Abstract
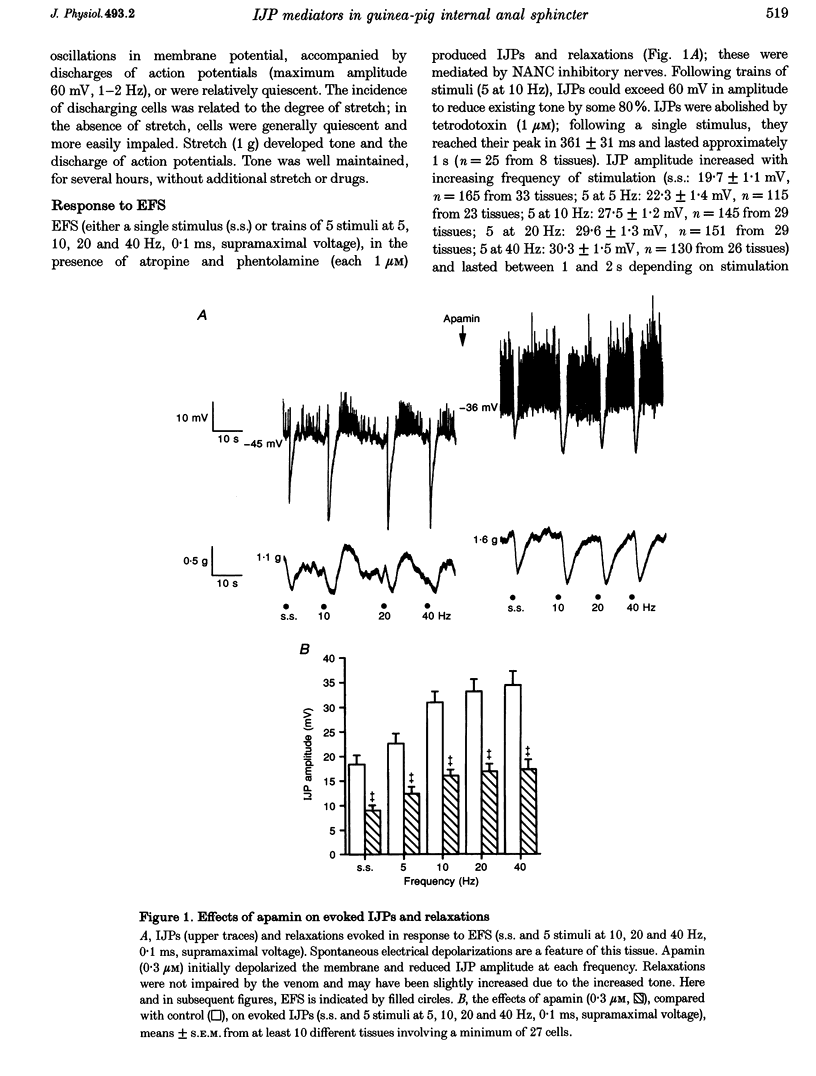
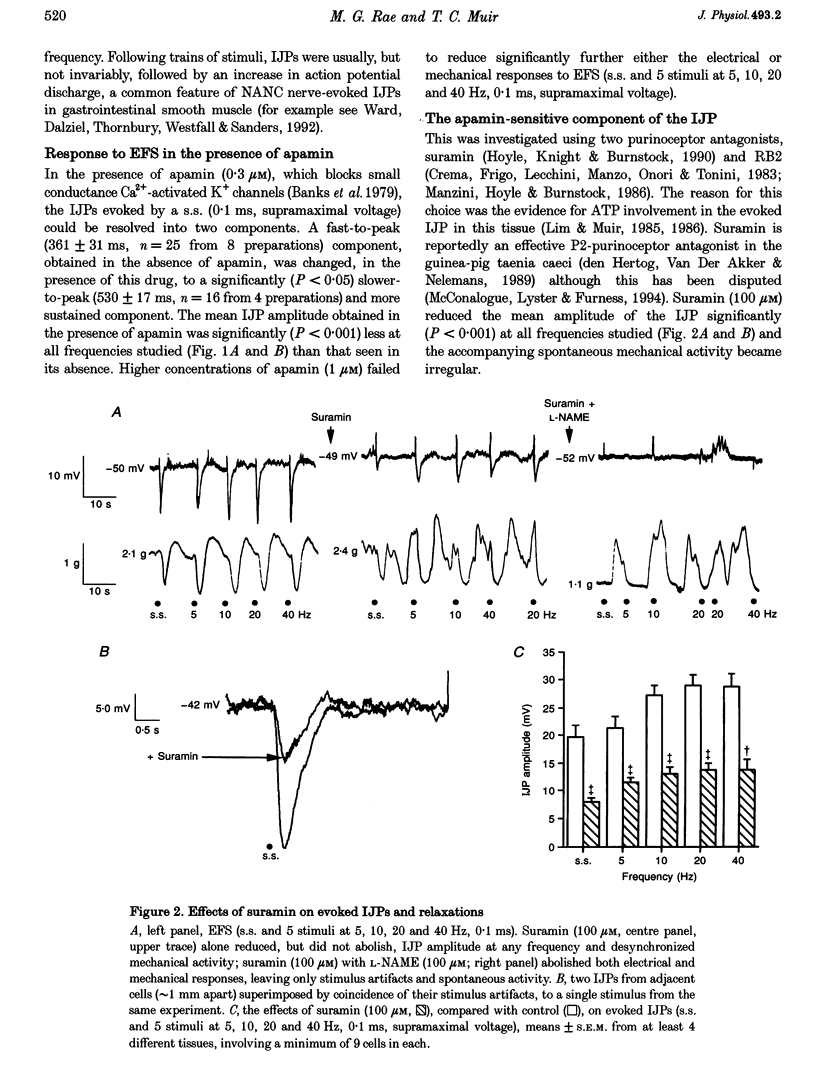
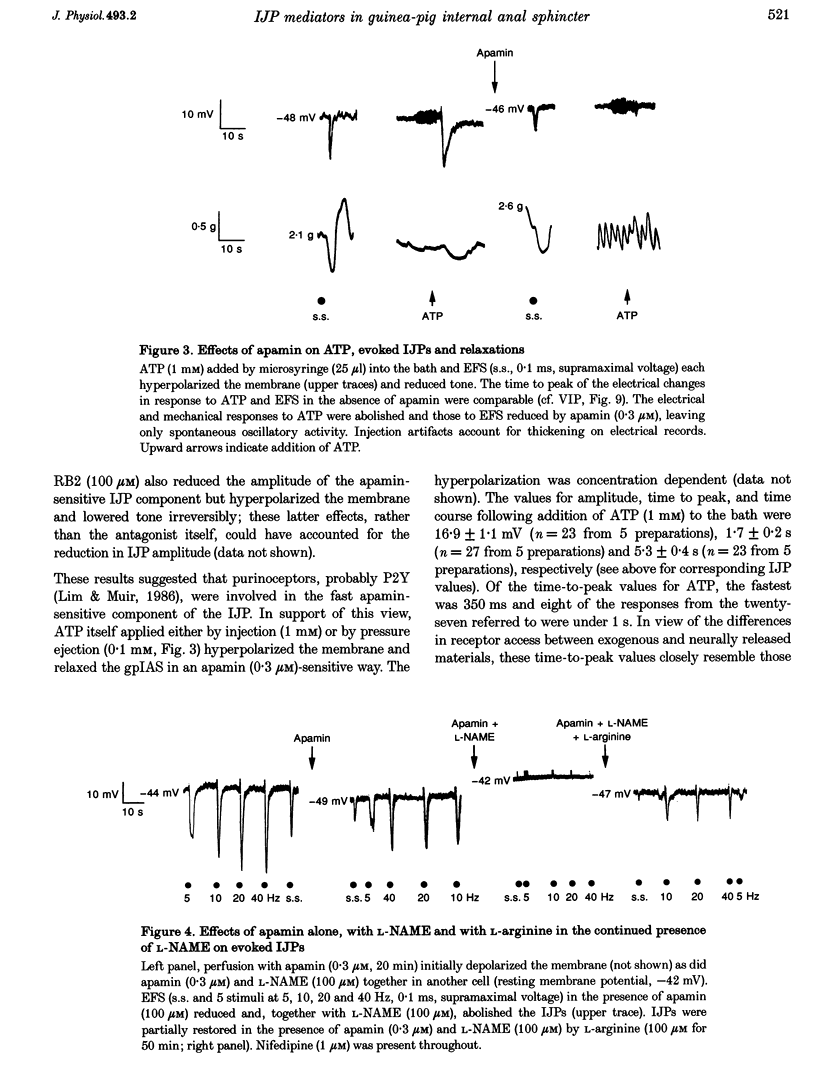
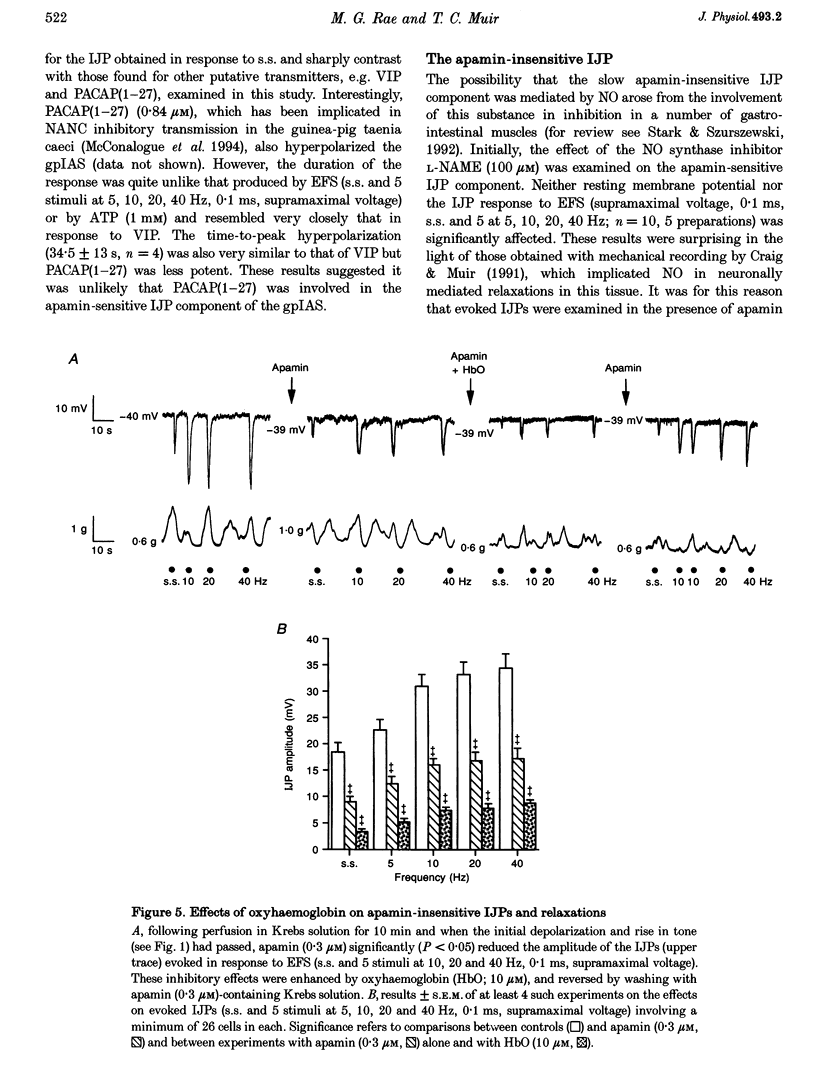
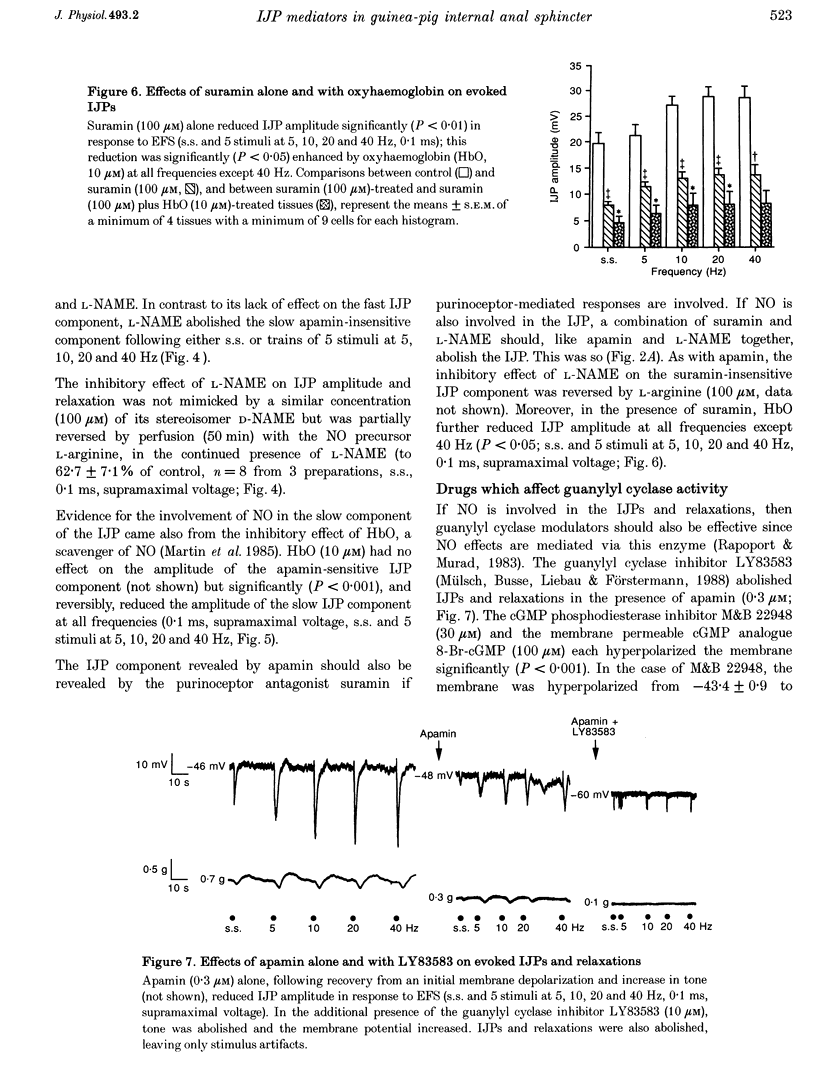
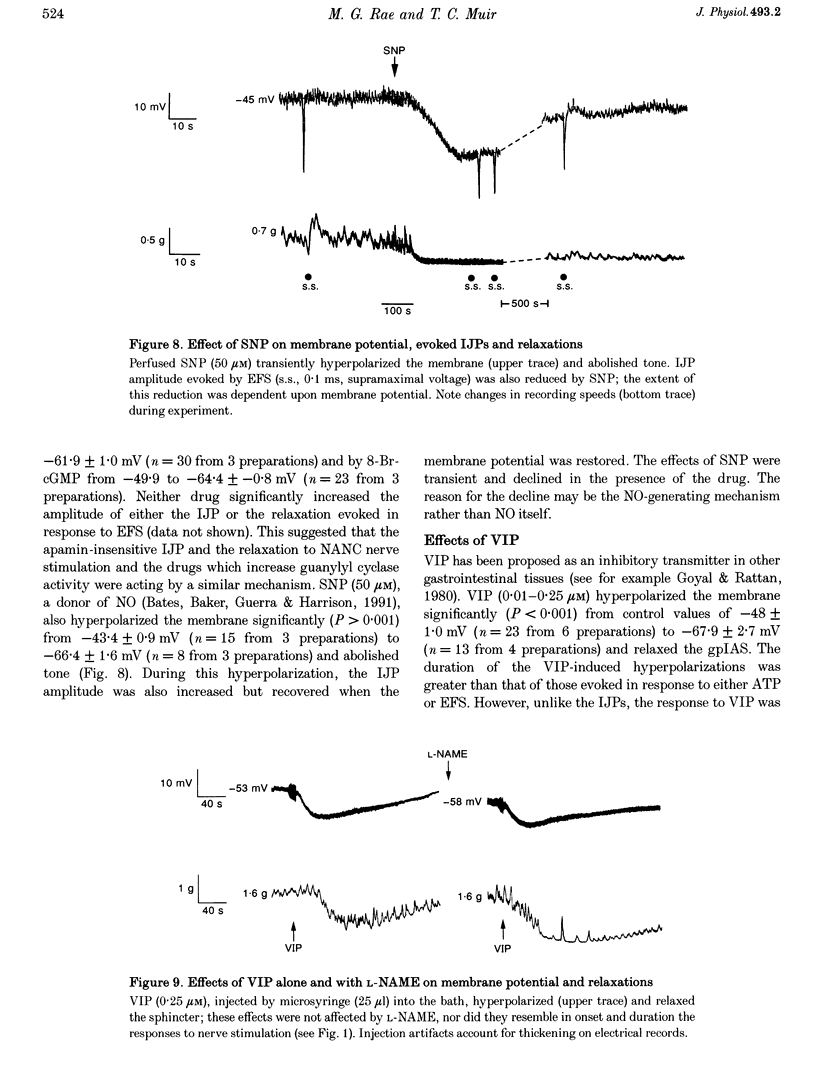
1. Inhibitory junction potentials (IJPs) and relaxations evoked in response to field stimulation (supramaximal voltage, 0.1 ms, single stimulus and 5 stimuli at 5-40 Hz) of non-adrenergic non-cholinergic (NANC) nerves with atropine and phentolamine (each 1 microM) were measured in the guinea-pig internal anal sphincter (gpIAS). The mean resting membrane potential was -44.2 +/- 0.2 mV (n = 1119 cells from 260 preparations). 2. NANC nerve stimulation evoked frequency-dependent IJPs (19.7 +/- 1.1 mV, n = 165, 33 tissues to a single stimulus) and relaxations. IJPs consisted of two tetrodotoxin (1 microM)-sensitive components: one was abolished by apamin (0.3 microM) and the P2-purinoceptor antagonist suramin (100 microM); the other, smaller in amplitude, was sensitive to inhibitors of nitric oxide synthase (NOS, e.g. L-NAME, 100 microM) and the nitric oxide (NO) scavenger oxyhaemoglobin (HbO, 10 microM). 3. ATP (1 mM), vasoactive intestinal polypeptide (VIP, 0.01-0.25 microM) and pituitary adenylate cyclase-activating peptide (PACAP(1-27), 0.84 microM) each hyperpolarized and relaxed the gpIAS; only ATP responses resembled the evoked IJPs in time course. 