Abstract
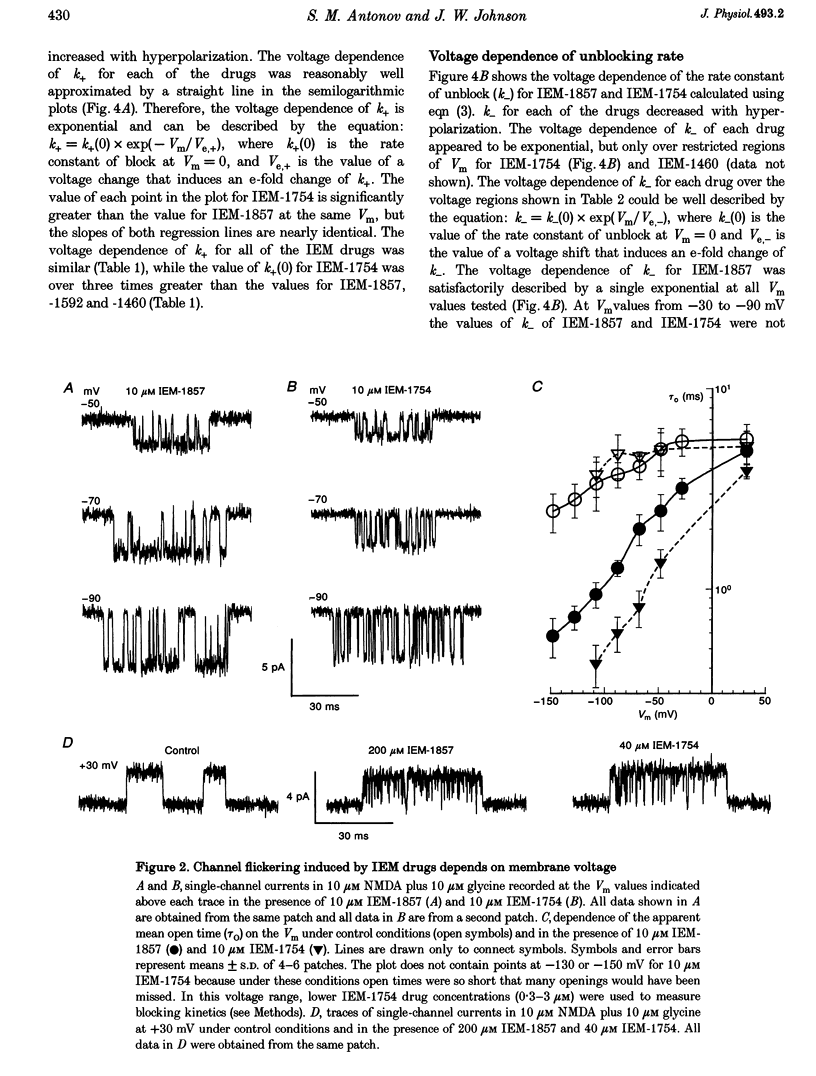
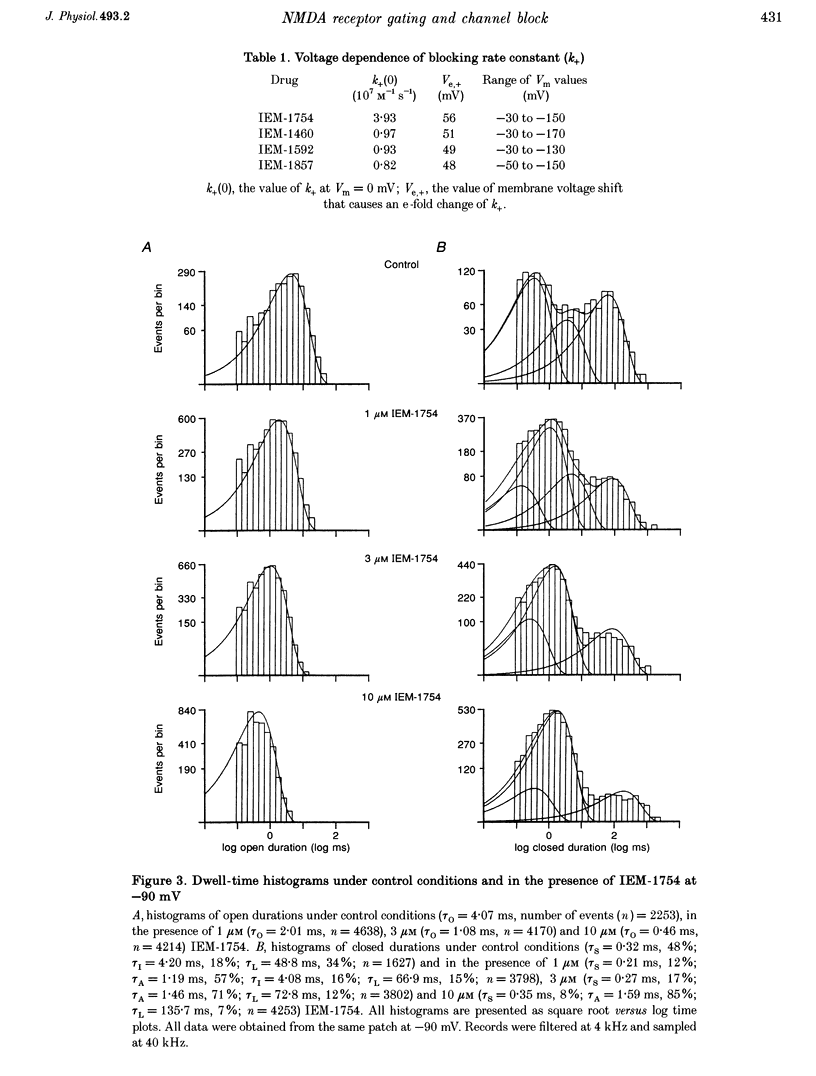
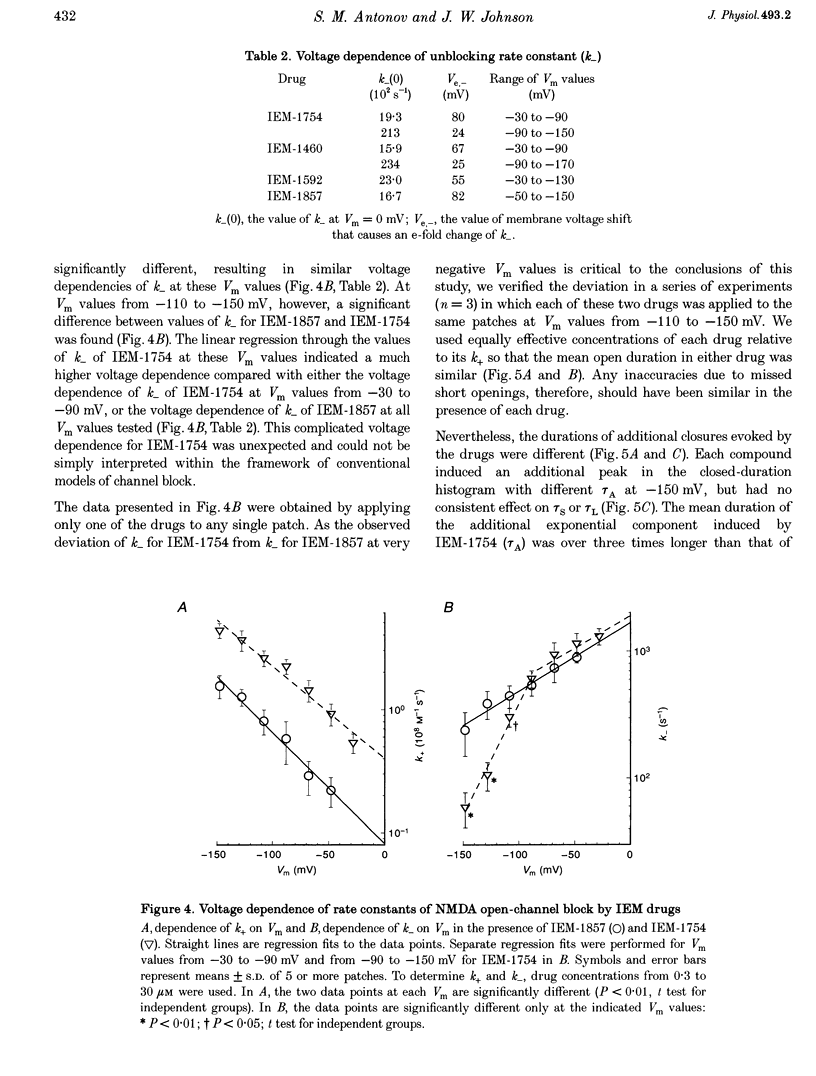
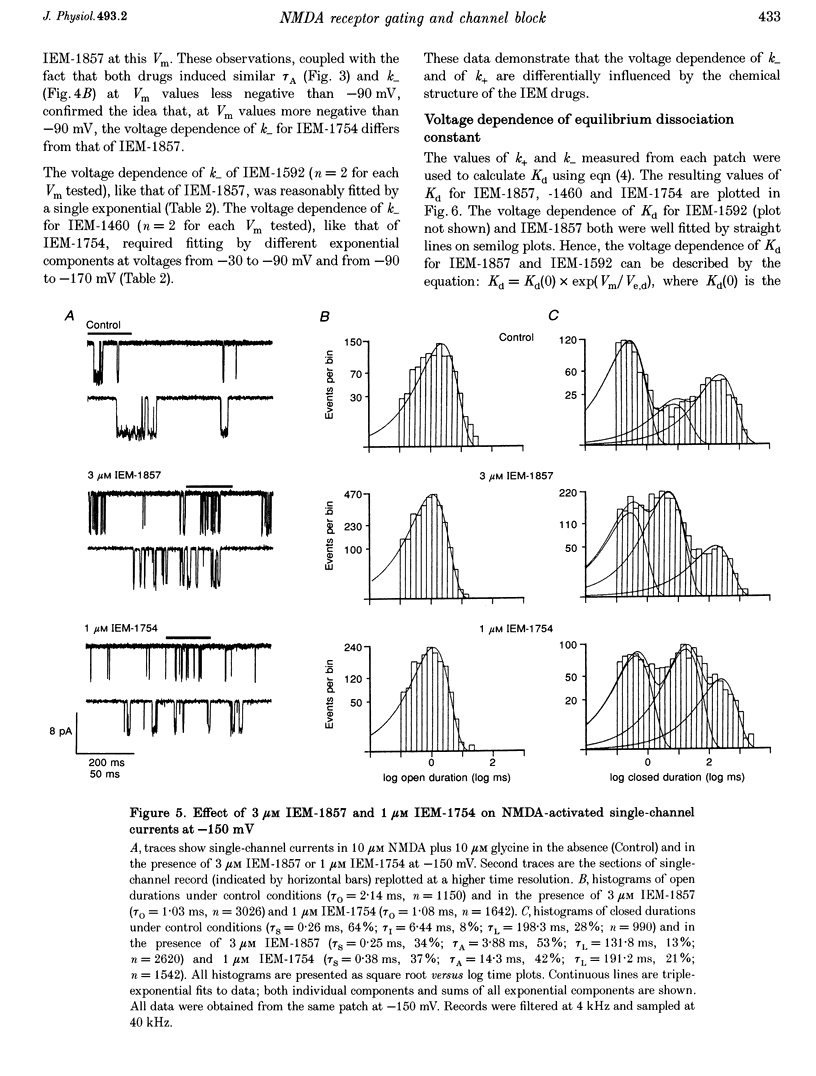
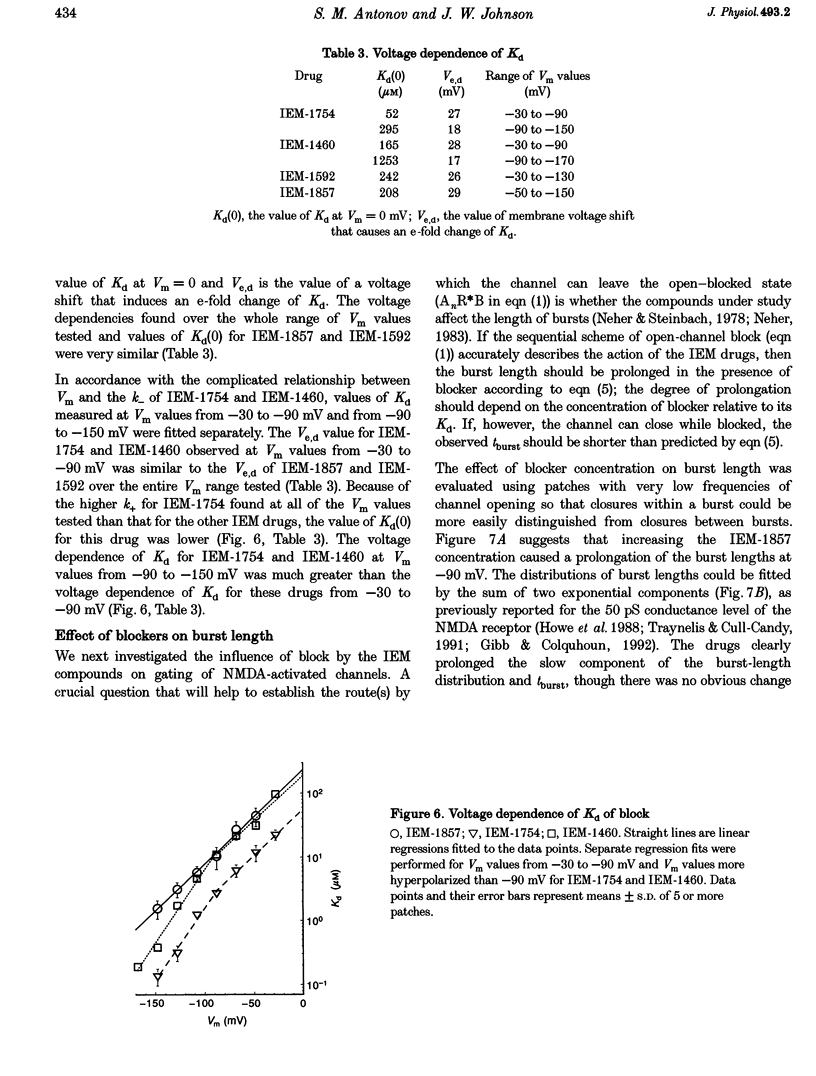
1. The mechanisms by which four adamantane derivatives (IEM-1857, -1592, -1460 and -1754) block the open NMDA-activated channel were studied at membrane voltages (Vm) from -170 to +30 mV. The rate constants of channel block (k+) and of channel unblock (k-) were measured from the fully resolvable flicker of single-channel currents induced by each compound. 2. The k+ of each compound exhibited a similar exponential dependence on voltage over the Vm range studied. 