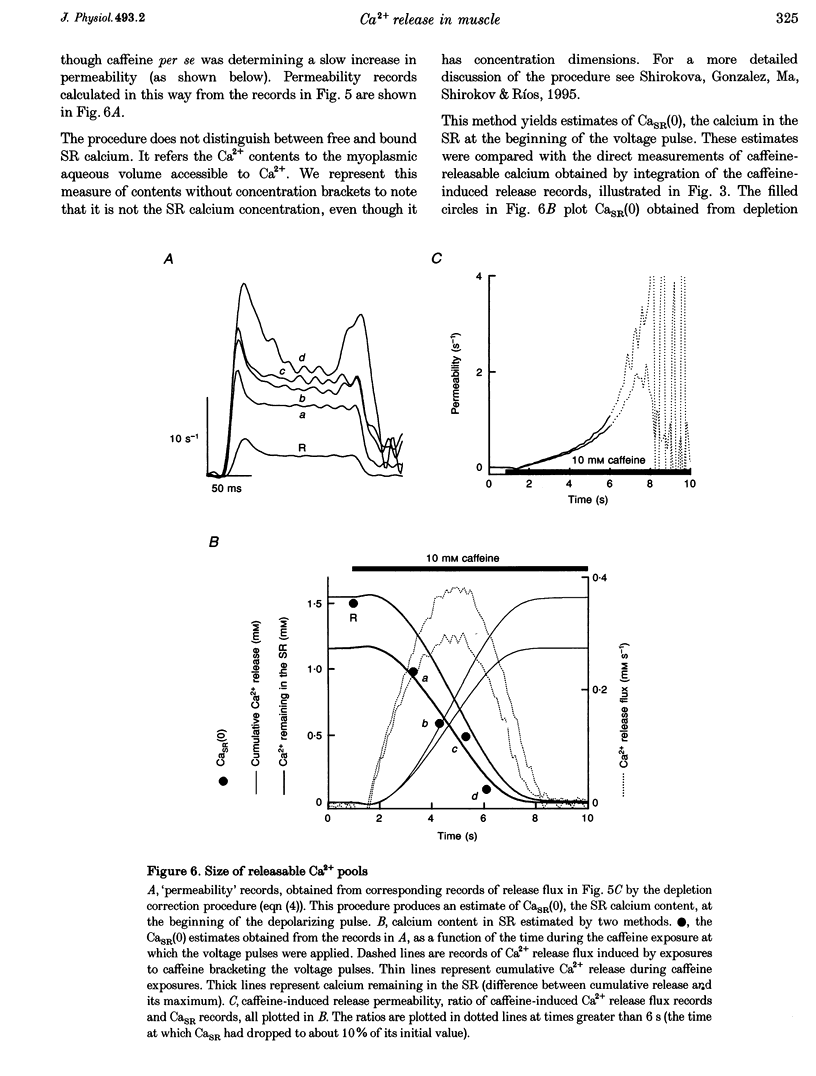
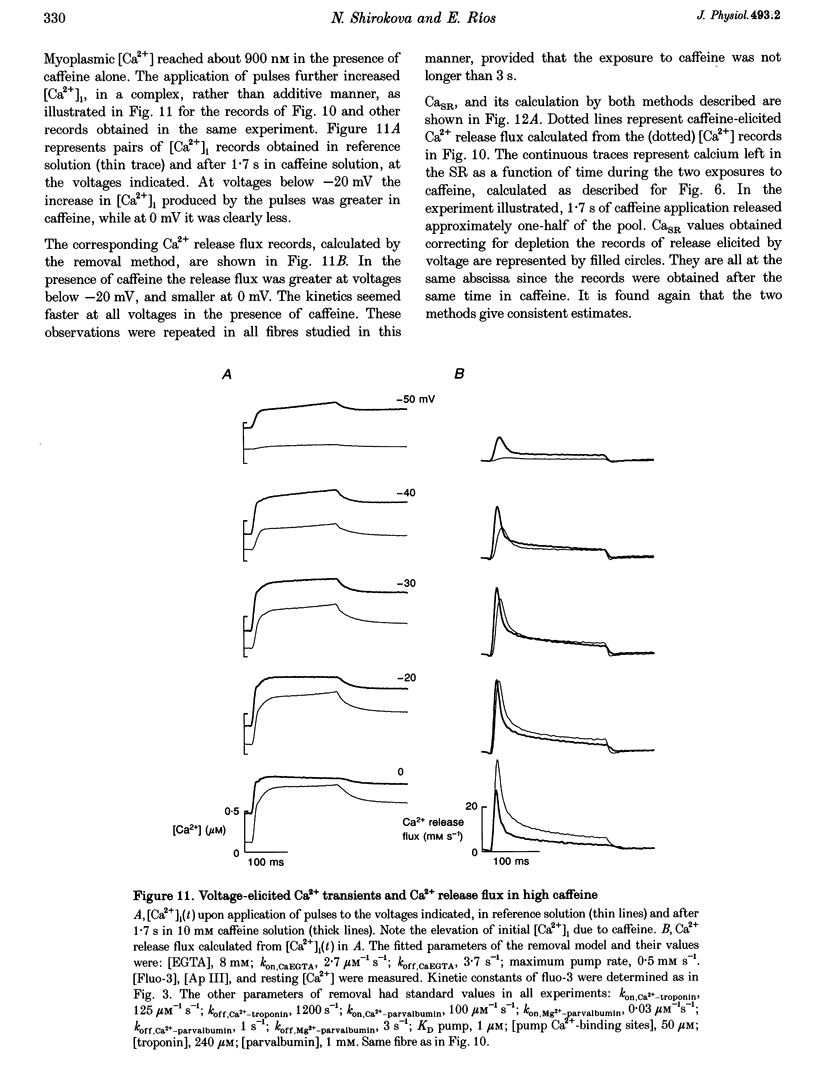
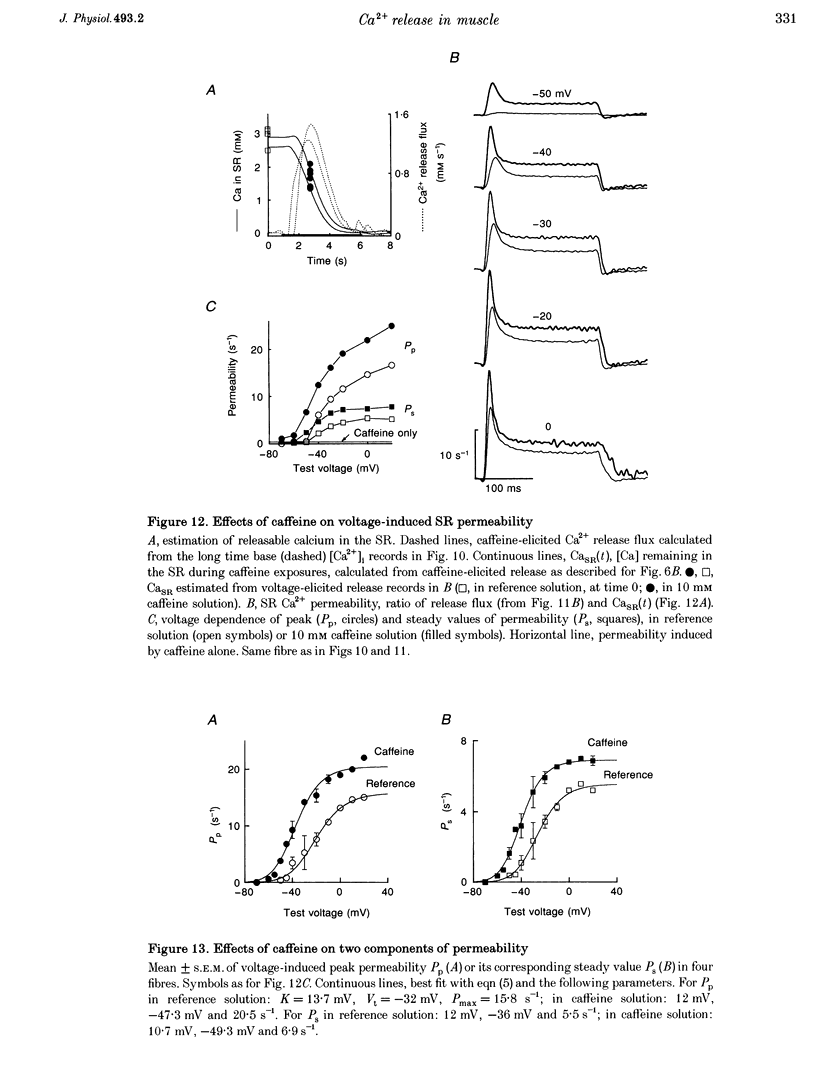
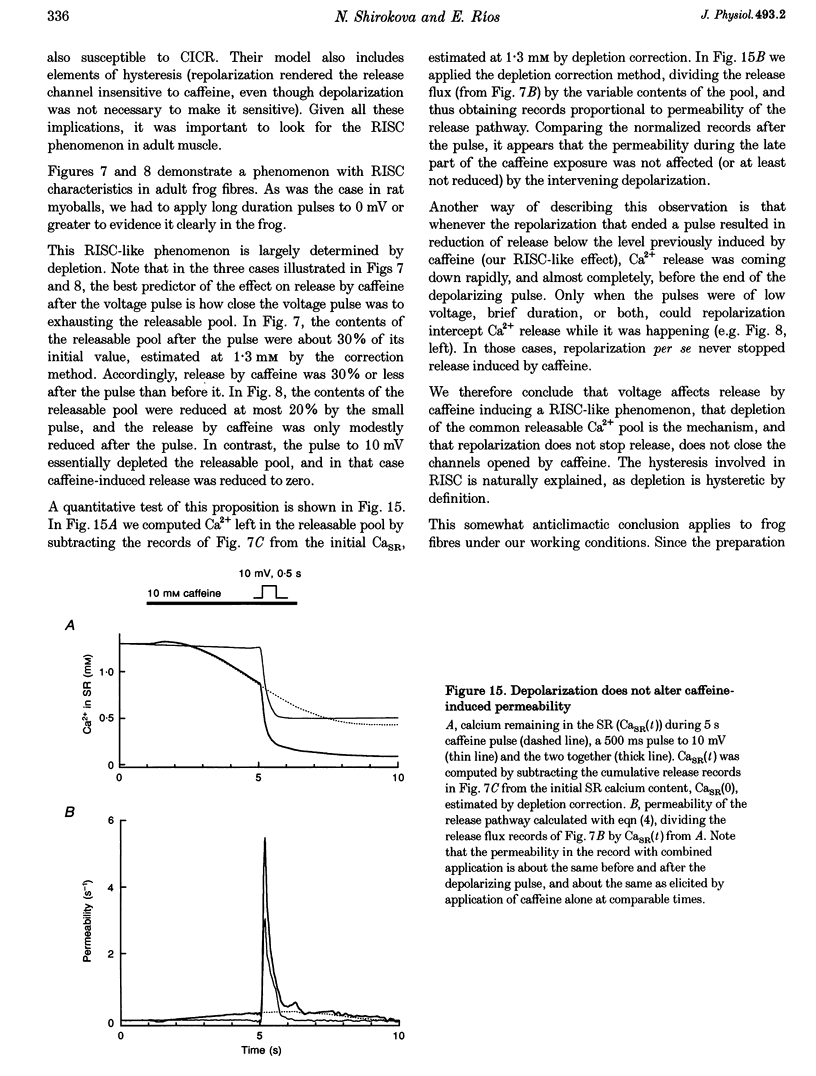
1. Using a fast flow, computer-controlled, two-Vaseline-gap chamber, single muscle fibres were subjected to 'pulses' of caffeine at Ca2+ releasing concentrations, combined with voltage-clamp depolarizations, while monitoring intracellular [Ca2+]. 2. Ca2+ release flux elicited by caffeine reached 2.5 mM s-1, or less, after 3 s of exposure, then decayed to zero. The caffeine-releasable pool of sarcoplasmic reticulum (SR) Ca2+ was 2.9 +/- 0.4 mM (mean +/- S.E.M., n = 10). 3. In parallel with release induced by caffeine, release induced by voltage pulses applied during a caffeine exposure increased in the first second of exposure, then decreased, to abolition after 5 s. 4. The amount of Ca2+ releasable by depolarizing pulses was always equal to the amount of Ca2+ in the caffeine-releasable pool. Therefore, there is a single releasable Ca2+ pool. This pool is well stirred-it takes much more time to lose its Ca2+ by release than to diffusionally homogenize its [Ca2+]. Its depletion explains quantitatively the decay of release induced by caffeine or voltage during an exposure to caffeine. 5. A 1.5 s pulse to 10 mV, applied during exposure to caffeine, resulted in large Ca2+ release and, upon repolarization, termination of the caffeine-induced release. This is similar to repolarization-induced stop of caffeine contracture (RISC) in embryonic murine myoballs. The permeability elicited by caffeine (ratio of flux to calcium in the releasable pool) was not affected by depolarizing pulses. Therefore, the mechanism of the RISC-like effect was Ca2+ depletion. 6. Caffeine-induced release did not depend on the holding potential. 