Abstract
Benzo[a]pyrene (BaP), a polycyclic aromatic hydrocarbon (PAH), is implicated in many developmental and behavioral adverse outcomes in offspring of exposed parents. The objective of this study was to investigate sex-dependent multigenerational effects of preconceptional effects of BaP exposure. Adult wild-type (5D) zebrafish were fed 708 μg BaP/g diet (measured) at a rate of 1% body weight twice/day (14 μg BaP/g fish/day) for 21 days. Fish were spawned using a crossover design, and parental (F0) behavior and reproductive indexes were measured. In offspring, behavioral effects were measured at 96 h post fertilization (hpf) in F1 & F2 larvae, and again when F1s were adults. Compared to controls, there was no significant effect on F0 adult behavior immediately following exposure, but locomotor activity was significantly increased in F1 adults of both sexes. Larval behavior (96 hpf, photomotor response assay) was significantly altered in both the F1 and F2 generations. To assess molecular changes associated with BaP exposure, we conducted transcriptome and DNA methylation profiling in F0 gametes (sperm and eggs) and F1 embryos (10 hpf) from all four crosses. Embryos resulting from the BaP male and control female cross had the most differentially expressed genes (DEGs) and differentially methylated regions (DMRs). Some DMRs were associated with genes encoding chromatin modifying enzymes suggesting regulation of chromatin conformation by DNA methylation. Overall, these results suggest that parental dietary BaP exposure significantly contributes to the multigenerational adverse outcomes.
Keywords: Benzo[a]Pyrene, Multigenerational, DNA Methylation, RNAseq, Behavior, PAH
1. Introduction
Susceptibility to disease can be set preconceptionally as a result of exposure to contaminants or nutritional deficits (Gómez-Roig et al., 2021; Grandjean et al., 2015; Heindel and Vandenberg, 2015). Benzo[a] pyrene (BaP), a carcinogenic polycyclic aromatic hydrocarbon (PAH), is a ubiquitous environmental contaminant derived from the incomplete combustion of carbon (Bukowska et al., 2022). Exposures to BaP occur from ingesting contaminated food, water, or inhalation of tobacco smoke or automotive or oven exhausts. In humans, prenatal exposure to PAHs, including BaP, are implicated in neural tube defects (Ren et al., 2011), growth deficits (Langlois et al., 2014; Siddiqui et al., 2008) and lower neurobehavioral development and higher odds of developmental delay in children as they mature (Cao et al., 2020; Perera et al., 2006, 2009, 2012).
The sex-dependence of parents’ preconceptional diets and contaminant exposures on next generation adverse outcomes is recognized in human and model organisms (Perez and Lehner, 2019; Schmidt, 2018). For example, there is an association between poor paternal nutrition and higher incidence of metabolic diseases such as type 2 diabetes in offspring (Braun et al., 2017; Ferguson-Smith and Patti, 2011). Some epidemiological studies have related paternal, but not maternal, exposure to PAHs/tobacco smoke with a higher risk of childhood cancers (Boffetta et al., 2000; Cordier et al., 2004; Ji et al., 1997). Recently, the POHaD paradigm (Paternal Origins of Health and Disease) was coined to highlight the need to consider the impacts of paternal exposure on offspring (Soubry, 2018). Thus, there is growing evidence that sex-specific environmental exposures during reproductive years can alter subsequent generations and epigenetic mechanisms are implicated in this process (Ambeskovic et al., 2020; Ho et al., 2017).
We have previously demonstrated in zebrafish that BaP dietary exposure caused developmental defects in F1 and F2 offspring (Corrales et al., 2014b), and that altered DNA methylation occurred in embryos exposed directly to BaP or via their parents (Corrales et al., 2014a; Fang et al., 2013a; Fang et al., 2013b). Prior studies in medaka and rodent models found paternal BaP exposure caused more severe adverse outcomes in offspring including epigenetic modifications and reproductive dysfunction (Jorge et al., 2021; Mohamed et al., 2010; Yin et al., 2020). As mentioned above, multigenerational neurobehavioral deficits following prenatal exposure to BaP are recognized in both human offspring and model organisms (Jedrychowski et al., 2015; Knecht et al., 2017; Zhang et al., 2022) however, the molecular mechanisms associated adverse outcomes following sex-dependent preconceptional exposure to BaP have not been fully elucidated. In this study, adult zebrafish were exposed to BaP via diet for 21-days and a crossover study design was used to assess sex-dependent effects on growth and behavior on F0 and subsequent generations (F1 and F2). In addition, we quantified transcriptional and DNA methylation changes in the exposed gametes (F0) and F1 embryos.
