Dear Editor,
Cas9 and Cas12a have been widely applied in genome engineering in both plant and human cells (Tang and Zhang, 2023). However, the relatively large sizes restrict their delivery into cells via viral vectors. As hypothetical ancestors of Cas9 and Cas12a, IscB and TnpB have been reported as RNA-guided DNA endonucleases suitable for genome editing in human cells (Han et al., 2023; Xiang et al., 2023). More recently, a eukaryotic RNA-guided endonuclease named Fanzor has demonstrated genome editing capabilities in human cells (Saito et al., 2023). These nucleases, such as IsDge10, are significantly smaller (∼390 amino acids for IsDge10) compared to Cas9 and Cas12a (e.g., ∼1300 amino acids for SpCas9). However, the applicability of TnpB, IscB, or Fanzor for plant genome engineering remained unexplored. Here, we evaluated a series of nucleases from the TnpB, IscB, and Fanzor families and successfully developed a miniature plant genome editor using IsDge10, a TnpB nuclease from Deinococcus geothermalis.
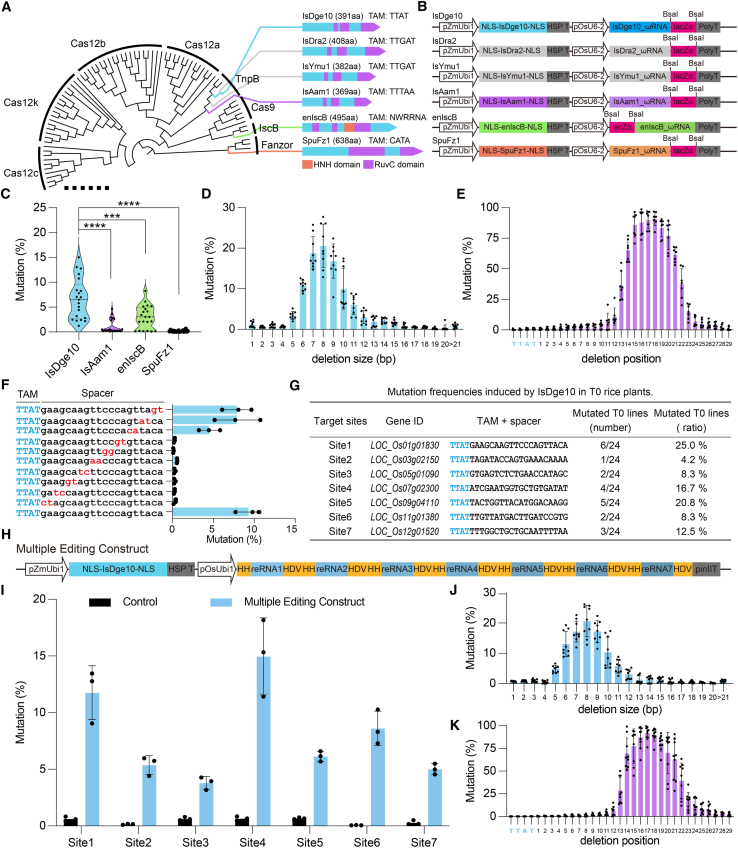
First, we selected six different nucleases from these three small nuclease families, including IsDge10, IsAam1, enIscB, and SpuFz1 (Figure 1A) (Han et al., 2023; Saito et al., 2023; Xiang et al., 2023), and optimized their codons for expression in rice. Next, we used the ZmUbi1 (RNA polymerase II) promoter to drive the expression of the nuclease gene and the OsU6-2 promoter for their respective guide RNAs (Figure 1B). We also developed a dual-fluorescence reporter system that simultaneously expresses GFP and mCherry. In this system, the green fluorescence from GFP serves as a normalization standard, and the red fluorescence from mCherry can be perturbated by targeted mutagenesis by any of these seven nuclease systems (Supplemental Figure 1A). This reporter system was co-transfected with the nuclease system into rice protoplasts, enabling preliminary assessments of the editing capabilities of our constructed nuclease systems. In our design, the mCherry gene was targeted at one site with the transposon-associated motif (TAM) by each corresponding nuclease (Figure 1A; Supplemental Table 1). The results showed that IsDge10, IsAam1, enIscB, and SpuFz1 each exhibited detectable editing activity in rice protoplasts, as indicated by the reduction of mCherry-to-GFP ratios, although their editing activities appeared lower than that of Cas9 (Supplemental Figures 1B and 1C). We then selected these four nucleases for further experiments.
Figure 1.
Development of the IsDge10 genome editing system in rice.
(A) Phylogenetic diagram illustrating the evolutionary relationships among TnpB, Cas9, Cas12, IscB, and Fanzor. The selected nucleases IsDge10, IsDra2, IsYmu1, IsAam1, enIscB, and SpuFz1 are highlighted and connected to their corresponding structural diagrams, showing their sizes and domains (HNH and RuvC).
(B) Schematics of the IsDge10, IsDra2, IsYmu1, IsAam1, enIscB, and SpuFz1 constructs used for genome editing in rice.