7. Whether caffeine was present or not, release activated by voltage remained always under voltage control, ending rapidly upon repolarization. A depolarizing pulse induced a release permeability with an early peak, followed by decay to a steady level. Caffeine (10 mM) shifted the mid-activation voltage of both peak and steady components by -15 mV and increased the steepness of their voltage dependence by 15%. The maximum permeability increased by 30% for the peak and 25% for the steady component (n = 5). These results neither support nor disprove the hypothesis that the peak of Ca2+ release is activated by Ca2+. 8. The similar potentiation by caffeine of both components of release, the continued ability of voltage to control release in the presence of caffeine, and its failure to alter caffeine-induced permeability indicate that caffeine and the voltage sensor enhance independently the channel's tendency to open.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. Activation of the contractile mechanism in striated muscle. Acta Physiol Scand. 1958 Oct 28;44(1):55–66. doi: 10.1111/j.1748-1716.1958.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Anderson K., Meissner G. T-tubule depolarization-induced SR Ca2+ release is controlled by dihydropyridine receptor- and Ca(2+)-dependent mechanisms in cell homogenates from rabbit skeletal muscle. J Gen Physiol. 1995 Mar;105(3):363–383. doi: 10.1085/jgp.105.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G., Fitts R., Pizarro G., Ríos E. Voltage sensors of the frog skeletal muscle membrane require calcium to function in excitation-contraction coupling. J Physiol. 1988 Apr;398:475–505. doi: 10.1113/jphysiol.1988.sp017053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G., Ríos E., Stéfani E. Effects of extracellular calcium on calcium movements of excitation-contraction coupling in frog skeletal muscle fibres. J Physiol. 1988 Apr;398:441–473. doi: 10.1113/jphysiol.1988.sp017052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunder D. G., Györke S., Dettbarn C., Palade P. Involvement of sarcoplasmic reticulum 'Ca2+ release channels' in excitation-contraction coupling in vertebrate skeletal muscle. J Physiol. 1992 Jan;445:759–778. doi: 10.1113/jphysiol.1992.sp018949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton J. D., Lännergren J., Westerblad H. Mechano-sensitive linkage in excitation-contraction coupling in frog skeletal muscle. J Physiol. 