2. Materials and methods
2.1. Fish care and handling
Wild-type 5D zebrafish (kindly provided by Dr. Robyn Tanguay at Oregon State University) were used in this study and raised under the approved Institutional Animal Care and Use Committee (IACUC) protocol (18–022). Fish were maintained in Aquatic Habitats ZF0601 Zebrafish Stand-Alone System (Aquatic Habitats, Apopka, FL) at a constant pH (7.0–7.6, 760 μS), temperature (25–28 °C), and 14:10 light-dark cycle. Fish were fed twice daily with Gemma 300 micro food (Skretting USA, UT). Sexually mature fish without any deformities or signs of disease were selected as breeders. Their eggs were collected, and larvae were raised to 4 months post fertilization (mpf) to obtain the F0 generation for the dietary exposure described below. All the experiments and exposure protocols were in accordance with IACUC approved guidelines and recommendations.
2.2. BaP dietary preparation and GC–MS
BaP-treated diet was prepared following a previously established protocol (Corrales et al., 2014b). To prepare the diet, 24 g of flake food were spiked with 18 mL of acetone containing BaP (0 or 1.667 μg/μL), equivalent to nominal BaP concentrations of 0 or 1250 μg/g food, respectively. The spiked flakes were immediately rotovapped to dryness and stored in amber vials at room temperature. Acetone and BaP were purchased from Fisher Scientific (Fair Lawn, NJ) and Supelco Analytical (Belfonte, PA), respectively.
BaP concentrations in the food were quantified using GC–MS following extraction with 2–3 mL methylene chloride. Approximately 10 mg of flakes were extracted at the beginning of the exposure and then weekly. A surrogate standard, BaP-d12, was added to each sample to obtain a final concentration of 0.2 μg/mL (surrogate percent recovery 140–205%). Samples were vortexed for 30 s and centrifuged for 7 min at 668 xg. Samples were then blown to dryness with N2 and brought back up with a known volume of hexane. An internal standard, fluorene-d10, was added to obtain a final concentration of 0.2 μg/mL to determine extraction efficiency. BaP was quantitated with GC–MS using selected ion mode (m/z 252). BaP was not identified in the acetone-treated control samples. Average measured BaP concentration of the treated flakes was 708 ± 26 μg BaP/g flake equivalent to 14 μg BaP/g fish/day.
2.3. BaP dietary exposure
Sexually mature (4 mpf) zebrafish were fed either acetone alone or BaP-treated TetraMin® Tropical Flakes (nominally 1250 μg BaP/g food equivalent to 25 μg BaP/g fish) (Exposure Timeline, Fig. 1A). Zebrafish (2 males and 2 females) in five replicate tanks per treatment group (N = 5 replicate tanks for a total 20 fish/group) were allowed to acclimate for a week at 25.5–28 °C and were fed twice daily with untreated flakes and Gemma 300 micro food. During the exposure, fish were fed 1% body weight twice daily of the corresponding dose of BaP-treated flake food and once daily Gemma 300 micro food for 21 days. To determine if the dietary exposure to BaP affected somatic growth, parental fish had their weight and length recorded at beginning and end of exposure and gonad and liver weight measured at the end. Weight and length were used to calculate Fulton’s condition factor, K (K = W/TL3 × 105; W = weight in g, TL = total body length in mm) which can reflect the well-being of fish (Clark et al., 2018).