3. The k- of IEM-1857 and IEM-1592 over the Vm range studied, and of IEM-1754 and IEM-1460 from -30 to -90 mV, exhibited similar exponential dependencies on voltage. However, the k- of IEM-1754 and IEM-1460 at Vm values more hyperpolarized than -90 mV were much more steeply voltage dependent, suggesting that at these Vm values the two drugs can occupy a deeper binding site. 4. Each of the drugs induced a concentration-dependent prolongation of the mean burst length at -90 mV, suggesting that while blocking they can interfere with channel closure. 5. The prolongation of mean burst length induced by the largest drug (IEM-1857) increased with hyperpolarization. The increase was consistent at each Vm with the predictions of the sequential scheme of block, suggesting that channel closure is prevented when IEM-1857 is bound. The prolongation of burst length induced by the smallest drug (IEM-1754) was less than predicted by the sequential scheme and the deviation increased with hyperpolarization. 6. The IEM-1857 concentration-dependence of number of blockages per unit open time had a slope equal to k+ at -150 mV. The IEM-1754 concentration-dependence of number of blockages per unit open time revealed a slope about two times less than k+ for this compound at -150 mV. 7. The mean patch current was not significantly altered by 3 microM IEM-1857 at Vm values from -90 to -150 mV, as expected of a drug that prevents channel closure when blocking. Mean patch current significantly decreased with hyperpolarization beyond -90 mV in the presence of 1 microM IEM-1754. 8. The data suggest that there are two blocking sites at different depths within the NMDA-activated channel. Channel closure is prevented when any of the IEM drugs occupy the shallow blocking site. Channel closure is permitted during occupation of a deeper blocking site that can be reached only by the smaller IEM drugs at hyperpolarized voltages.
