Abstract
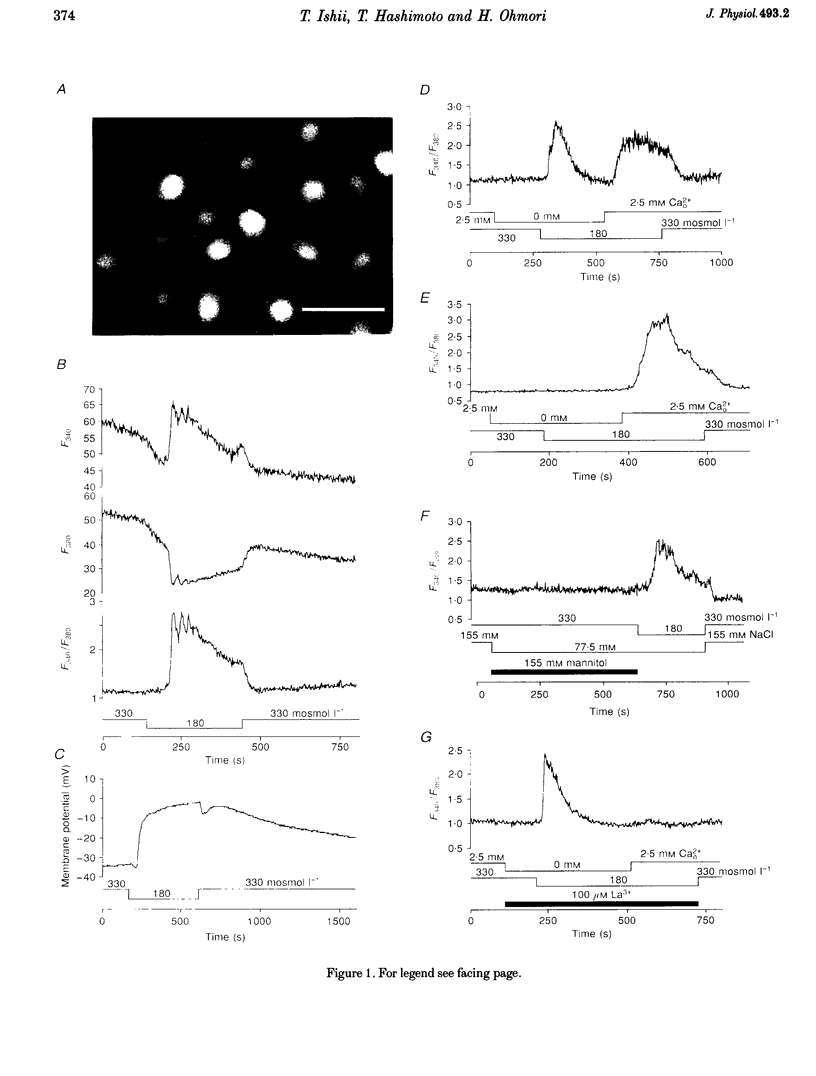
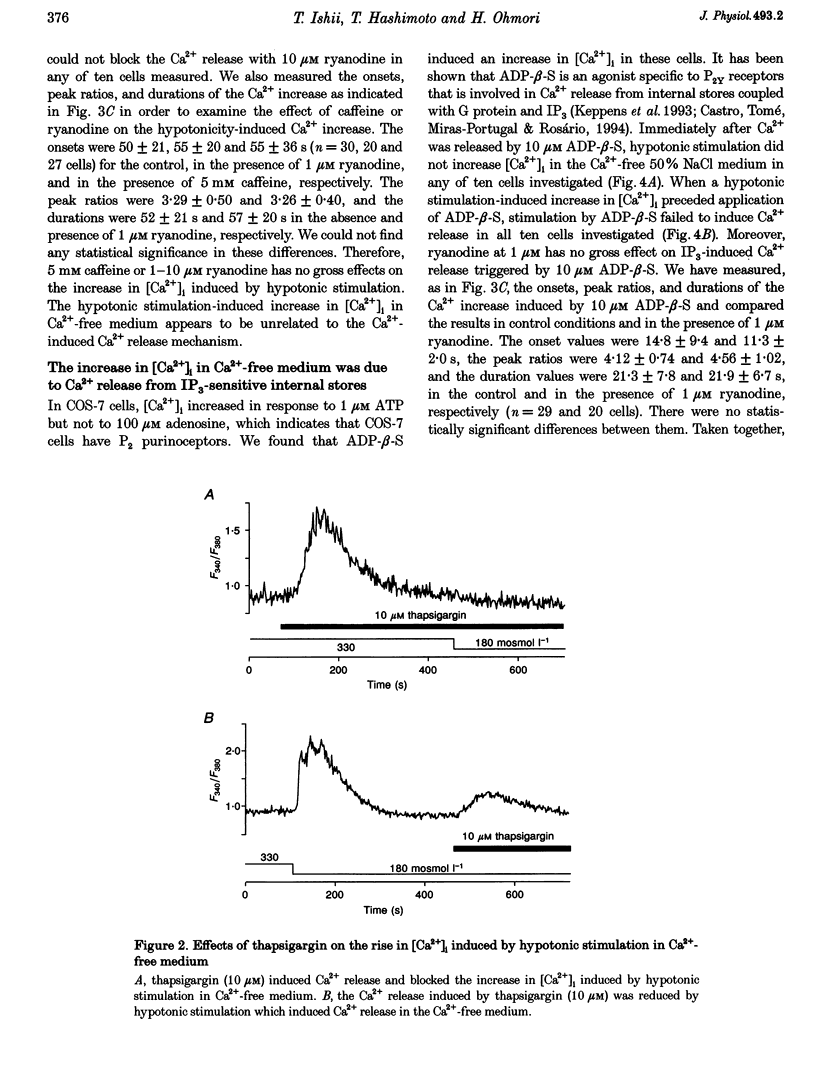
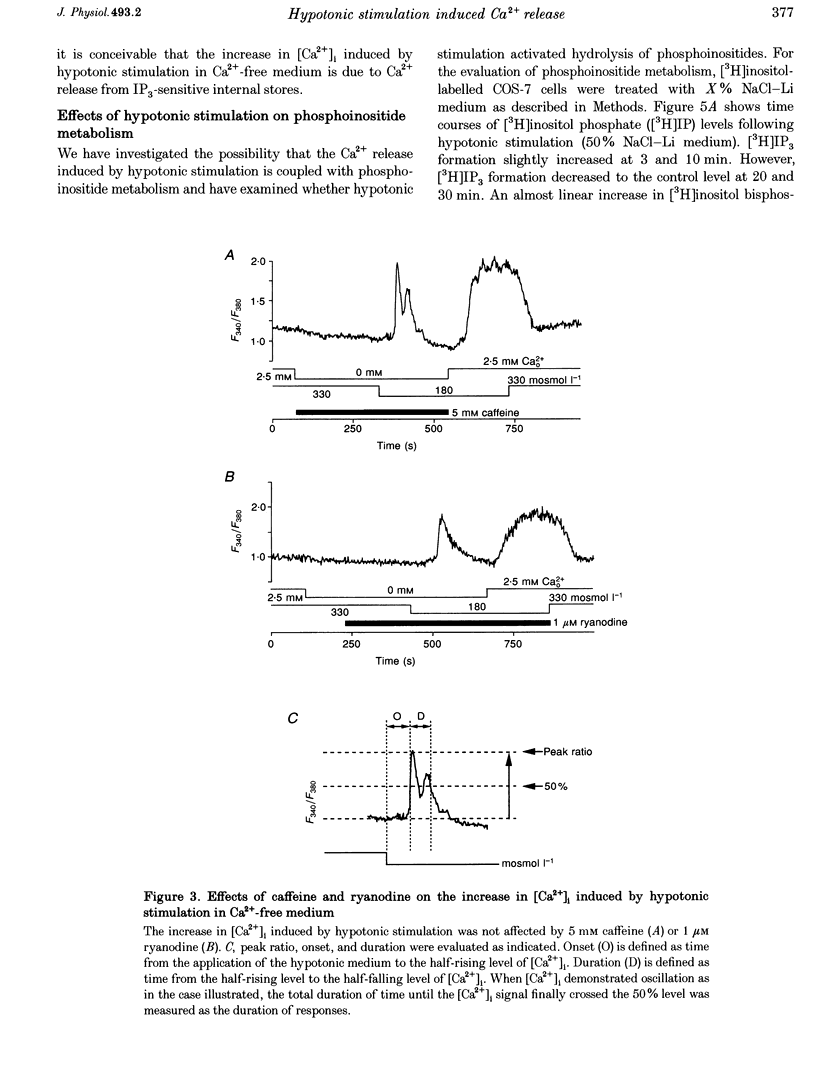
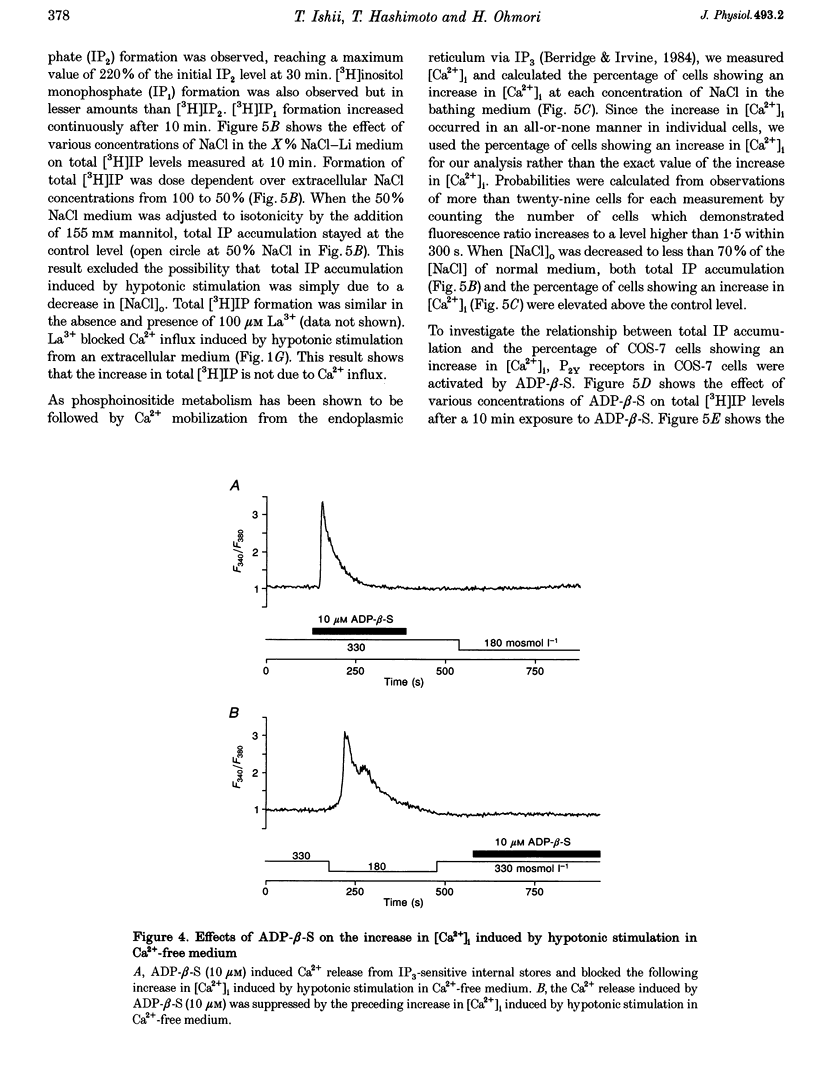
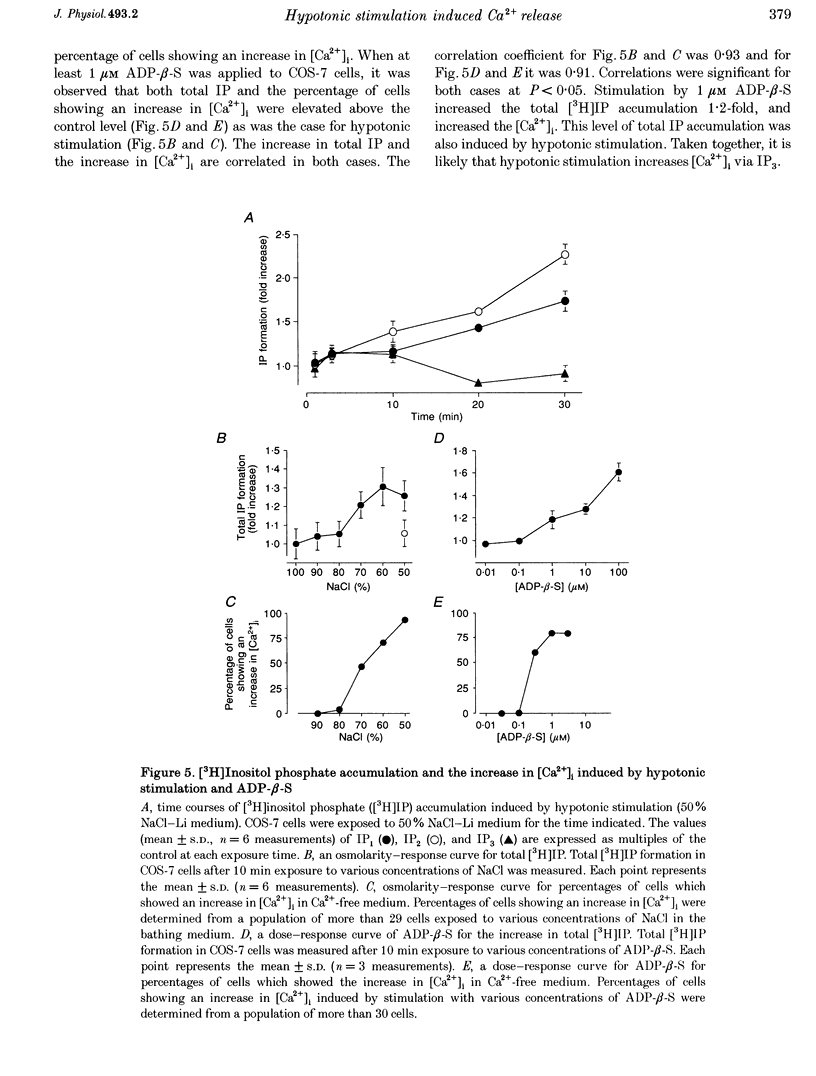
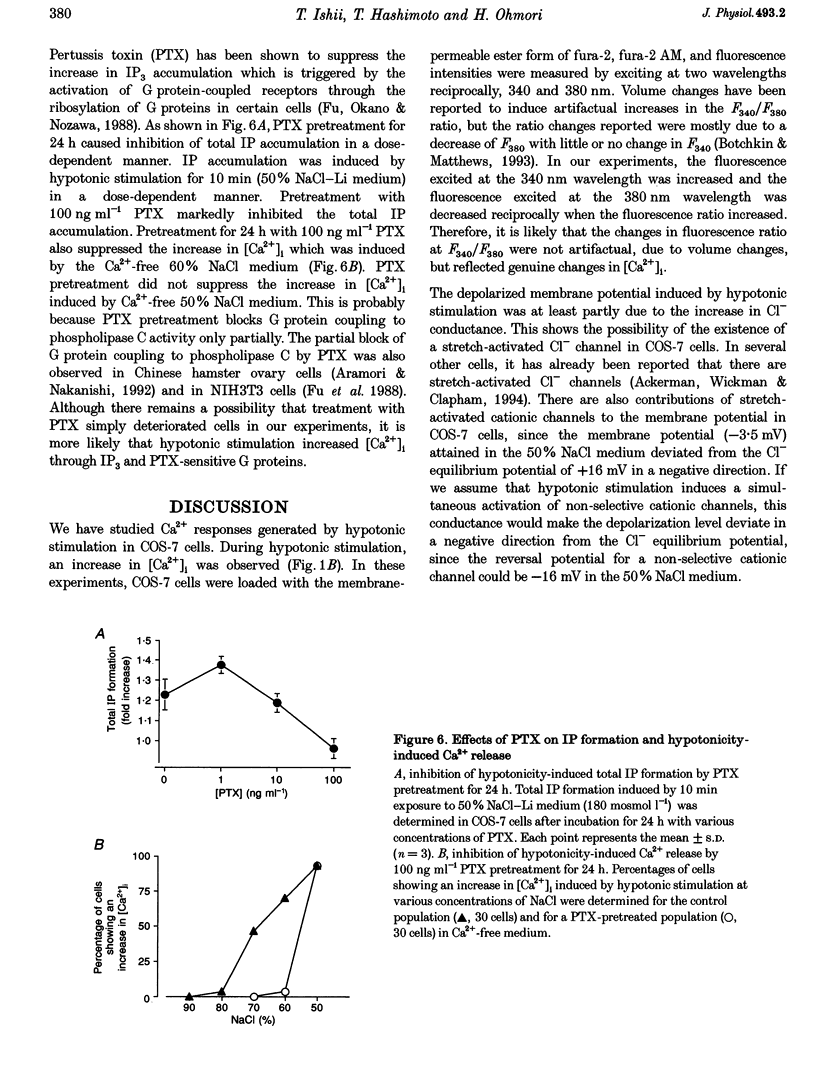
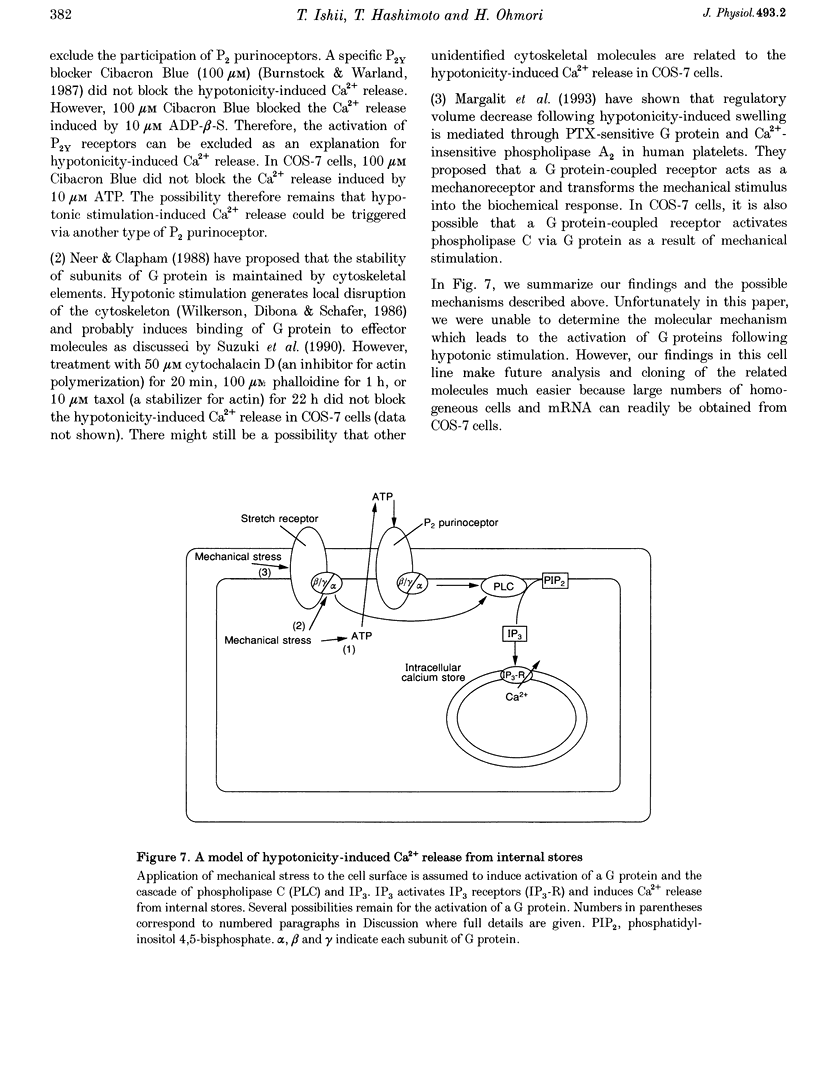
1. Hypotonic stimulation (180 +/- 5 mosmol l-1) increased [Ca2+]i in fura-2-loaded Green monkey kidney cells (COS-7 cells) and depolarized the membrane. 2. COS-7 cells were depolarized up to -3.5 +/- 4.4 mV from a resting membrane potential of -35.2 +/- 2.3 mV in response to hypotonic stimulation, when the patch electrode was filled with a 160 mM KCl-0.5 mM EGTA-based intracellular medium. 3. The increase in [Ca2+]i induced by hypotonic stimulation was divided into two phases. One was transient and oscillatory, and observed in Ca(2+)-free medium; the other was persistent, blocked by 100 microM La3+, and observed only in Ca(2+)-containing medium. 4. The increase in [Ca2+]i in Ca(2+)-free medium was blocked by pretreatment with 10 microM thapsigargin. The increase in [Ca2+]i induced by 10 microM thapsigargin was reduced after hypotonic stimulation which induced an increase in [Ca2+]i in Ca(2+)-free medium. 