1995 May 1;484(Pt 3):737–742. doi: 10.1113/jphysiol.1995.sp020699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. Effects of glycerol treatment and maintained depolarization on charge movement in skeletal muscle. J Physiol. 1976 Jan;254(2):285–316. doi: 10.1113/jphysiol.1976.sp011233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernoch L., Jacquemond V., Schneider M. F. Microinjection of strong calcium buffers suppresses the peak of calcium release during depolarization in frog skeletal muscle fibers. J Gen Physiol. 1993 Feb;101(2):297–333. doi: 10.1085/jgp.101.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delay M., Ribalet B., Vergara J. Caffeine potentiation of calcium release in frog skeletal muscle fibres. J Physiol. 1986 Jun;375:535–559. doi: 10.1113/jphysiol.1986.sp016132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Regenerative calcium release within muscle cells. Science. 1970 Jan 2;167(3914):58–59. doi: 10.1126/science.167.3914.58. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Kenney L. J., Varriano-Marston E. The structure of calsequestrin in triads of vertebrate skeletal muscle: a deep-etch study. J Cell Biol. 1987 Jul;105(1):49–56. doi: 10.1083/jcb.105.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González A., Ríos E. Perchlorate enhances transmission in skeletal muscle excitation-contraction coupling. J Gen Physiol. 1993 Sep;102(3):373–421. doi: 10.1085/jgp.102.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F. 'Quantal' Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS Lett. 1990 Apr 9;263(1):5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Jacquemond V., Csernoch L., Klein M. G., Schneider M. F. Voltage-gated and calcium-gated calcium release during depolarization of skeletal muscle fibers. Biophys J. 1991 Oct;60(4):867–873. doi: 10.1016/S0006-3495(91)82120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong D. S., Pape P. C., Chandler W. K., Baylor S. M. Reduction of calcium inactivation of sarcoplasmic reticulum calcium release by fura-2 in voltage-clamped cut twitch fibers from frog muscle. J Gen Physiol. 