Full text
PDF




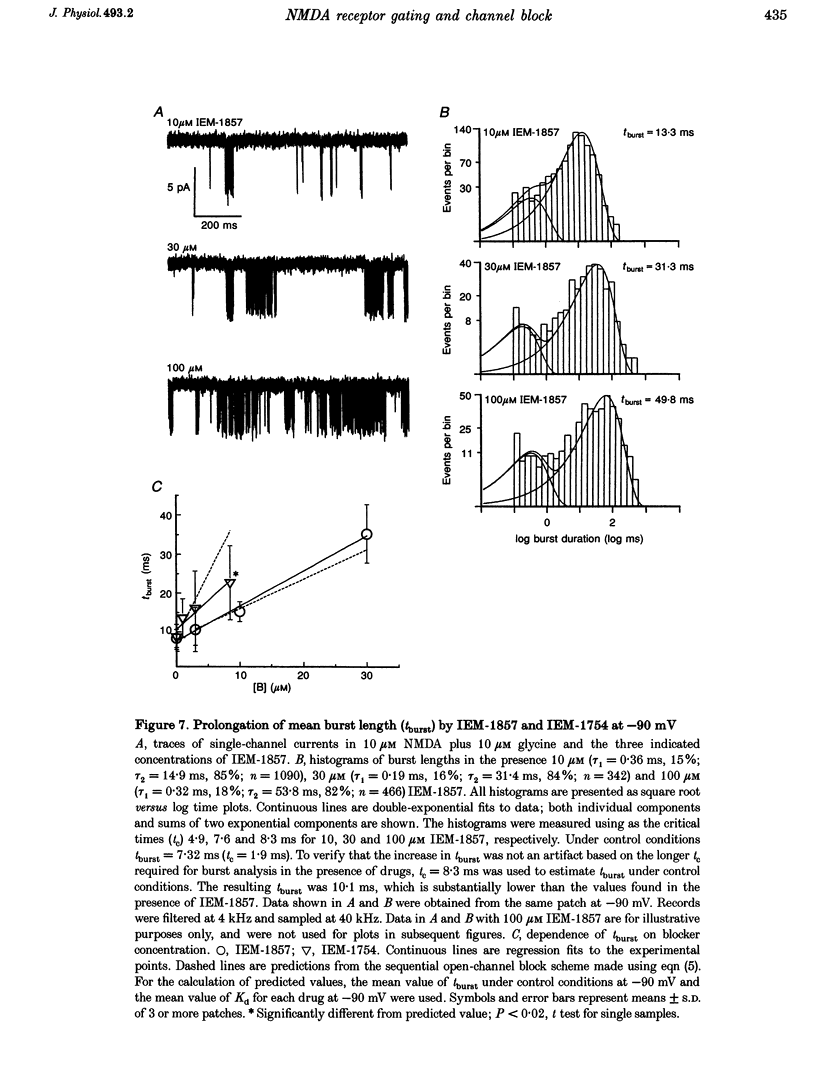





Selected References
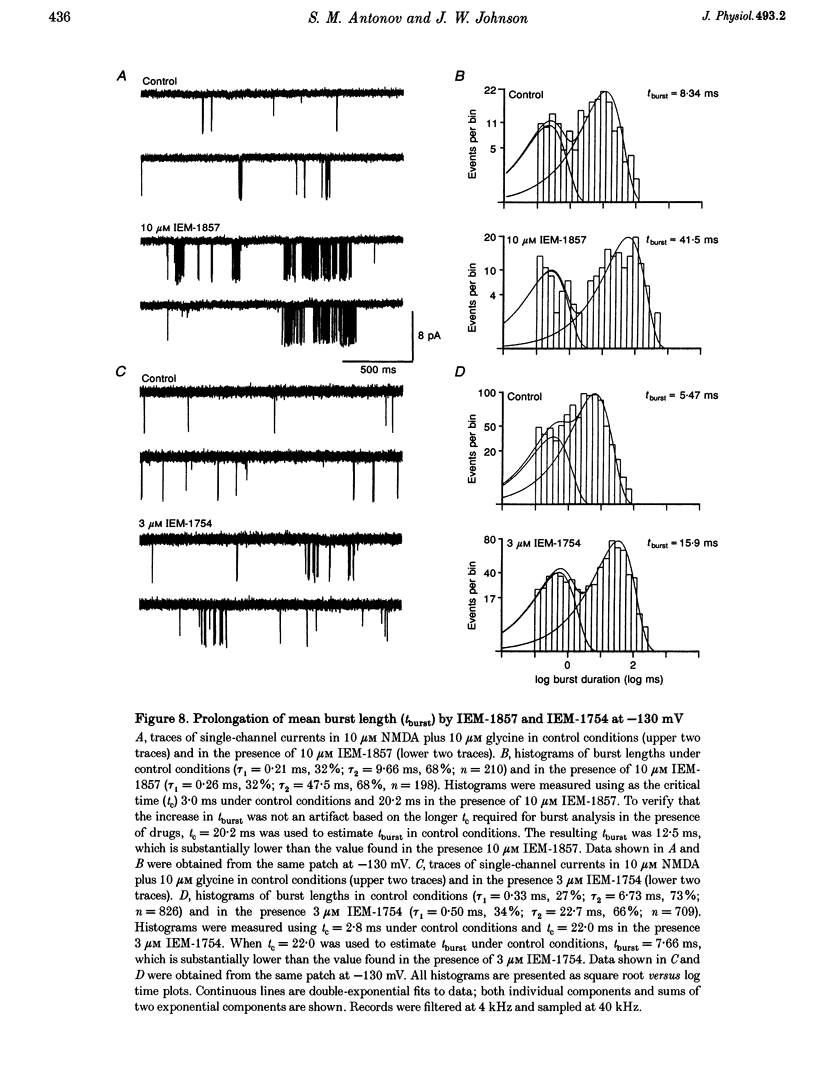
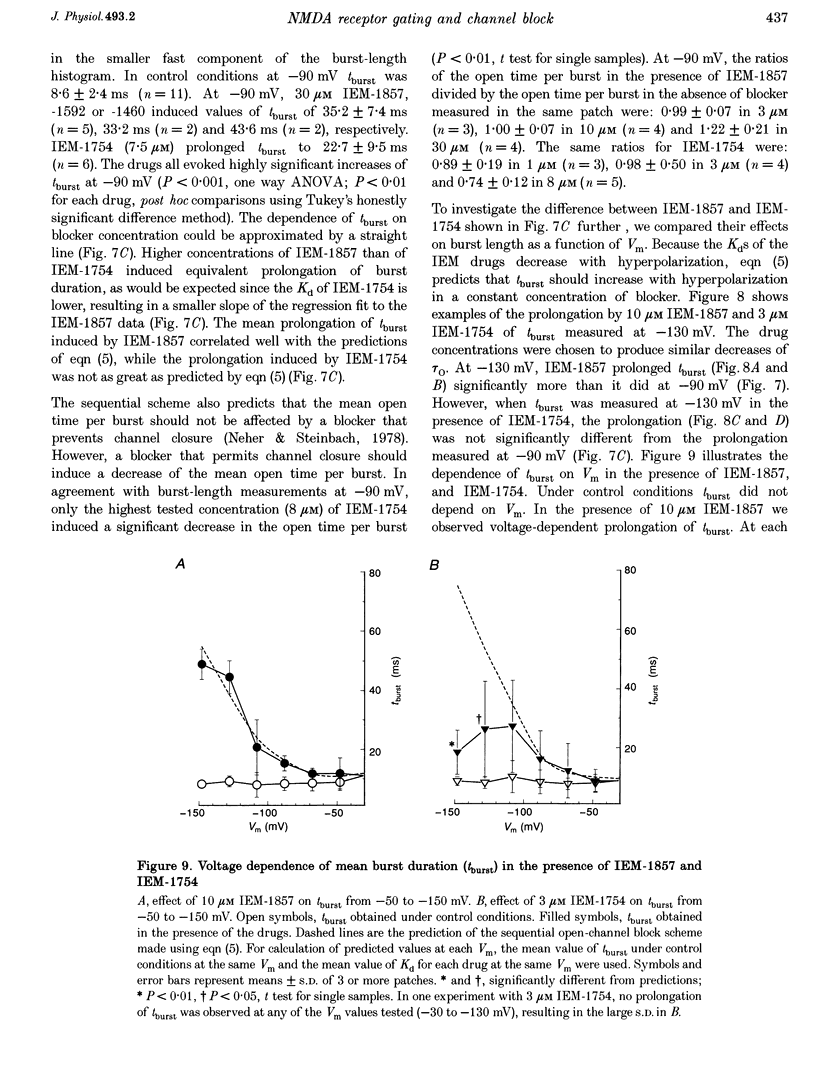
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov S. M., Johnson J. W., Lukomskaya N. Y., Potapyeva N. N., Gmiro V. E., Magazanik L. G. Novel adamantane derivatives act as blockers of open ligand-gated channels and as anticonvulsants. Mol Pharmacol. 1995 Mar;47(3):558–567. [PubMed] [Google Scholar]
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste M., Mayer M. L. Trapping of glutamate and glycine during open channel block of rat hippocampal neuron NMDA receptors by 9-aminoacridine. J Physiol. 1995 Mar 1;483(Pt 2):367–384. doi: 10.1113/jphysiol.1995.sp020591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N., Schoepfer R., Monyer H., Ruppersberg J. P., Günther W., Seeburg P. H., Sakmann B. Control by asparagine residues of calcium permeability and magnesium blockade in the NMDA receptor. Science. 1992 Sep 4;257(5075):1415–1419. doi: 10.1126/science.1382314. [DOI] [PubMed] [Google Scholar]
- Chen H. S., Pellegrini J. W., Aggarwal S. K., Lei S. Z., Warach S., Jensen F. E., Lipton S. A. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992 Nov;12(11):4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb A. J., Colquhoun D. Activation of N-methyl-D-aspartate receptors by L-glutamate in cells dissociated from adult rat hippocampus. J Physiol. 1992 Oct;456:143–179. doi: 10.1113/jphysiol.1992.sp019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich K. J., Beardsley D., Yue D. T. Ultra-deep blockade of Na+ channels by a quaternary ammonium ion: catalysis by a transition-intermediate state? J Physiol. 1993 Nov;471:319–341. doi: 10.1113/jphysiol.1993.sp019903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. R., Colquhoun D., Cull-Candy S. G. On the kinetics of large-conductance glutamate-receptor ion channels in rat cerebellar granule neurons. Proc R Soc Lond B Biol Sci. 1988 May 23;233(1273):407–422. doi: 10.1098/rspb.1988.0030. [DOI] [PubMed] [Google Scholar]