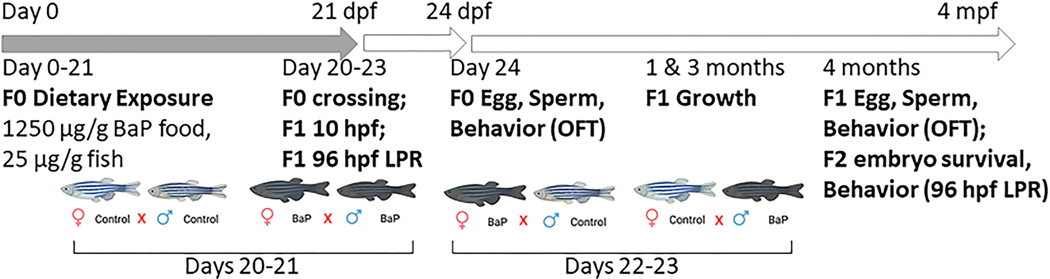
Fig. 1.
Exposure timeline and methodology. Adult 5D zebrafish were exposed to acetone- or BaP-treated flakes for 21 days. On days 20–23 a cross-over mating design was used to generate F1 embryos of the four crosses (Cm x Cf, Cm x Bf, Bm x Cf, and Bm x Bf). On day 24, F0 open field test (OFT) behavior was assessed prior to collection of eggs or sperm for RNASeq and RRBS. F1 embryos were raised to 10 hpf and isolated for RNASeq and RRBS. At 96 hpf, behavior of F1 larvae was assessed in a larval photomotor response assay (LPR). F1 growth was measured at 1, 3, and 4 mpf. At 4 mpf, adult F1 OFT was done, F1s mated, and F2 embryo survival and behavior (96 hpf; LPR) was measured.
At the end of day 21, a crossover breeding design was implemented to assess sex-specific contributions namely: 1) control males x control females (Cm x Cf), 2) control males x BaP females (Cm x Bf), 3) BaP males x control females (Bm x Cf), and 4) BaP males x BaP females (Bm x Bf). On days 22 and 23, eggs were collected to determine reproductive success. On day 24 (females) and day 25 (males) F0 parental behavior in an open field test (OFT) was assessed. In adult zebrafish, open field tests (OFT) in a novel environment are utilized to determine locomotor activity and thigmotaxis (wall hugging to assess anxiety) (Champagne et al., 2010). After OFT, fish were euthanized, and gonad, liver, and brain samples were collected and biobanked. Sperm or eggs from the BaP-exposed and control fish, along with 10 hpf embryos from each cross (4 crosses) were collected for RNAseq and DNA methylation analysis (graphical abstract and Fig. 1).
2.4. Quantification of BaP exposure induced multigenerational effects
On days 22 and 23, from each parental tank, the total number and viability of eggs were determined and a subset of 40 fertilized eggs (200 per group) were placed in 12-well plates (5 embryos/well containing 5 mL of egg water (sterilized deionized water; 60 ppm Instant Ocean; pH 7.6) with 0.05% methylene blue). Embryo survival and time to hatch was measured at 24, 48, 72, and 96 h post fertilization (hpf). Water changes in the wells were done once daily until 96 hpf.
Larval F1 zebrafish (96 hpf) from all four parental crosses (Cm x Cf, Cm x Bf, Bm x Cf, and Bm x Bf) were assessed for morphological defects and larval behaviors. At 96 hpf, a subset of 50 larvae per treatment (10 larval fish per tank, 5 tanks per treatment) were examined for developmental deformities: body shape, tail, pericardial edema, swim bladder, and yolk sac edema. Ten larvae per tank were anesthetized in buffered MS-222 and immediately placed on a microscope slide with a chamber containing 3% methylcellulose. Digital still micrographs were taken with a MicroFire camera and PictureFrame 2.3 software (Optronics®, Goleta, CA) attached to a Zeiss Stemi 2000-C stereomicroscope. Lateral images were recorded for each fish. All measurements of the larval fish were completed by blind reviewers. Deformities were evaluated and quantified in NIH ImageJ. Body length and optic vesicle areas were measured in pixels by tracing the boundaries of the space. Presence or absence of pericardial edema, yolk sac edema, body axis curvature, and swim bladder inflation were recorded. Pixel area or distance was converted to mm2 or mm based on the calibrated magnification (Corrales et al., 2014b).