1993 Aug;102(2):333–370. doi: 10.1085/jgp.102.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino Y., Osakabe M., Shimizu H. Ca2+-induced Ca2+ release from fragmented sarcoplasmic reticulum: Ca2+-dependent passive Ca2+ efflux. J Biochem. 1983 Oct;94(4):1111–1118. doi: 10.1093/oxfordjournals.jbchem.a134454. [DOI] [PubMed] [Google Scholar]
- Konishi M., Kurihara S. Effects of caffeine on intracellular calcium concentrations in frog skeletal muscle fibres. J Physiol. 1987 Feb;383:269–283. doi: 10.1113/jphysiol.1987.sp016408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs L., Rios E., Schneider M. F. Measurement and modification of free calcium transients in frog skeletal muscle fibres by a metallochromic indicator dye. J Physiol. 1983 Oct;343:161–196. doi: 10.1113/jphysiol.1983.sp014887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács L., Szücs G. Effect of caffeine on intramembrane charge movement and calcium transients in cut skeletal muscle fibres of the frog. J Physiol. 1983 Aug;341:559–578. doi: 10.1113/jphysiol.1983.sp014824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau H. C., Oetliker H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J Physiol. 1968 Jan;194(1):51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty I., Robert M., Villaz M., De Jongh K., Lai Y., Catterall W. A., Ronjat M. Biochemical evidence for a complex involving dihydropyridine receptor and ryanodine receptor in triad junctions of skeletal muscle. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2270–2274. doi: 10.1073/pnas.91.6.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Isolation and characterization of two types of sarcoplasmic reticulum vesicles. Biochim Biophys Acta. 1975 Apr 21;389(1):51–68. doi: 10.1016/0005-2736(75)90385-5. [DOI] [PubMed] [Google Scholar]
