Abstract
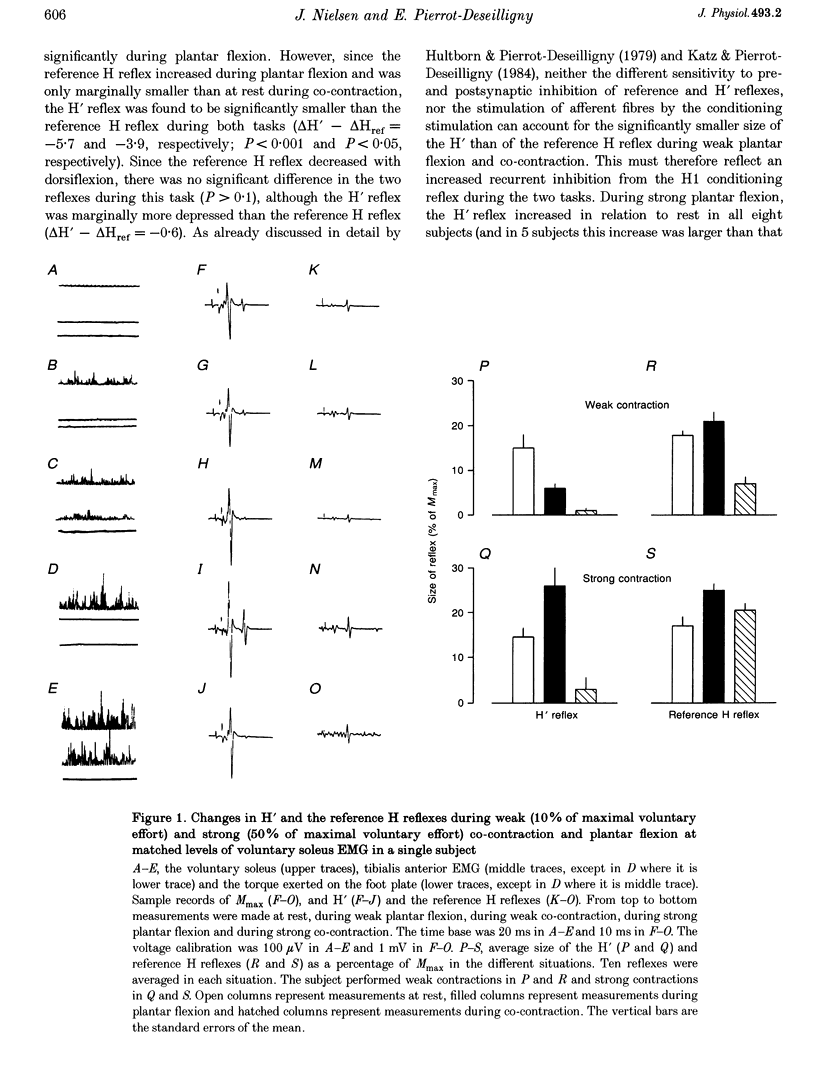
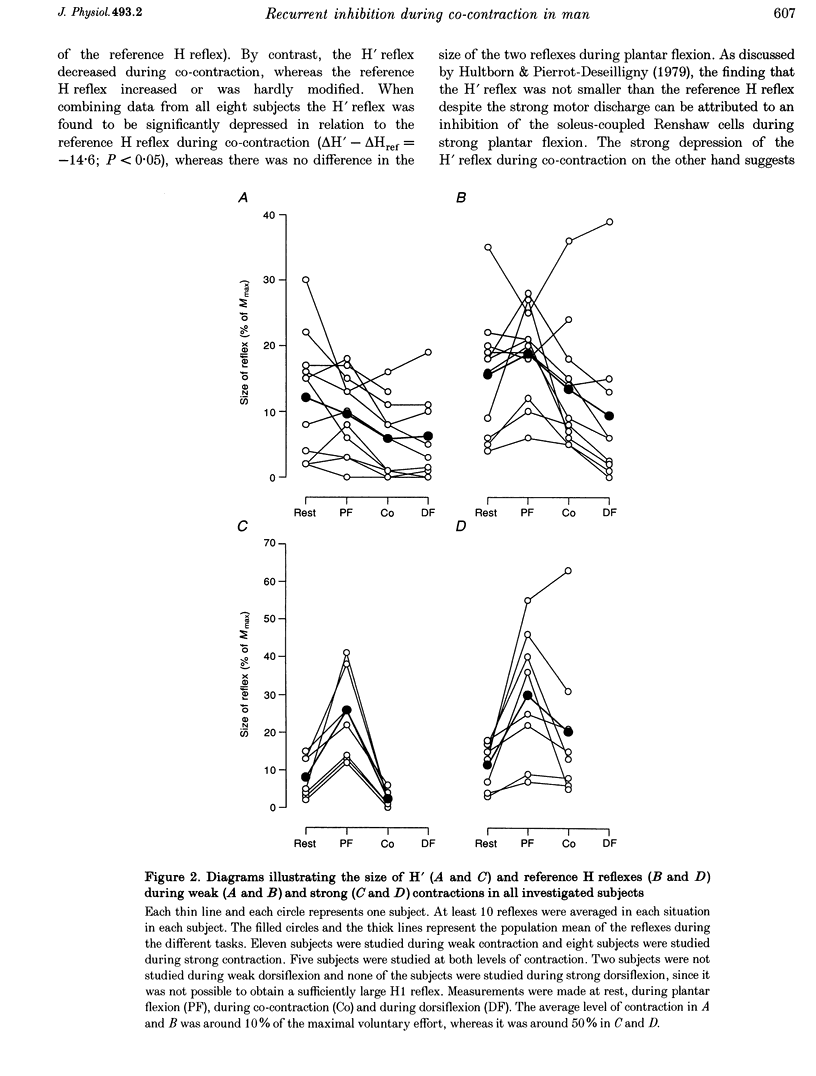
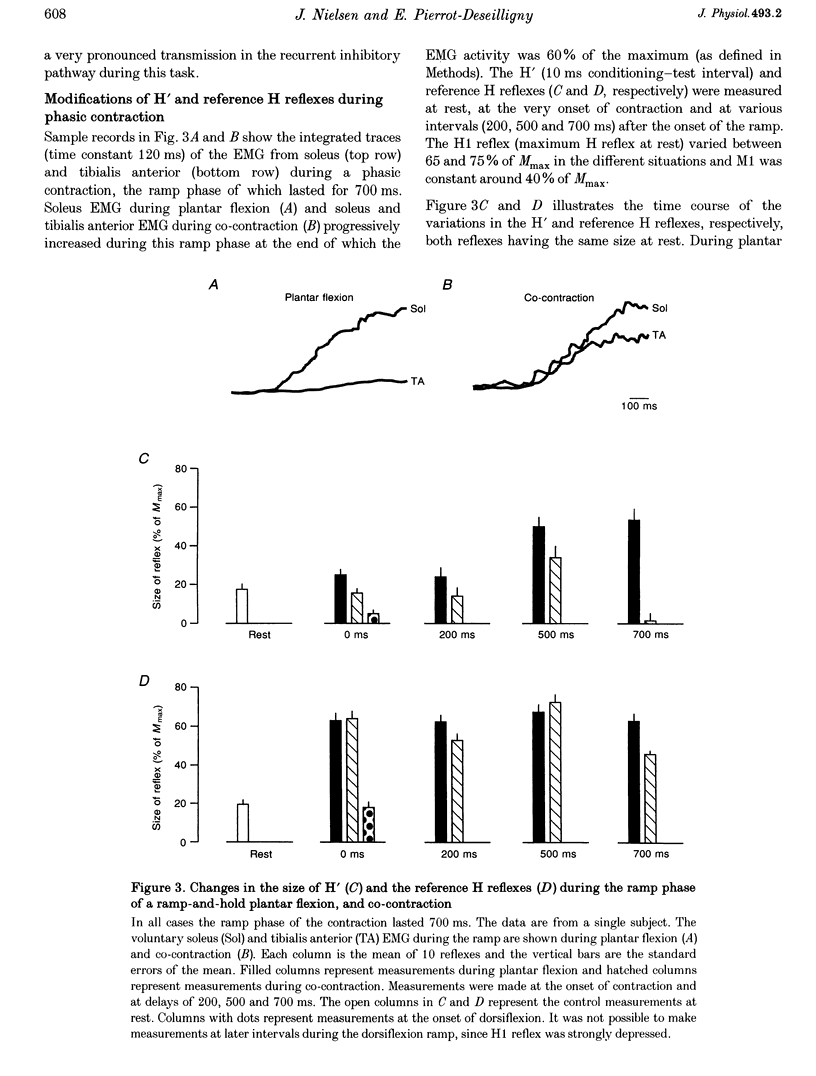
1. The amount of recurrent inhibition onto soleus motoneurones was compared during plantar flexion and co-contraction of antagonistic ankle plantar and dorsiflexors at matched levels of background activity in the soleus muscle. 2. During weak plantar flexion and co-contraction (less than 10% of maximal voluntary plantar flexion effort) a test reflex discharge (H' reflex), which was conditioned by a previous reflex discharge, was found to be significantly more depressed in relation to rest than an unconditioned reference H reflex. During strong plantar flexion (more than 50% of maximal voluntary plantar flexion effort) the H' reflex either increased more or to the same extent as the reference H reflex in relation to rest. In contrast to this, the H' reflex was strongly depressed during co-contraction, whereas the reference H reflex was not significantly different from its resting value. 3. At the end of the ramp phase of a phasic contraction, large variations of the H' reflex were observed during plantar flexion (large increase in relation to rest) and during co-contraction (marked decrease), whereas the reference H reflex was facilitated in the two situations. 4. These observations provide evidence that soleus-coupled Renshaw cells are differently regulated during co-contraction and plantar flexion. It is suggested that the Renshaw cells are inhibited during strong plantar flexion but not during strong co-contraction. The functional significance of the findings is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anastasijević R., Vuco J. The relative dependence of the activity of Renshaw cells on recurrent pathways during contraction of the triceps muscle. Pflugers Arch. 1978 Nov 30;377(3):255–261. doi: 10.1007/BF00584281. [DOI] [PubMed] [Google Scholar]