- Huettner J. E., Bean B. P. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. A quantitative description of NMDA receptor-channel kinetic behavior. J Neurosci. 1990 Jun;10(6):1830–1837. doi: 10.1523/JNEUROSCI.10-06-01830.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Calcium permeability of the N-methyl-D-aspartate receptor channel in hippocampal neurons in culture. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11573–11577. doi: 10.1073/pnas.90.24.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N. W., Pallotta B. S. Burst kinetics of single NMDA receptor currents in cell-attached patches from rat brain cortical neurons in culture. J Physiol. 1995 Jul 15;486(Pt 2):411–426. doi: 10.1113/jphysiol.1995.sp020822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Smerin Y., Johnson J. W. Effects of intracellular Mg2+ on channel gating and steady-state responses of the NMDA receptor in cultured rat neurons. J Physiol. 1996 Feb 15;491(Pt 1):137–150. doi: 10.1113/jphysiol.1996.sp021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F., Stevens C. F. Both open and closed NMDA receptor channels desensitize. J Neurosci. 1994 Apr;14(4):2153–2160. doi: 10.1523/JNEUROSCI.14-04-02153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J. F., Bartlett M. C., Mody I., Pahapill P., Reynolds J. N., Salter M. W., Schneiderman J. H., Pennefather P. S. Actions of ketamine, phencyclidine and MK-801 on NMDA receptor currents in cultured mouse hippocampal neurones. J Physiol. 