Abstract
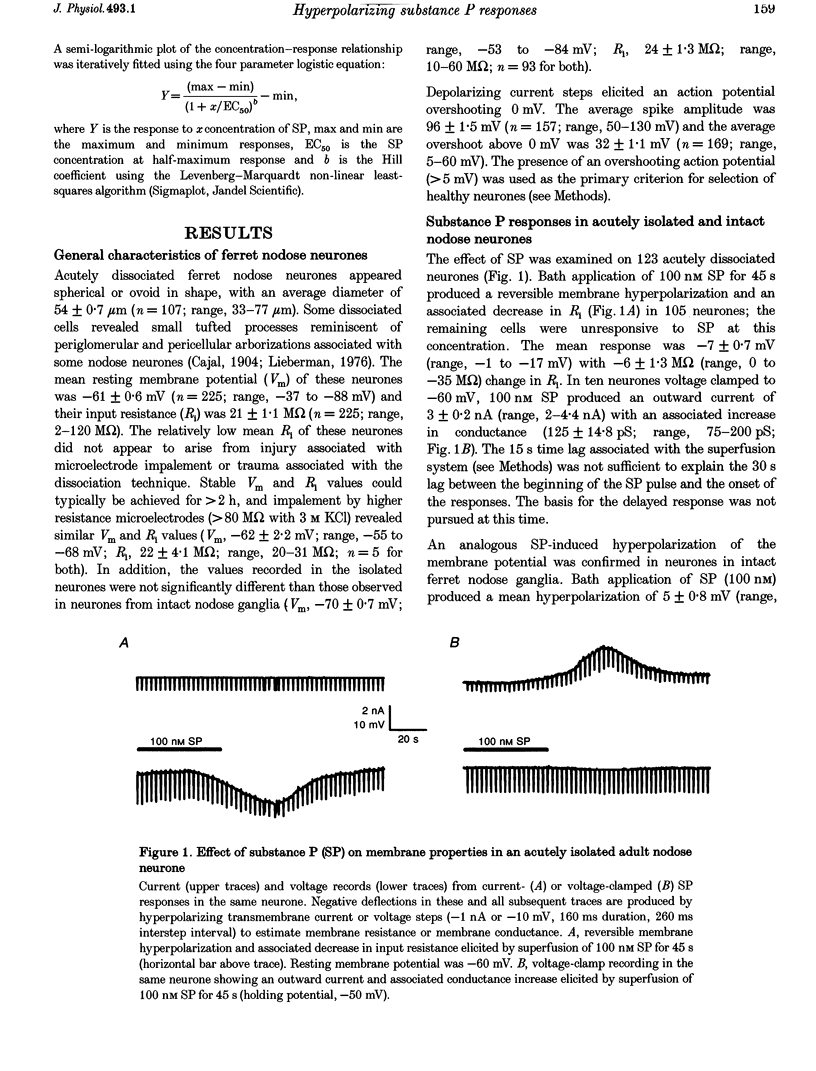
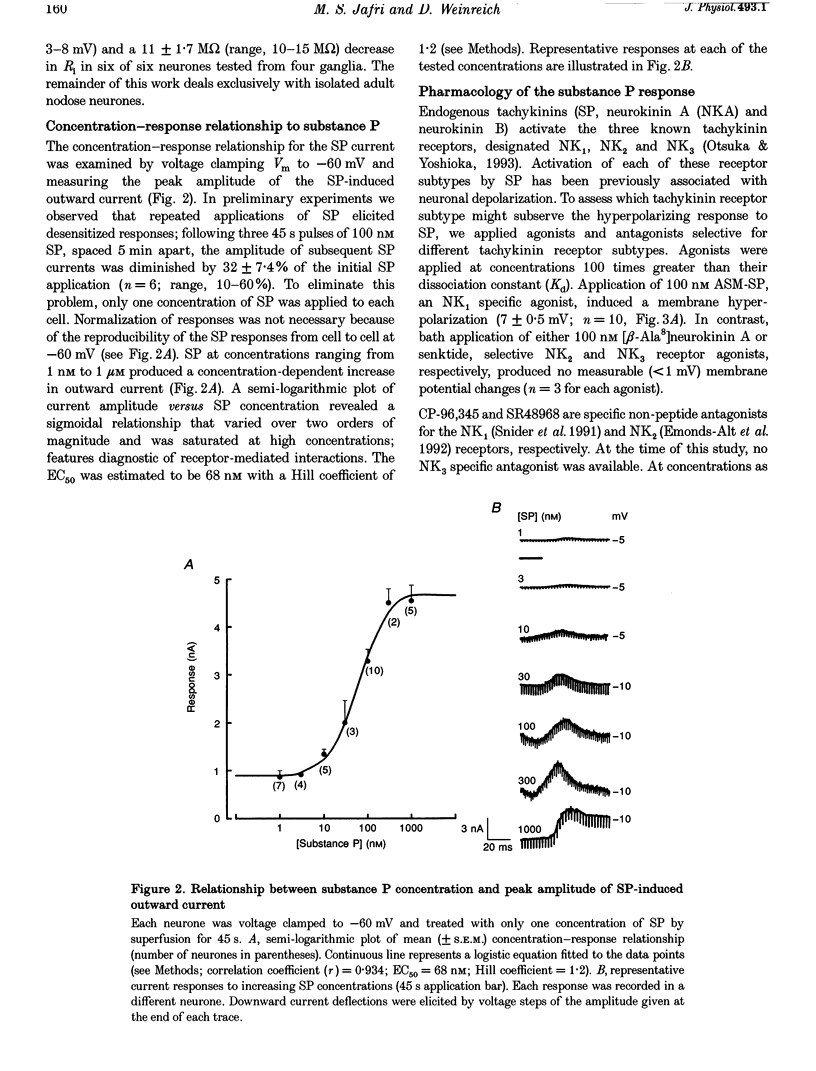
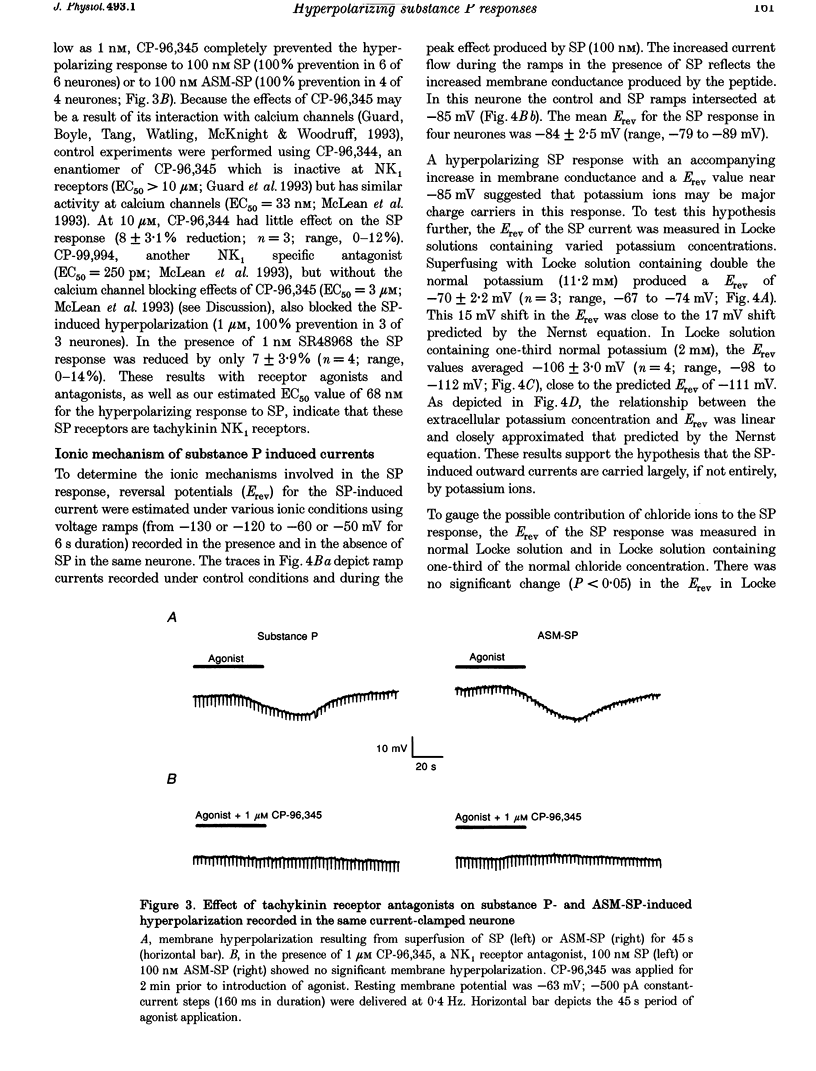
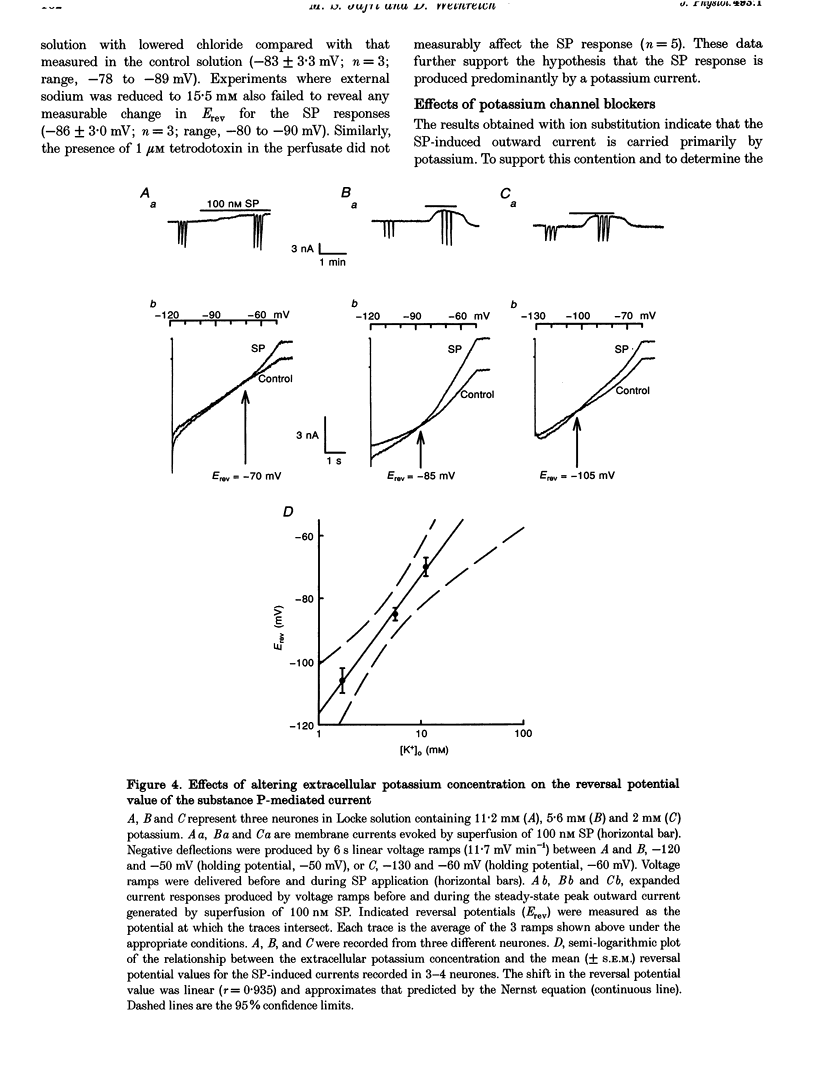
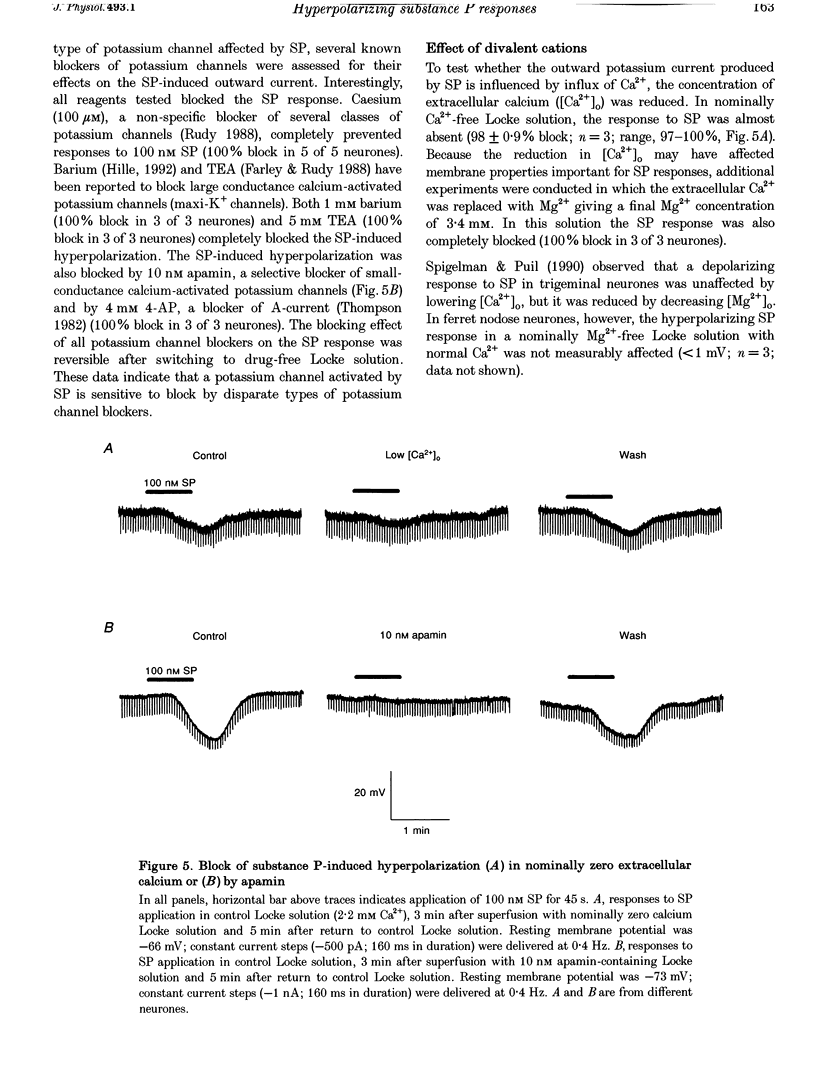
1. Intracellular recordings were made in intact and in acutely dissociated vagal afferent neurones (nodose ganglion cells) of the ferret to investigate the effects of substance P(SP). 2. In current-clamp recordings, SP (100 nM) applied by superfusion hyperpolarized the membrane potential (7 +/- 0.7 mV; mean +/- S.E.M.; n = 105) and decreased the input resistance in 80% of the neurones. With voltage-clamp recording, SP produced an outward current of 3 +/- 0.2 nA (n = 10). 3. The SP current was concentration dependent with an estimated EC50 of 68 nM. The SP-induced hyperpolarization or current was mimicked by the tachykinin receptor NK1 agonist Ac-[Arg6, Sar9, Met(O2)11]SP(6-11) (ASM-SP; 100 nM; n = 10) and blocked by the NK1 antagonist CP-96,345 (10 nM; n = 6), but not by the NK2 antagonist SR48968 (100 nM; n = 4). No measurable change in membrane potential or input resistance was observed with application of either [beta-Ala8]neurokinin A or senktide, selective NK2 and NK3 receptor agonists, respectively (100 nM; n = 3 for each agonist). 4. The reversal potential (Erev) for the SP outward current was -85 +/- 2.5 mV (n = 4). The Erev for the SP response shifted in a Nernstian manner with changes in extracellular potassium concentration. Alterations in extracellular sodium or chloride concentrations had no significant effect on the Erev for the SP response (n = 3 for each ion). 5. Nominally Ca(2+)-free external solution abolished the SP response. Removal of magnesium from the extracellular solution had no effect on the response. 