5. The increase in [Ca2+]i in Ca(2+)-free medium was not affected by treatment with 5 mM caffeine or 1-10 microM ryanodine. Neither caffeine nor ryanodine induced an increase in [Ca2+]i. 6. Adenosine 5'-O-2-thiodiphosphate (ADP-beta-S; a P2Y receptor agonist) induced an increase in [Ca2+]i in Ca(2+)-free medium and caused phosphoinositide breakdown in COS-7 cells. Exposure to 10 microM ADP-beta-S blocked the increase in [Ca2+]i induced in the Ca(2+)-free medium by hypotonic stimulation. The results of summary points 4, 5, and 6 suggest that the increase in [Ca2+]i induced by hypotonic stimulation is due to Ca2+ release from inositol 1,4,5-trisphosphate (IP3)-sensitive internal stores. 7. The hypotonic stimulation-activated hydrolysis of phosphoinositides was decreased by pertussis toxin (PTX) in a dose-dependent manner. 8. These observations strongly suggest that hypotonic stimulation induced an increase in [Ca2+]i in Ca(2+)-free medium through activation of cascades using PTX-sensitive guanine nucleotide binding protein (G protein) and IP3.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman M. J., Wickman K. D., Clapham D. E. Hypotonicity activates a native chloride current in Xenopus oocytes. J Gen Physiol. 1994 Feb;103(2):153–179. doi: 10.1085/jgp.103.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramori I., Nakanishi S. Signal transduction and pharmacological characteristics of a metabotropic glutamate receptor, mGluR1, in transfected CHO cells. Neuron. 1992 Apr;8(4):757–765. doi: 10.1016/0896-6273(92)90096-v. [DOI] [PubMed] [Google Scholar]