(C) Comparison of the mutation rates of the IsDge10, IaAam1, enIscB, and SpuFz1 systems in rice protoplasts.
(D) Deletion size profiles for three representative target sites in rice.
(E) Deletion position profiles for three representative target sites in rice.
(F) Assessment of targeting specificity using mismatched guide RNAs at a representative target site in rice protoplasts.
(G) Genome editing efficiency of IsDge10 in stable rice lines at seven target sites.
(H) Schematics of the dual RNA polymerase II promoter-based and multiplexed IsDge10 system for genome editing in rice.
(I) Multiplexed editing of seven target sites in rice protoplasts.
(J) Deletion size profiles for three representative multiplexed target sites in rice.
(K) Deletion position profile for three representative multiplexed target sites in rice. Each dot represents a biological replicate. Data are presented as mean values ± SD. Data were analyzed using a two-tailed unpaired t-test. ∗∗∗P < 0.001 and ∗∗∗∗P < 0.0001. Solid line, median; dashed line, quartiles.
To evaluate the editing efficiency of these four systems at endogenous sites in rice, we selected seven sites per nuclease system and assessed the outcomes of targeted mutagenesis in rice protoplasts using next-generation sequencing of PCR amplicons (Figure 1A; Supplemental Table 1). Our data showed that IsDge10 exhibited an editing efficiency ranging from 2.20% to 15.04% across the seven target sites. In contrast, enIscB achieved 2.05%–8.27% editing efficiency at five out of seven sites, IsAam1 exhibited 2.36%–4.65% editing efficiency at two out of seven sites, and SpuFz1 showed no detectable editing activity (Figure 1C and Supplemental Figure 2). These results suggest that IsDge10 is superior to the enIscB, IsAam1, and SpuFz1 systems for genome editing in rice. Further analysis showed that IsDge10 primarily generated deletions ranging from 6 to 10 bp in size, occurring 13 to –23 bp away from the TAM (Figures 1D and 1E and Supplemental Figures 3–5). This cleavage pattern of IsDge10 is similar to those observed with Cas12 nucleases, but differs from that of enIscB, which cleaves proximally to the TAM-a characteristic consistent with enlscB’s evolutionary relationship to the Cas9 group (Supplemental Figures 6 and 7). This suggests that IsDge10, like Cas12 nucleases, produces off-set DNA double-strand breaks distal to the TAM sites.
To assess the specificity of IsDge10, we focused on a high-activity target, site 01, and designed a series of protospacers with two adjacent mutations at various positions (Supplemental Table 1; Supplemental Figure 2A). Analysis of rice protoplasts showed that permutations of every two nucleotides from positions 1–14 bp within the protospacer completely abolished the editing activity of IsDge10, whereas mutations at positions 15–16 bp within the protospacer led to ∼50% reduction in editing frequency (Figure 1F). In contrast, mutations at positions 17–20 bp of the protospacer did not significantly affect the editing activity of IsDge10 (Figure 1F). These findings delineate the core functional length of a spacer for IsDge10 and confirmed its high specificity as a nuclease.
We then tested whether IsDge10 could generate edits in stable rice lines. The same transfer DNA constructs targeting the seven sites were used for the stable transformation of rice. The analysis of T0 generation plants revealed successful editing at all seven sites, with mutation efficiencies ranging from 4.2% to 25% (Figure 1G). The mutations predominantly consisted of deletions of 5–10 bp or longer (Supplemental Figure 8), consistent with the editing profile observed in rice protoplasts (Figure 1D). Notably, only monoallelic mutations were detected in these rice lines (Supplemental Figure 9). Assuming that biallelic knockout of these genes is non-lethal, these findings indicate the potential for enhancing IsDge10 to achieve biallelic editing in rice.
To develop a multiplexed IsDge10 genome editing system, we adopted the dual RNA polymerase II promoter expression systems previously used for Cas12a, Cas12b, and Cas12j2, as well as their guide RNAs (Tang et al., 2017, 2019; Ming et al., 2020; Liu et al., 2022; Zheng et al., 2023; Zhou et al., 2023). The IsDge10 protein was expressed under the ZmUbi1 promoter, and the seven guide RNAs were expressed under the OsUbi1 promoter and processed by the HH (hammer head)-HDV (hepatitis delta virus) dual ribozyme system to form mature “guide RNA–ωRNA” complexes (Figure 1H). Interestingly, this multiplexed construct exhibited higher editing efficiencies at all seven target sites compared to those achieved using the OsU6-2 promoter for guide RNA expression (Figure 1C and Supplemental Figure 2A), with efficiencies ranging from 4.3% to 18.2% in rice protoplasts (Figure 1I). The deletions were typically 6–10 bp in length and occurred 13–23 bp away from the TAM (Figures 1J and 1K and Supplemental Figures 10 and 11). These results confirm that IsDge10 can edit multiple sites simultaneously using this robust dual polymerase II promoter system.