6. Caesium (100 microM), barium (1 mM), tetraethylammonium (TEA; 5 mM), apamin (10 nM) and 4-aminopyridine (4-AP; 4 mM) each completely prevented the SP response (n > or = 3 for each). 7. These results indicate that SP, via an NK1 receptor, can induce a Ca(2+)-dependent outward potassium current which hyperpolarizes the resting membrane potential of vagal afferent somata.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blatz A. L., Magleby K. L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986 Oct 23;323(6090):718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- Christian E. P., Togo J. A., Naper K. E., Koschorke G., Taylor G. A., Weinreich D. A retrograde labeling technique for the functional study of airway-specific visceral afferent neurons. J Neurosci Methods. 1993 Apr;47(1-2):147–160. doi: 10.1016/0165-0270(93)90031-l. [DOI] [PubMed] [Google Scholar]
- Dalsgaard C. J., Vincent S. R., Hökfelt T., Lundberg J. M., Dahlström A., Schultzberg M., Dockray G. J., Cuello A. C. Coexistence of cholecystokinin- and substance P-like peptides in neurons of the dorsal root ganglia of the rat. Neurosci Lett. 1982 Nov 30;33(2):159–163. doi: 10.1016/0304-3940(82)90244-0. [DOI] [PubMed] [Google Scholar]
- De Koninck Y., Henry J. L. Substance P-mediated slow excitatory postsynaptic potential elicited in dorsal horn neurons in vivo by noxious stimulation. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11344–11348. doi: 10.1073/pnas.88.24.11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray A., Pinnock R. D. Effects of substance P on adult rat sensory ganglion neurones in vitro. Neurosci Lett. 1982 Nov 16;33(1):61–66. doi: 10.1016/0304-3940(82)90130-6. [DOI] [PubMed] [Google Scholar]
