Abstract
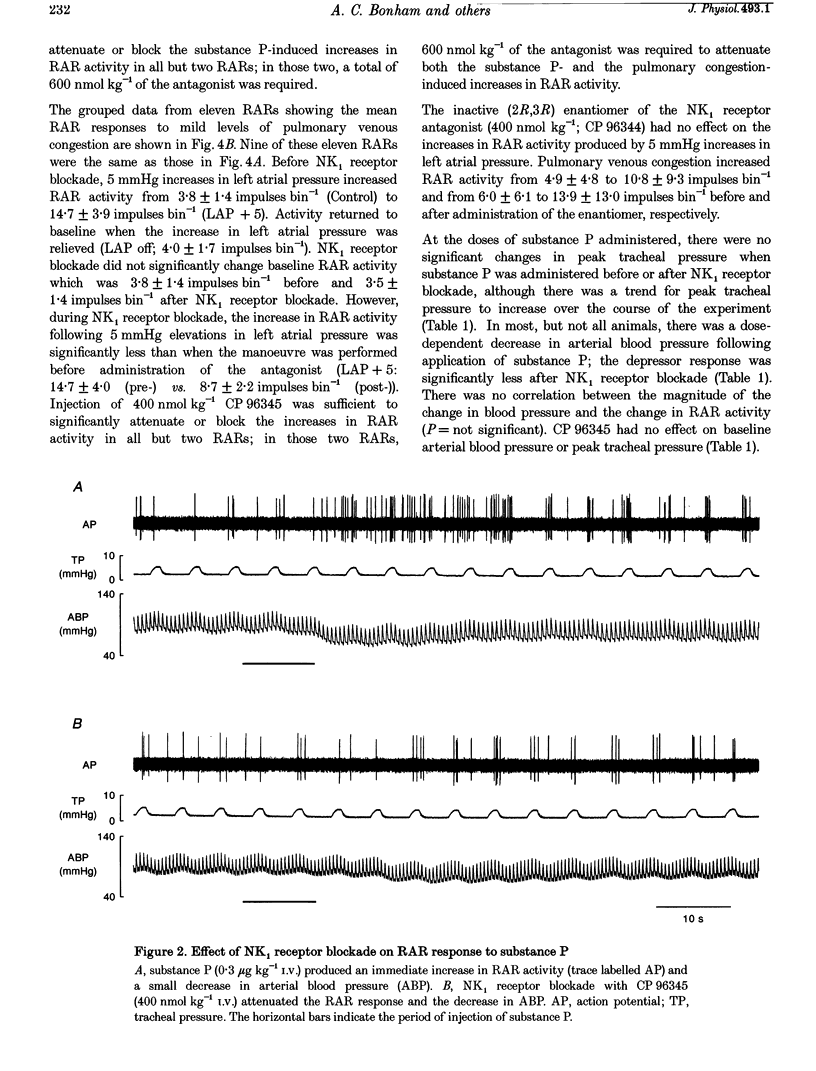
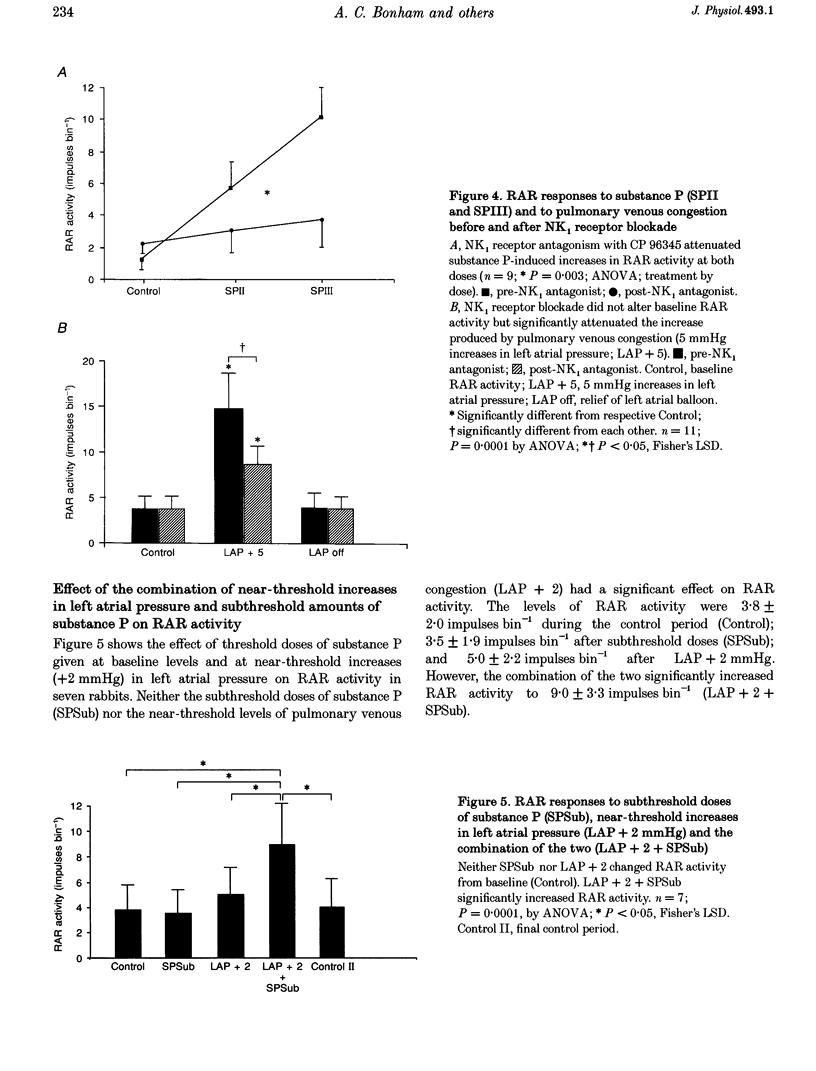
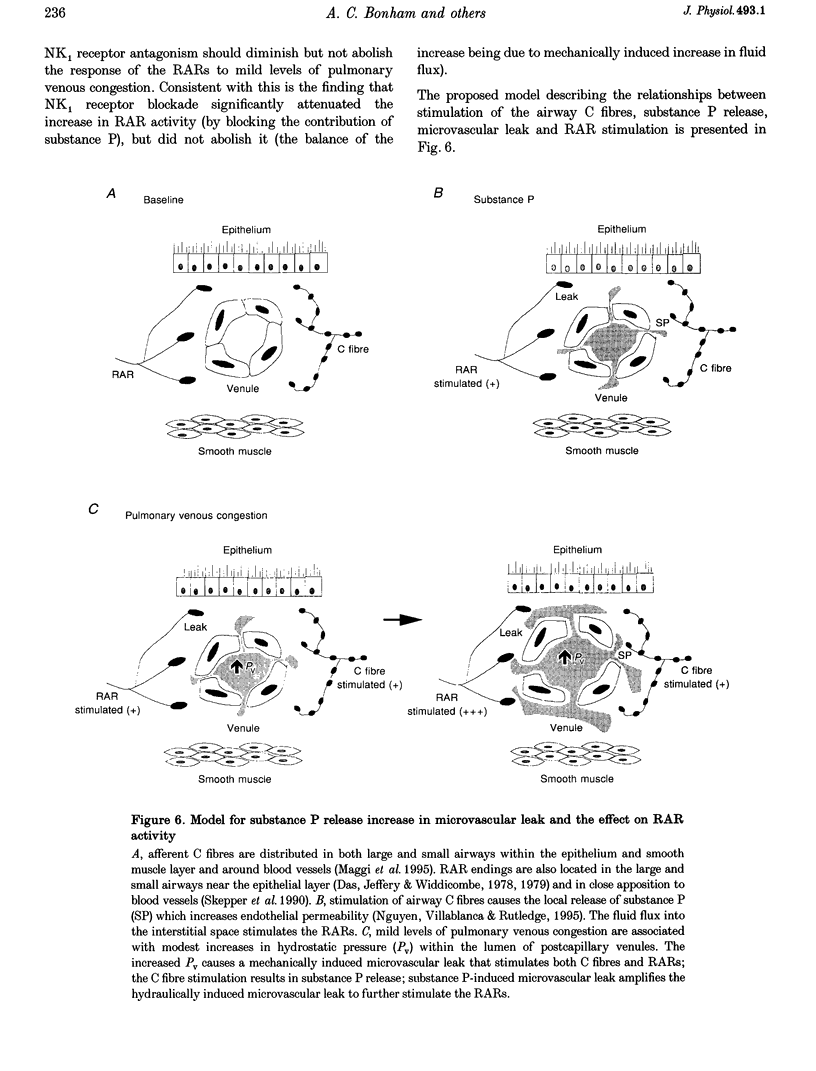
1. This study tested the hypothesis that substance P stimulates rapidly adapting receptors (RARs), contributes to the increase in RAR activity produced by mild pulmonary congestion, and evokes an augmented response from RARs when combined with near-threshold levels of pulmonary congestion. 2. RAR activity, peak tracheal pressure, arterial blood pressure and left atrial pressure were measured in paralysed, anaesthetized and ventilated rabbits. Substance P was given i.v. in one-half log incremental doses to a maximum of 3 micrograms kg-1. Mild pulmonary congestion was produced by inflating a balloon in the left atrium to increase left atrial pressure by 5 mmHg. Near-threshold levels of pulmonary congestion were produced by increasing left atrial pressure by 2 mmHg. 3. Substance P produced dose-dependent increases in RAR activity. The highest dose given increased the activity from 1.3 +/- 0.5 to 11.0 +/- 3.1 impulses bin-1. Increases in left atrial pressure of 5 mmHg increased RAR activity from 3.8 +/- 1.4 to 14.7 +/- 3.9 impulses bin-1. Blockade of NK1 receptors with CP 96345 significantly attenuated RAR responses to substance P and to mild pulmonary congestion. 4. Doses of substance P, which alone had no effect, stimulated the RARs when delivered during near-threshold levels of pulmonary congestion. 5. The findings suggest that substance P augments the stimulatory effect of mild pulmonary congestion on RAR activity, most probably by enhancing hydraulically induced microvascular leak.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonham A. C., Ravi K., Wilson C. G., Zhang Z., Kappagoda C. T. Pulmonary venous congestion augments respiratory motoneuronal responses to cigarette smoke in rabbit. J Appl Physiol (1985) 1995 Mar;78(3):1145–1157. doi: 10.1152/jappl.1995.78.3.1145. [DOI] [PubMed] [Google Scholar]
- Coleridge H. M., Coleridge J. C., Ginzel K. H., Baker D. G., Banzett R. B., Morrison M. A. Stimulation of 'irritant' receptors and afferent C-fibres in the lungs by prostaglandins. Nature. 1976 Dec 2;264(5585):451–453. doi: 10.1038/264451a0. [DOI] [PubMed] [Google Scholar]