1991 Jan;432:483–508. doi: 10.1113/jphysiol.1991.sp018396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C. G., Ogden D. C., Colquhoun D. The actions of suxamethonium (succinyldicholine) as an agonist and channel blocker at the nicotinic receptor of frog muscle. J Physiol. 1990 Sep;428:155–174. doi: 10.1113/jphysiol.1990.sp018205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. Bis-quaternary ammonium blockers as structural probes of the sarcoplasmic reticulum K+ channel. J Gen Physiol. 1982 May;79(5):869–891. doi: 10.1085/jgp.79.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Masaki H., Yamakura T., Mishina M. Identification by mutagenesis of a Mg(2+)-block site of the NMDA receptor channel. Nature. 1992 Aug 20;358(6388):673–675. doi: 10.1038/358673a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The charge carried by single-channel currents of rat cultured muscle cells in the presence of local anaesthetics. J Physiol. 1983 Jun;339:663–678. doi: 10.1113/jphysiol.1983.sp014741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Rock D. M., Macdonald R. L. The polyamine diaminodecane (DA-10) produces a voltage-dependent flickery block of single NMDA receptor channels. Neurosci Lett. 1992 Sep 14;144(1-2):111–115. doi: 10.1016/0304-3940(92)90728-p. [DOI] [PubMed] [Google Scholar]
- Rogawski M. A. Therapeutic potential of excitatory amino acid antagonists: channel blockers and 2,3-benzodiazepines. Trends Pharmacol Sci. 1993 Sep;14(9):325–331. doi: 10.1016/0165-6147(93)90005-5. [DOI] [PubMed] [Google Scholar]
- Sakurada K., Masu M., Nakanishi S. Alteration of Ca2+ permeability and sensitivity to Mg2+ and channel blockers by a single amino acid substitution in the N-methyl-D-aspartate receptor. J Biol Chem. 1993 Jan 5;268(1):410–415. [PubMed] [Google Scholar]
- Subramaniam S., Donevan S. D., Rogawski M. A. Hydrophobic interactions of n-alkyl diamines with the N-methyl-D-aspartate receptor: voltage-dependent and -independent blocking sites. Mol Pharmacol. 1994 Jan;45(1):117–124. [PubMed] [Google Scholar]