- Ducreux C., Puizillout J. J. A-current modifies the spike of C-type neurones in the rabbit nodose ganglion. J Physiol. 1995 Jul 15;486(Pt 2):439–451. doi: 10.1113/jphysiol.1995.sp020824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. L., Undem B. J. Antigen-induced enhancement of noncholinergic contractile responses to vagus nerve and electrical field stimulation in guinea pig isolated trachea. J Pharmacol Exp Ther. 1992 Aug;262(2):646–653. [PubMed] [Google Scholar]
- Emonds-Alt X., Vilain P., Goulaouic P., Proietto V., Van Broeck D., Advenier C., Naline E., Neliat G., Le Fur G., Brelière J. C. A potent and selective non-peptide antagonist of the neurokinin A (NK2) receptor. Life Sci. 1992;50(15):PL101–PL106. doi: 10.1016/0024-3205(92)90352-p. [DOI] [PubMed] [Google Scholar]
- Farley J., Rudy B. Multiple types of voltage-dependent Ca2+-activated K+ channels of large conductance in rat brain synaptosomal membranes. Biophys J. 1988 Jun;53(6):919–934. doi: 10.1016/S0006-3495(88)83173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry S. E. Tachykinin receptors mediating airway macromolecular secretion. Life Sci. 1991;48(17):1609–1618. doi: 10.1016/0024-3205(91)90120-z. [DOI] [PubMed] [Google Scholar]
- Guard S., Boyle S. J., Tang K. W., Watling K. J., McKnight A. T., Woodruff G. N. The interaction of the NK1 receptor antagonist CP-96,345 with L-type calcium channels and its functional consequences. Br J Pharmacol. 1993 Sep;110(1):385–391. doi: 10.1111/j.1476-5381.1993.tb13821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helke C. J., Hill K. M. Immunohistochemical study of neuropeptides in vagal and glossopharyngeal afferent neurons in the rat. Neuroscience. 1988 Aug;26(2):539–551. doi: 10.1016/0306-4522(88)90166-2. [DOI] [PubMed] [Google Scholar]
- Henry J. L., Sessle B. J., Lucier G. E., Hu J. W. Effects of substance P on nociceptive and non-nociceptive trigeminal brain stem neurons. Pain. 1980 Feb;8(1):33–45. doi: 10.1016/0304-3959(80)90088-3. [DOI] [PubMed] [Google Scholar]