- Coleridge H. M., Coleridge J. C. Pulmonary reflexes: neural mechanisms of pulmonary defense. Annu Rev Physiol. 1994;56:69–91. doi: 10.1146/annurev.ph.56.030194.000441. [DOI] [PubMed] [Google Scholar]
- Coleridge J. C., Coleridge H. M., Schelegle E. S., Green J. F. Acute inhalation of ozone stimulates bronchial C-fibers and rapidly adapting receptors in dogs. J Appl Physiol (1985) 1993 May;74(5):2345–2352. doi: 10.1152/jappl.1993.74.5.2345. [DOI] [PubMed] [Google Scholar]
- Das R. M., Jeffery P. K., Widdicombe J. G. Experimental degeneration of intra-epithelia nerve fibres in cat airways. J Anat. 1979 Mar;128(Pt 2):259–267. [PMC free article] [PubMed] [Google Scholar]
- Das R. M., Jeffrey P. K., Widdicombe J. G. The epithelial innervation of the lower respiratory tract of the cat. J Anat. 1978 May;126(Pt 1):123–131. [PMC free article] [PubMed] [Google Scholar]
- Delay-Goyet P., Lundberg J. M. Cigarette smoke-induced airway oedema is blocked by the NK1 antagonist, CP-96,345. Eur J Pharmacol. 1991 Oct 2;203(1):157–158. doi: 10.1016/0014-2999(91)90808-4. [DOI] [PubMed] [Google Scholar]
- Eglezos A., Giuliani S., Viti G., Maggi C. A. Direct evidence that capsaicin-induced plasma protein extravasation is mediated through tachykinin NK1 receptors. Eur J Pharmacol. 1991 Dec 17;209(3):277–279. doi: 10.1016/0014-2999(91)90183-q. [DOI] [PubMed] [Google Scholar]
- Hargreaves M., Ravi K., Kappagoda C. T. Effect of bradykinin on respiratory rate in anaesthetized rabbits; role of rapidly adapting receptors. J Physiol. 1993 Aug;468:501–513. doi: 10.1113/jphysiol.1993.sp019784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves M., Ravi K., Kappagoda C. T. Responses of slowly and rapidly adapting receptors in the airways of rabbits to changes in the Starling forces. J Physiol. 1991 Jan;432:81–97. doi: 10.1113/jphysiol.1991.sp018377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves M., Ravi K., Senaratne M. P., Kappagoda C. T. Responses of airway rapidly adapting receptors to bradykinin before and after administration of enalapril in rabbits. Clin Sci (Lond) 1992 Oct;83(4):399–407. doi: 10.1042/cs0830399. [DOI] [PubMed] [Google Scholar]