- Bussel B., Pierrot-Deseilligny E. Inhibition of human motoneurons, probably of Renshaw origin, elicited by an orthodromic motor discharge. J Physiol. 1977 Jul;269(2):319–339. doi: 10.1113/jphysiol.1977.sp011904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz E. E., Cheney P. D. Functional relations between primate motor cortex cells and muscles: fixed and flexible. Ciba Found Symp. 1987;132:98–117. doi: 10.1002/9780470513545.ch7. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Relative contribution from different nerves to recurrent depression of Ia IPSPs in motoneurones. J Physiol. 1971 Jul;215(3):637–664. doi: 10.1113/jphysiol.1971.sp009489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Pierrot-Deseilligny E. Changes in recurrent inhibition during voluntary soleus contractions in man studied by an H-reflex technique. J Physiol. 1979 Dec;297(0):229–251. doi: 10.1113/jphysiol.1979.sp013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Pierrot-Deseilligny E., Wigström H. Recurrent inhibition and afterhyperpolarization following motoneuronal discharge in the cat. J Physiol. 1979 Dec;297(0):253–266. doi: 10.1113/jphysiol.1979.sp013038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INMAN V. T., RALSTON H. J., SAUNDERS J. B., FEINSTEIN B., WRIGHT E. W., Jr Relation of human electromyogram to muscular tension. Electroencephalogr Clin Neurophysiol. 1952 May;4(2):187–194. doi: 10.1016/0013-4694(52)90008-4. [DOI] [PubMed] [Google Scholar]
- Katz R., Pierrot-Deseilligny E. Facilitation of soleus-coupled Renshaw cells during voluntary contraction of pretibial flexor muscles in man. J Physiol. 1984 Oct;355:587–603. doi: 10.1113/jphysiol.1984.sp015440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn M., Yang J. F., Prochazka A. Human H-reflexes are smaller in difficult beam walking than in normal treadmill walking. Exp Brain Res. 1990;83(1):22–28. doi: 10.1007/BF00232189. [DOI] [PubMed] [Google Scholar]
- Mazzocchio R., Rossi A. Further evidence for Renshaw inhibition in man. A combined electrophysiological and pharmacological approach. Neurosci Lett. 1989 Nov 20;106(1-2):131–136. doi: 10.1016/0304-3940(89)90214-0. [DOI] [PubMed] [Google Scholar]