- Ishimatsu M. Substance P produces an inward current by suppressing voltage-dependent and -independent K+ currents in bullfrog primary afferent neurons. Neurosci Res. 1994 Feb;19(1):9–20. doi: 10.1016/0168-0102(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Katz D. M., Karten H. J. Substance P in the vagal sensory ganglia: localization in cell bodies and pericellular arborizations. J Comp Neurol. 1980 Sep 15;193(2):549–564. doi: 10.1002/cne.901930216. [DOI] [PubMed] [Google Scholar]
- Kummer W., Fischer A., Kurkowski R., Heym C. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience. 1992 Aug;49(3):715–737. doi: 10.1016/0306-4522(92)90239-x. [DOI] [PubMed] [Google Scholar]
- Leal-Cardoso H., Koschorke G. M., Taylor G., Weinreich D. Electrophysiological properties and chemosensitivity of acutely isolated nodose ganglion neurons of the rabbit. J Auton Nerv Syst. 1993 Oct;45(1):29–39. doi: 10.1016/0165-1838(93)90359-3. [DOI] [PubMed] [Google Scholar]
- Lidberg P., Edin R., Lundberg J. M., Dahlström A., Rosell S., Folkers K., Ahlman H. The involvement of substance P in the vagal control of the feline pylorus. Acta Physiol Scand. 1982 Feb;114(2):307–309. doi: 10.1111/j.1748-1716.1982.tb06987.x. [DOI] [PubMed] [Google Scholar]
- Lindh B., Dalsgaard C. J., Elfvin L. G., Hökfelt T., Cuello A. C. Evidence of substance P immunoreactive neurons in dorsal root ganglia and vagal ganglia projecting to the guinea pig pylorus. Brain Res. 1983 Jun 20;269(2):365–369. doi: 10.1016/0006-8993(83)90148-8. [DOI] [PubMed] [Google Scholar]