- Ashmore J. F., Ohmori H. Control of intracellular calcium by ATP in isolated outer hair cells of the guinea-pig cochlea. J Physiol. 1990 Sep;428:109–131. doi: 10.1113/jphysiol.1990.sp018203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard E. A., Burnstock G., Webb T. E. G protein-coupled receptors for ATP and other nucleotides: a new receptor family. Trends Pharmacol Sci. 1994 Mar;15(3):67–70. doi: 10.1016/0165-6147(94)90280-1. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Boyer J. L., Downes C. P., Harden T. K. Kinetics of activation of phospholipase C by P2Y purinergic receptor agonists and guanine nucleotides. J Biol Chem. 1989 Jan 15;264(2):884–890. [PubMed] [Google Scholar]
- Burnstock G., Warland J. J. P2-purinoceptors of two subtypes in the rabbit mesenteric artery: reactive blue 2 selectively inhibits responses mediated via the P2y-but not the P2x-purinoceptor. Br J Pharmacol. 1987 Feb;90(2):383–391. doi: 10.1111/j.1476-5381.1987.tb08968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro E., Tomé A. R., Miras-Portugal M. T., Rosário L. M. Single-cell fura-2 microfluorometry reveals different purinoceptor subtypes coupled to Ca2+ influx and intracellular Ca2+ release in bovine adrenal chromaffin and endothelial cells. Pflugers Arch. 1994 Apr;426(6):524–533. doi: 10.1007/BF00378530. [DOI] [PubMed] [Google Scholar]