4. The guanylyl cyclase inhibitor LY83583 (10 microM) abolished apamin-insensitive IJPs and relaxations. The cGMP phosphodiesterase inhibitor M&B 22948 (30 microM) and 8-Br-cGMP (100 microM) each hyperpolarized the gpIAS. 5. Two components comprise the IJP and relaxation evoked in response to NANC nerve stimulation in the gpIAS. One, sensitive to apamin, resembles the response to ATP and is modulated by purinoceptor antagonists; the other, apamin and suramin insensitive, is inhibited by NO antagonists.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks B. E., Brown C., Burgess G. M., Burnstock G., Claret M., Cocks T. M., Jenkinson D. H. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979 Nov 22;282(5737):415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- Bates J. N., Baker M. T., Guerra R., Jr, Harrison D. G. Nitric oxide generation from nitroprusside by vascular tissue. Evidence that reduction of the nitroprusside anion and cyanide loss are required. Biochem Pharmacol. 1991 Dec 11;42 (Suppl):S157–S165. doi: 10.1016/0006-2952(91)90406-u. [DOI] [PubMed] [Google Scholar]
- Bolotina V. M., Najibi S., Palacino J. J., Pagano P. J., Cohen R. A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994 Apr 28;368(6474):850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Burleigh D. E. Ng-nitro-L-arginine reduces nonadrenergic, noncholinergic relaxations of human gut. Gastroenterology. 1992 Feb;102(2):679–683. doi: 10.1016/0016-5085(92)90120-n. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Overview. Purinergic mechanisms. Ann N Y Acad Sci. 1990;603:1–18. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- Bywater R. A., Taylor G. S. Non-cholinergic excitatory and inhibitory junction potentials in the circular smooth muscle of the guinea-pig ileum. J Physiol. 1986 May;374:153–164. doi: 10.1113/jphysiol.1986.sp016072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capiod T., Ogden D. C. The properties of calcium-activated potassium ion channels in guinea-pig isolated hepatocytes. J Physiol. 1989 Feb;409:285–295. doi: 10.1113/jphysiol.1989.sp017497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crema A., Frigo G. M., Lecchini S., Manzo L., Onori L., Tonini M. Purine receptors in the guinea-pig internal anal sphincter. Br J Pharmacol. 1983 Mar;78(3):599–603. doi: 10.1111/j.1476-5381.1983.tb08820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Hertog A., Van den Akker J., Nelemans A. Suramin and the inhibitory junction potential in taenia caeci of the guinea-pig. Eur J Pharmacol. 1989 Dec 7;173(2-3):207–209. doi: 10.1016/0014-2999(89)90522-0. [DOI] [PubMed] [Google Scholar]