- Melzer W., Rios E., Schneider M. F. A general procedure for determining the rate of calcium release from the sarcoplasmic reticulum in skeletal muscle fibers. Biophys J. 1987 Jun;51(6):849–863. doi: 10.1016/S0006-3495(87)83413-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W., Rios E., Schneider M. F. Time course of calcium release and removal in skeletal muscle fibers. Biophys J. 1984 Mar;45(3):637–641. doi: 10.1016/S0006-3495(84)84203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaki K., Kasai M. Fast release of calcium from sarcoplasmic reticulum vesicles monitored by chlortetracycline fluorescence. J Biochem. 1983 Oct;94(4):1101–1109. doi: 10.1093/oxfordjournals.jbchem.a134453. [DOI] [PubMed] [Google Scholar]
- Oyamada H., Iino M., Endo M. Effects of ryanodine on the properties of Ca2+ release from the sarcoplasmic reticulum in skinned skeletal muscle fibres of the frog. J Physiol. 1993 Oct;470:335–348. doi: 10.1113/jphysiol.1993.sp019861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade P. Drug-induced Ca2+ release from isolated sarcoplasmic reticulum. I. Use of pyrophosphate to study caffeine-induced Ca2+ release. J Biol Chem. 1987 May 5;262(13):6135–6141. [PubMed] [Google Scholar]
- Pape P. C., Jong D. S., Chandler W. K., Baylor S. M. Effect of fura-2 on action potential-stimulated calcium release in cut twitch fibers from frog muscle. J Gen Physiol. 1993 Aug;102(2):295–332. doi: 10.1085/jgp.102.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios E., Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987 Feb 19;325(6106):717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Ladine J., Liu Q. Y., Meissner G. Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Arch Biochem Biophys. 1988 Nov 15;267(1):75–86. doi: 10.1016/0003-9861(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Ríos E., Ma J. J., González A. The mechanical hypothesis of excitation-contraction (EC) coupling in skeletal muscle. J Muscle Res Cell Motil. 1991 Apr;12(2):127–135. doi: 10.1007/BF01774031. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Simon B. J., Szucs G. Depletion of calcium from the sarcoplasmic reticulum during calcium release in frog skeletal muscle. J Physiol. 