In summary, our study establishes IsDge10 is a novel and compact transposon-associated TnpB nuclease suitable for genome editing in rice. Compared to other compact nucleases tested, IsDge10 exhibits robust genome editing activity in rice and requires only a simple TTAT TAM. Although the current IsDge10 system does not yet match the efficiency of the widely used Cas9 and Cas12a systems, this study paves the way for further enhancements through protein engineering and evolutionary approaches. As one of the smallest nucleases functional in plants, IsDge10 holds great potential for various applications, including multi-nuclease combination editing, integration with diverse effectors to develop tools for transcriptional and epigenetic regulation, and incorporation into viral vectors for plant genome engineering.
Funding
This research was supported by the Biological Breeding-Major Projects (2023ZD04076) awarded to X.T. and Y.Z., and the National Natural Science Foundation of China (award nos. 32270433, 32101205, and 32072045) to Y.Z., X.Z., and X.T. It was also supported by the NSF Plant Genome Research Program (IOS-2029889 and IOS-2132693) to Y.Q.
Acknowledgments
No conflict of interest is declared.
Author contributions
Y.Z. conceived the project and designed the experiments. R.Z., X.T., and Y.H. generated all the constructs. R.Z. performed the rice protoplast transformation and analyzed the mutation frequencies in protoplasts. R.Z., W.W., Y.W., D.W., and X.Z. conducted rice stable transformations. Y.L. revised the manuscript. Y.Z., Y.Q., and R.Z. analyzed the data and wrote the manuscript with input from all authors. All authors have read and approved the final version of the manuscript.
Published: August 21, 2024
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Supplemental information is available at Plant Communications Online.
Contributor Information
Yiping Qi, Email: yiping@umd.edu.
Yong Zhang, Email: zhangyong916@swu.edu.cn.
Supplemental information
References
- Han D., Xiao Q., Wang Y., Zhang H., Dong X., Li G., Kong X., Wang S., Song J., Zhang W., et al. Development of miniature base editors using engineered IscB nickase. Nat. Methods. 2023;20:1029–1036. doi: 10.1038/s41592-023-01898-9. [DOI] [PubMed] [Google Scholar]
- Liu S., Sretenovic S., Fan T., Cheng Y., Li G., Qi A., Tang X., Xu Y., Guo W., Zhong Z., et al. Hypercompact CRISPR-Cas12j2 (CasPhi) enables genome editing, gene activation, and epigenome editing in plants. Plant Commun. 2022;3:100453. doi: 10.1016/j.xplc.2022.100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming M., Ren Q., Pan C., He Y., Zhang Y., Liu S., Zhong Z., Wang J., Malzahn A.A., Wu J., et al. CRISPR-Cas12b enables efficient plant genome engineering. Nat. Plants. 2020;6:202–208. doi: 10.1038/s41477-020-0614-6. [DOI] [PubMed] [Google Scholar]
- Saito M., Xu P., Faure G., Maguire S., Kannan S., Altae-Tran H., Vo S., Desimone A., Macrae R.K., Zhang F. Fanzor is a eukaryotic programmable RNA-guided endonuclease. Nature. 2023;620:660–668. doi: 10.1038/s41586-023-06356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Zhang Y. Beyond knockouts: fine-tuning regulation of gene expression in plants with CRISPR-Cas-based promoter editing. New Phytol. 2023;239:868–874. doi: 10.1111/nph.19020. [DOI] [PubMed] [Google Scholar]
- Tang X., Lowder L.G., Zhang T., Malzahn A.A., Zheng X., Voytas D.F., Zhong Z., Chen Y., Ren Q., Li Q., et al. A CRISPR-Cpf1 system for efficient genome editing and transcriptional repression in plants. Nat. Plants. 2017;3:17018. doi: 10.1038/nplants.2017.18. [DOI] [PubMed] [Google Scholar]
- Tang X., Ren Q., Yang L., Bao Y., Zhong Z., He Y., Liu S., Qi C., Liu B., Wang Y., et al. Single transcript unit CRISPR 2.0 systems for robust Cas9 and Cas12a mediated plant genome editing. Plant Biotechnol. J. 2019;17:1431–1445. doi: 10.1111/pbi.13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang G., Li Y., Sun J., Huo Y., Cao S., Cao Y., Guo Y., Yang L., Cai Y., Zhang Y.E., et al. Evolutionary mining and functional characterization of TnpB nucleases identify efficient miniature genome editors. Nat. Biotechnol. 2023;42:745–757. doi: 10.1038/s41587-023-01857-x. [DOI] [PubMed] [Google Scholar]
- Zheng X., Zhang S., Liang Y., Zhang R., Liu L., Qin P., Zhang Z., Wang Y., Zhou J., Tang X., et al. Loss-function mutants of OsCKX gene family based on CRISPR-Cas systems revealed their diversified roles in rice. Plant Genome. 2023;16:e20283. doi: 10.1002/tpg2.20283. [DOI] [PubMed] [Google Scholar]
- Zhou J., Liu G., Zhao Y., Zhang R., Tang X., Li L., Jia X., Guo Y., Wu Y., Han Y., et al. An efficient CRISPR-Cas12a promoter editing system for crop improvement. Nat. Plants. 2023;9:588–604. doi: 10.1038/s41477-023-01384-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.