- Du C., Murray J., Bates J. N., Conklin J. L. Nitric oxide: mediator of NANC hyperpolarization of opossum esophageal smooth muscle. Am J Physiol. 1991 Dec;261(6 Pt 1):G1012–G1016. doi: 10.1152/ajpgi.1991.261.6.G1012. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J. Vasoactive intestinal polypeptide: measurement, distribution and putative neurotransmitter function. Digestion. 1979;19(3):149–169. doi: 10.1159/000198339. [DOI] [PubMed] [Google Scholar]
- Goyal R. K., Rattan S., Said S. I. VIP as a possible neurotransmitter of non-cholinergic non-adrenergic inhibitory neurones. Nature. 1980 Nov 27;288(5789):378–380. doi: 10.1038/288378a0. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Knight G. E., Burnstock G. Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br J Pharmacol. 1990 Mar;99(3):617–621. doi: 10.1111/j.1476-5381.1990.tb12979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. P., Muir T. C. Mechanisms underlying the electrical and mechanical responses of the guinea-pig internal anal sphincter to field stimulation and to drugs. Br J Pharmacol. 1985 Oct;86(2):427–437. doi: 10.1111/j.1476-5381.1985.tb08912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. P., Muir T. C. Neuroeffector transmission in the guinea-pig internal anal sphincter: an electrical and mechanical study. Eur J Pharmacol. 