- Dassouli A., Sülpice J. C., Roux S., Crozatier B. Stretch-induced inositol trisphosphate and tetrakisphosphate production in rat cardiomyocytes. J Mol Cell Cardiol. 1993 Aug;25(8):973–982. doi: 10.1006/jmcc.1993.1109. [DOI] [PubMed] [Google Scholar]
- Fu T., Okano Y., Nozawa Y. Bradykinin-induced generation of inositol 1,4,5-trisphosphate in fibroblasts and neuroblastoma cells: effect of pertussis toxin, extracellular calcium, and down-regulation of protein kinase C. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1429–1435. doi: 10.1016/s0006-291x(88)81035-0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hazama A., Okada Y. Ca2+ sensitivity of volume-regulatory K+ and Cl- channels in cultured human epithelial cells. J Physiol. 1988 Aug;402:687–702. doi: 10.1113/jphysiol.1988.sp017229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S., Vandekerckhove A., De Wulf H. Characterization of the effects of adenosine 5'-[beta-thio]-diphosphate in rat liver. Br J Pharmacol. 1993 Mar;108(3):663–668. doi: 10.1111/j.1476-5381.1993.tb12858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansman J. B., Hallam T. J., Rink T. J. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers? 1987 Feb 26-Mar 4Nature. 325(6107):811–813. doi: 10.1038/325811a0. [DOI] [PubMed] [Google Scholar]