- Ling E. A., Yick T. Y., Ng G. L., Wong W. C. Immunocytochemical localisation of substance P in vagal ganglion cells and pericellular arborisations in the monkey. J Anat. 1992 Aug;181(Pt 1):61–71. [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Hökfelt T., Kewenter J., Pettersson G., Ahlman H., Edin R., Dahlström A., Nilsson G., Terenius L., Uvnäs-Wallensten K. Substance P-, VIP-, and enkephalin-like immunoreactivity in the human vagus nerve. Gastroenterology. 1979 Sep;77(3):468–471. [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- McCarthy P. W., Lawson S. N. Cell type and conduction velocity of rat primary sensory neurons with substance P-like immunoreactivity. Neuroscience. 1989;28(3):745–753. doi: 10.1016/0306-4522(89)90019-5. [DOI] [PubMed] [Google Scholar]
- McLean S., Ganong A., Seymour P. A., Snider R. M., Desai M. C., Rosen T., Bryce D. K., Longo K. P., Reynolds L. S., Robinson G. Pharmacology of CP-99,994; a nonpeptide antagonist of the tachykinin neurokinin-1 receptor. J Pharmacol Exp Ther. 1993 Oct;267(1):472–479. [PubMed] [Google Scholar]
- Murase K., Randić M. Actions of substance P on rat spinal dorsal horn neurones. J Physiol. 1984 Jan;346:203–217. doi: 10.1113/jphysiol.1984.sp015017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M., Yoshioka K. Neurotransmitter functions of mammalian tachykinins. Physiol Rev. 1993 Apr;73(2):229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- Renzetti L. M., Shenvi A., Buckner C. K. Nonadrenergic, noncholinergic contractile responses of the guinea pig hilar bronchus involve the preferential activation of tachykinin neurokinin2 receptors. J Pharmacol Exp Ther. 1992 Sep;262(3):957–963. [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Saria A., Martling C. R., Yan Z., Theodorsson-Norheim E., Gamse R., Lundberg J. M. Release of multiple tachykinins from capsaicin-sensitive sensory nerves in the lung by bradykinin, histamine, dimethylphenyl piperazinium, and vagal nerve stimulation. Am Rev Respir Dis. 1988 Jun;137(6):1330–1335. doi: 10.1164/ajrccm/137.6.1330. [DOI] [PubMed] [Google Scholar]