1986 Aug 22;128(1-2):17–24. doi: 10.1016/0014-2999(86)90552-2. [DOI] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- McKirdy H. C. Innervation of internal anal sphincter--in vitro studies. Int J Colorectal Dis. 1992 Feb;7(1):43–44. doi: 10.1007/BF01647661. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Busse R., Liebau S., Förstermann U. LY 83583 interferes with the release of endothelium-derived relaxing factor and inhibits soluble guanylate cyclase. J Pharmacol Exp Ther. 1988 Oct;247(1):283–288. [PubMed] [Google Scholar]
- O'Kelly T., Brading A., Mortensen N. Nerve mediated relaxation of the human internal anal sphincter: the role of nitric oxide. Gut. 1993 May;34(5):689–693. doi: 10.1136/gut.34.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Endothelium-dependent and nitrovasodilator-induced relaxation of vascular smooth muscle: role of cyclic GMP. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(4-5):281–296. [PubMed] [Google Scholar]
- Soediono P., Burnstock G. Contribution of ATP and nitric oxide to NANC inhibitory transmission in rat pyloric sphincter. Br J Pharmacol. 1994 Nov;113(3):681–686. doi: 10.1111/j.1476-5381.1994.tb17046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M. E., Szurszewski J. H. Role of nitric oxide in gastrointestinal and hepatic function and disease. Gastroenterology. 