- Kappagoda C. T., Man G. C., Teo K. K. Behaviour of canine pulmonary vagal afferent receptors during sustained acute pulmonary venous pressure elevation. J Physiol. 1987 Dec;394:249–265. doi: 10.1113/jphysiol.1987.sp016869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappagoda C. T., Ravi K. Plasmapheresis affects responses of slowly and rapidly adapting airway receptors to pulmonary venous congestion in dogs. J Physiol. 1989 Sep;416:79–91. doi: 10.1113/jphysiol.1989.sp017750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J. A., Sant'Ambrogio G., Widdicombe J. Afferent neural pathways in cough and reflex bronchoconstriction. J Appl Physiol (1985) 1988 Sep;65(3):1007–1023. doi: 10.1152/jappl.1988.65.3.1007. [DOI] [PubMed] [Google Scholar]
- Lei Y. H., Barnes P. J., Rogers D. F. Inhibition of neurogenic plasma exudation in guinea-pig airways by CP-96,345, a new non-peptide NK1 receptor antagonist. Br J Pharmacol. 1992 Feb;105(2):261–262. doi: 10.1111/j.1476-5381.1992.tb14243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y. H., Barnes P. J., Rogers D. F. Mechanisms and modulation of airway plasma exudation after direct inhalation of cigarette smoke. Am J Respir Crit Care Med. 1995 Jun;151(6):1752–1762. doi: 10.1164/ajrccm.151.6.7767517. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Donnerer J., Tsuchiya M., Nagahisa A. The non-peptide tachykinin antagonist, CP-96,345, is a potent inhibitor of neurogenic inflammation. Br J Pharmacol. 1992 Mar;105(3):527–530. doi: 10.1111/j.1476-5381.1992.tb09013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Martling C. R., Saria A., Folkers K., Rosell S. Cigarette smoke-induced airway oedema due to activation of capsaicin-sensitive vagal afferents and substance P release. Neuroscience. 1983 Dec;10(4):1361–1368. doi: 10.1016/0306-4522(83)90117-3. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Giachetti A., Dey R. D., Said S. I. Neuropeptides as regulators of airway function: vasoactive intestinal peptide and the tachykinins. Physiol Rev. 1995 Apr;75(2):277–322. doi: 10.1152/physrev.1995.75.2.277. [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Yamasaki M., Kanno T., Nagayama T., Tanno M., Shimizu T. Substance P antagonist does not block the stimulation of rapidly adapting pulmonary stretch receptors by ammonia. Lung. 1994;172(1):31–45. doi: 10.1007/BF00186167. [DOI] [PubMed] [Google Scholar]