- Leung A. Y., Wong P. Y. Ca2+ release in cultured rat epididymal cells during hypoosmotic swelling. Pflugers Arch. 1993 Oct;425(1-2):77–81. doi: 10.1007/BF00374506. [DOI] [PubMed] [Google Scholar]
- Margalit A., Livne A. A., Funder J., Granot Y. Initiation of RVD response in human platelets: mechanical-biochemical transduction involves pertussis-toxin-sensitive G protein and phospholipase A2. J Membr Biol. 1993 Dec;136(3):303–311. doi: 10.1007/BF00233669. [DOI] [PubMed] [Google Scholar]
- McCarty N. A., O'Neil R. G. Calcium-dependent control of volume regulation in renal proximal tubule cells: I. Swelling-activated Ca2+ entry and release. J Membr Biol. 1991 Aug;123(2):149–160. doi: 10.1007/BF01998085. [DOI] [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem. 1986 May 15;261(14):6300–6306. [PubMed] [Google Scholar]
- Nachshen D. A. Selectivity of the Ca binding site in synaptosome Ca channels. Inhibition of Ca influx by multivalent metal cations. J Gen Physiol. 1984 Jun;83(6):941–967. doi: 10.1085/jgp.83.6.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y., Tsuchida K., Negishi M., Ito S., Nakanishi S. Direct linkage of three tachykinin receptors to stimulation of both phosphatidylinositol hydrolysis and cyclic AMP cascades in transfected Chinese hamster ovary cells. J Biol