1992 Dec;103(6):1928–1949. doi: 10.1016/0016-5085(92)91454-c. [DOI] [PubMed] [Google Scholar]
- Tøttrup A., Glavind E. B., Svane D. Involvement of the L-arginine-nitric oxide pathway in internal anal sphincter relaxation. Gastroenterology. 1992 Feb;102(2):409–415. doi: 10.1016/0016-5085(92)90084-c. [DOI] [PubMed] [Google Scholar]
- Tøttrup A., Ny L., Alm P., Larsson B., Forman A., Andersson K. E. The role of the L-arginine/nitric oxide pathway for relaxation of the human lower oesophageal sphincter. Acta Physiol Scand. 1993 Dec;149(4):451–459. doi: 10.1111/j.1748-1716.1993.tb09642.x. [DOI] [PubMed] [Google Scholar]
- Ward S. M., Dalziel H. H., Thornbury K. D., Westfall D. P., Sanders K. M. Nonadrenergic, noncholinergic inhibition and rebound excitation in canine colon depend on nitric oxide. Am J Physiol. 1992 Feb;262(2 Pt 1):G237–G243. doi: 10.1152/ajpgi.1992.262.2.G237. [DOI] [PubMed] [Google Scholar]
- Ward S. M., McKeen E. S., Sanders K. M. Role of nitric oxide in non-adrenergic, non-cholinergic inhibitory junction potentials in canine ileocolonic sphincter. Br J Pharmacol. 1992 Apr;105(4):776–782. doi: 10.1111/j.1476-5381.1992.tb09056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