- Traynelis S. F., Cull-Candy S. G. Pharmacological properties and H+ sensitivity of excitatory amino acid receptor channels in rat cerebellar granule neurones. J Physiol. 1991 Feb;433:727–763. doi: 10.1113/jphysiol.1991.sp018453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel A., Burnashev N., Sakmann B. Dimensions of the narrow portion of a recombinant NMDA receptor channel. Biophys J. 1995 Mar;68(3):866–875. doi: 10.1016/S0006-3495(95)80263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobjev V. S., Sharonova I. N. Tetrahydroaminoacridine blocks and prolongs NMDA receptor-mediated responses in a voltage-dependent manner. Eur J Pharmacol. 1994 Feb 21;253(1-2):1–8. doi: 10.1016/0014-2999(94)90750-1. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakura T., Mori H., Masaki H., Shimoji K., Mishina M. Different sensitivities of NMDA receptor channel subtypes to non-competitive antagonists. Neuroreport. 1993 Jun;4(6):687–690. doi: 10.1097/00001756-199306000-00021. [DOI] [PubMed] [Google Scholar]
- Zamponi G. W., French R. J. Transcainide causes two modes of open-channel block with different voltage sensitivities in batrachotoxin-activated sodium channels. Biophys J. 1994 Sep;67(3):1028–1039. doi: 10.1016/S0006-3495(94)80568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M. M., Dani J. A. Ionic permeability characteristics of the N-methyl-D-aspartate receptor channel. J Gen Physiol. 1994 Feb;103(2):231–248. doi: 10.1085/jgp.103.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M. M., Dani J. A. Structural basis for explaining open-channel blockade of the NMDA receptor. J Neurosci. 1995 Feb;15(2):1446–1454. doi: 10.1523/JNEUROSCI.15-02-01446.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