Chem. 1992 Feb 5;267(4):2437–2442. [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Nitschke R., Leipziger J., Greger R. Intracellular Ca2+ transients in HT29 cells induced by hypotonic cell swelling. Pflugers Arch. 1993 May;423(3-4):274–279. doi: 10.1007/BF00374406. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Mechanical stimulation and Fura-2 fluorescence in the hair bundle of dissociated hair cells of the chick. J Physiol. 1988 May;399:115–137. doi: 10.1113/jphysiol.1988.sp017071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol. 1985 Feb;359:189–217. doi: 10.1113/jphysiol.1985.sp015581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oike M., Droogmans G., Nilius B. Mechanosensitive Ca2+ transients in endothelial cells from human umbilical vein. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2940–2944. doi: 10.1073/pnas.91.8.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F. Baroreceptor mechanisms at the cellular level. Fed Proc. 1987 Jan;46(1):12–16. [PubMed] [Google Scholar]
- Schmid A., Dehlinger-Kremer M., Schulz I., Gögelein H. Voltage-dependent InsP3-insensitive calcium channels in membranes of pancreatic endoplasmic reticulum vesicles. Nature. 1990 Jul 26;346(6282):374–376. doi: 10.1038/346374a0. [DOI] [PubMed] [Google Scholar]