- Mazzocchio R., Rossi A., Rothwell J. C. Depression of Renshaw recurrent inhibition by activation of corticospinal fibres in human upper and lower limb. J Physiol. 1994 Dec 1;481(Pt 2):487–498. doi: 10.1113/jphysiol.1994.sp020457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S., Pierrot-Deseilligny E., Simonetta-Moreau M. Pattern of heteronymous recurrent inhibition in the human lower limb. Exp Brain Res. 1994;102(1):149–159. doi: 10.1007/BF00232447. [DOI] [PubMed] [Google Scholar]
- Nielsen J., Kagamihara Y. Differential projection of the sural nerve to early and late recruited human tibialis anterior motor units: change of recruitment gain. Acta Physiol Scand. 1993 Apr;147(4):385–401. doi: 10.1111/j.1748-1716.1993.tb09515.x. [DOI] [PubMed] [Google Scholar]
- Nielsen J., Kagamihara Y. Synchronization of human leg motor units during co-contraction in man. Exp Brain Res. 1994;102(1):84–94. doi: 10.1007/BF00232441. [DOI] [PubMed] [Google Scholar]
- Nielsen J., Kagamihara Y. The regulation of disynaptic reciprocal Ia inhibition during co-contraction of antagonistic muscles in man. J Physiol. 1992 Oct;456:373–391. doi: 10.1113/jphysiol.1992.sp019341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J., Petersen N., Deuschl G., Ballegaard M. Task-related changes in the effect of magnetic brain stimulation on spinal neurones in man. J Physiol. 1993 Nov;471:223–243. doi: 10.1113/jphysiol.1993.sp019899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piercey M. F., Goldfarb J. Discharge patterns of Renshaw cells evoked by volleys in ipsilateral cutaneous and high-threshold muscle afferents and their relationship to reflexes recorded in ventral roots. J Neurophysiol. 1974 Mar;37(2):294–302. doi: 10.1152/jn.1974.37.2.294. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Katz R., Morin C. Evidence of Ib inhibition in human subjects. Brain Res. 1979 Apr 20;166(1):176–179. doi: 10.1016/0006-8993(79)90660-7. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Morin C., Bergego C., Tankov N. Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Exp Brain Res. 1981;42(3-4):337–350. doi: 10.1007/BF00237499. [DOI] [PubMed] [Google Scholar]
- Tyler A. E., Hutton R. S. Was Sherrington right about co-contractions? Brain Res. 1986 Apr 2;370(1):171–175. doi: 10.1016/0006-8993(86)91119-4. [DOI] [PubMed] [Google Scholar]