- Sharma N. R., Davis M. J. Mechanism of substance P-induced hyperpolarization of porcine coronary artery endothelial cells. Am J Physiol. 1994 Jan;266(1 Pt 2):H156–H164. doi: 10.1152/ajpheart.1994.266.1.H156. [DOI] [PubMed] [Google Scholar]
- Snider R. M., Constantine J. W., Lowe J. A., 3rd, Longo K. P., Lebel W. S., Woody H. A., Drozda S. E., Desai M. C., Vinick F. J., Spencer R. W. A potent nonpeptide antagonist of the substance P (NK1) receptor. Science. 1991 Jan 25;251(4992):435–437. doi: 10.1126/science.1703323. [DOI] [PubMed] [Google Scholar]
- Spigelman I., Puil E. Ionic mechanism of substance P actions on neurons in trigeminal root ganglia. J Neurophysiol. 1990 Jul;64(1):273–281. doi: 10.1152/jn.1990.64.1.273. [DOI] [PubMed] [Google Scholar]
- Starke K., Göthert M., Kilbinger H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev. 1989 Jul;69(3):864–989. doi: 10.1152/physrev.1989.69.3.864. [DOI] [PubMed] [Google Scholar]
- Thompson S. Aminopyridine block of transient potassium current. J Gen Physiol. 1982 Jul;80(1):1–18. doi: 10.1085/jgp.80.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undem B. J., Weinreich D. Electrophysiological properties and chemosensitivity of guinea pig nodose ganglion neurons in vitro. J Auton Nerv Syst. 1993 Jul;44(1):17–33. doi: 10.1016/0165-1838(93)90375-5. [DOI] [PubMed] [Google Scholar]
- Webber S. E. Receptors mediating the effects of substance P and neurokinin A on mucus secretion and smooth muscle tone of the ferret trachea: potentiation by an enkephalinase inhibitor. Br J Pharmacol. 1989 Dec;98(4):1197–1206. doi: 10.1111/j.1476-5381.1989.tb12665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