- Shigemoto T., Ohmori H. Muscarinic agonists and ATP increase the intracellular Ca2+ concentration in chick cochlear hair cells. J Physiol. 1990 Jan;420:127–148. doi: 10.1113/jphysiol.1990.sp017904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Kawahara K., Ogawa A., Morita T., Kawaguchi Y., Kurihara S., Sakai O. [Ca2+]i rises via G protein during regulatory volume decrease in rabbit proximal tubule cells. Am J Physiol. 1990 Mar;258(3 Pt 2):F690–F696. doi: 10.1152/ajprenal.1990.258.3.F690. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voogd T. E., Vansterkenburg E. L., Wilting J., Janssen L. H. Recent research on the biological activity of suramin. Pharmacol Rev. 1993 Jun;45(2):177–203. [PubMed] [Google Scholar]
- Wilkerson E. H., DiBona D. R., Schafer J. A. Analysis of structural changes during hypotonic swelling in Ehrlich ascites tumor cells. Am J Physiol. 1986 Jul;251(1 Pt 1):C104–C114. doi: 10.1152/ajpcell.1986.251.1.C104. [DOI] [PubMed] [Google Scholar]
- Yang X. C., Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989 Feb 24;243(4894 Pt 1):1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- Yokohama H., Tanaka T., Ito S., Negishi M., Hayashi H., Hayaishi O. Prostaglandin E receptor enhancement of catecholamine release may be mediated by phosphoinositide metabolism in bovine adrenal chromaffin cells. J Biol Chem. 1988 Jan 25;263(3):1119–1122. [PubMed] [Google Scholar]