1987 Nov;392:167–192. doi: 10.1113/jphysiol.1987.sp016775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokova N., García J., Pizarro G., Ríos E. Ca2+ release from the sarcoplasmic reticulum compared in amphibian and mammalian skeletal muscle. J Gen Physiol. 1996 Jan;107(1):1–18. doi: 10.1085/jgp.107.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokova N., González A., Ma J., Shirokov R., Ríos E. Properties and roles of an intramembranous charge mobilized at high voltages in frog skeletal muscle. J Physiol. 1995 Jul 15;486(Pt 2):385–400. doi: 10.1113/jphysiol.1995.sp020820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokova N., Ríos E. Caffeine enhances intramembranous charge movement in frog skeletal muscle by increasing cytoplasmic Ca2+ concentration. J Physiol. 1996 Jun 1;493(Pt 2):341–356. doi: 10.1113/jphysiol.1996.sp021387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon B. J., Hill D. A. Charge movement and SR calcium release in frog skeletal muscle can be related by a Hodgkin-Huxley model with four gating particles. Biophys J. 1992 May;61(5):1109–1116. doi: 10.1016/S0006-3495(92)81920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon B. J., Klein M. G., Schneider M. F. Caffeine slows turn-off of calcium release in voltage clamped skeletal muscle fibers. Biophys J. 1989 Apr;55(4):793–797. doi: 10.1016/S0006-3495(89)82878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., Gonzalez-Serratos H. G., Shuman H., McClellan G., Somlyo A. P. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol. 1981 Sep;90(3):577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda N. Involvement of Mg2+ in terminating Ca2+ release in cultured rat skeletal muscle. FEBS Lett. 1995 Feb 13;359(2-3):223–228. doi: 10.1016/0014-5793(95)00047-d. [DOI] [PubMed] [Google Scholar]
- Suda N., Penner R. Membrane repolarization stops caffeine-induced Ca2+ release in skeletal muscle cells. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5725–5729. doi: 10.1073/pnas.91.12.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]