- Ravi K., Bonham A. C., Kappagoda C. T. Effect of pulmonary lymphatic obstruction on respiratory rate and airway rapidly adapting receptor activity in rabbits. J Physiol. 1994 Oct 1;480(Pt 1):163–170. doi: 10.1113/jphysiol.1994.sp020350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi K., Kappagoda C. T., Bonham A. C. Pulmonary congestion enhances responses of lung rapidly adapting receptors to cigarette smoke in rabbit. J Appl Physiol (1985) 1994 Dec;77(6):2633–2640. doi: 10.1152/jappl.1994.77.6.2633. [DOI] [PubMed] [Google Scholar]
- Ravi K., Kappagoda C. T. Responses of pulmonary C-fibre and rapidly adapting receptor afferents to pulmonary congestion and edema in dogs. Can J Physiol Pharmacol. 1992 Jan;70(1):68–76. doi: 10.1139/y92-010. [DOI] [PubMed] [Google Scholar]
- Snider R. M., Constantine J. W., Lowe J. A., 3rd, Longo K. P., Lebel W. S., Woody H. A., Drozda S. E., Desai M. C., Vinick F. J., Spencer R. W. A potent nonpeptide antagonist of the substance P (NK1) receptor. Science. 1991 Jan 25;251(4992):435–437. doi: 10.1126/science.1703323. [DOI] [PubMed] [Google Scholar]
- Turner C. R., Stow R. B., Hubbs S. J., Gomes B. C., Williams J. C. Acrolein increases airway sensitivity to substance P and decreases NEP activity in guinea pigs. J Appl Physiol (1985) 1993 Apr;74(4):1830–1839. doi: 10.1152/jappl.1993.74.4.1830. [DOI] [PubMed] [Google Scholar]
- WIDDICOMBE J. G. Receptors in the trachea and bronchi of the cat. J Physiol. 1954 Jan;123(1):71–104. doi: 10.1113/jphysiol.1954.sp005034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Y., Håkanson R. (+/-)-CP-96,345, a selective tachykinin NK1 receptor antagonist, has non-specific actions on neurotransmission. Br J Pharmacol. 1992 Nov;107(3):762–765. doi: 10.1111/j.1476-5381.1992.tb14520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe J. G. Neurophysiology of the cough reflex. Eur Respir J. 1995 Jul;8(7):1193–1202. doi: 10.1183/09031936.95.08071193. [DOI] [PubMed] [Google Scholar]
- Yiamouyiannis C. A., Stengel P. W., Cockerham S. L., Silbaugh S. A. Pulmonary actions of the neurokinin1-specific agonist [Sar9,Met(O2)11]-substance P. Neuropeptides. 1995 Jan;28(1):35–42. doi: 10.1016/0143-4179(95)90072-1. [DOI] [PubMed] [Google Scholar]