At 96 hpf, 10 larvae per tank (total of 50 larvae/treatment) were placed in a 96 well plate with 1 larva/well (~300 μL/well) to assess photomotor response behavior (LPR) using ZebraBox (ViewPoint, Montreal, Canada). Zebrafish LPR activity, indicative of altered locomotor and/or anxiety-like activity in response to alternating light, is known to be altered following BaP exposure (Knecht et al., 2017). Larval behavior (distance moved) was observed during a 30 min duration with light:dark cycling (0–10 min, 100% light; 10–20 min, dark, 0% light; and 20–30 min, 100% light) (Kirla et al., 2016).
At 120 hpf, larvae were transferred to 3 L tanks with 20 larvae per tank; 10 tanks per treatment group. Mortality was checked weekly until 30 days post fertilization (dpf). At 30 dpf, length and weight of 40 fish per treatment were measured, and fish were thinned to 20 fish per tank; 5 tanks per treatment group. At 90 dpf, length and weight of 30 fish per treatment were measured, and fish were thinned to 10 fish (5 male and 5 female) per tank; 4 tanks per treatment group. At 120 dpf, reproductive success of the F1 generation was assessed, F1 endpoints were collected (weight, length, fecundity, OFT), and F2 fish were processed in the same manner as the F1 larvae until 96 hpf (developmental deformities, LPR).
2.5. Quantification of BaP exposure induced effects in F0 generation
At the termination of the F0 exposure and after the F1 offspring were collected as described above, F0 open field behavior (OFT) was assessed in 10 fish/sex/group following similar methods to (Pandelides et al., 2020). Briefly, fish were acclimated to a darkened behavioral testing room (27–28 °C) for 10 min. Individual fish were then transferred to a system water-filled bucket (diameter 23.5 cm, depth 24.8 cm) and their exploration behavior responses were video-captured from overhead for 5 min with Noldus Ethovision 14 software. The testing area was illuminated to 9 lx. At the completion of the trial, the fish were removed from the open field arena and placed in a holding container until euthanasia. OFT was used to determine if exposure affected locomotor activity (mean velocity in mm/s), freezing time (in s, duration of freezing bouts, i.e. time periods with swimming velocities below 1 mm/s), and time spent in the periphery of the tank (as % of the total active swimming period, with swimming speeds >0.1 mm/s) as a measure for thigmotaxis (Sireeni et al., 2020; Steenbergen et al., 2011). The swim arena was divided into two regions: periphery (1 fish length from the edge, outer 50% of the arena) and center (inner 50% of the arena).
2.6. RNA and DNA isolation
Total RNA and genomic DNA were isolated from the same tissue using a Quick-DNA/RNA Miniprep Plus Kit (Zymo, Cat # D7005) following the manufacturer’s instructions. Each treatment condition had 4 biological replicates. F0 sperm and egg samples (control and BaP-exposed) and the 10 hpf F1 embryos from the four crosses were sequenced. For F0 sperm, DNA was isolated, but there was not enough total RNA to sequence the transcriptome.
2.7. RNA sequencing analysis
Total RNA library preparation and sequencing (RNAseq) was conducted by Zymo research laboratories using approximately 250 ng of total RNA. Ribosomal RNA was removed using the method described by (Bogdanova et al., 2011). Libraries were prepared using the Zymo-Seq RiboFree Total RNA Library Prep Kit (Cat # R3000) according to the manufacturer’s instructions. Libraries were sequenced on an Illumina HiSeq to a sequencing depth of >50 million read pairs (150 bp paired-end sequencing) per sample.
Raw data files were assessed for quality using FastQC (Andrews, 2010) prior to pre-processing. Trimmomatic was used for pre-processing to remove any remaining adaptor sequences and reads with low sequence quality (Phred score <20) (Bolger et al., 2014). Trimmed sequence reads were mapped and aligned to the zebrafish genome using Salmon (Patro et al., 2017). The number of reads mapped to the annotated regions of the genome were also obtained with Salmon. Statistical analysis was conducted using DESeq2 (Love et al., 2014). Only genes with false discovery rate (FDR) of <5% were considered to be differentially expressed. Differentially expressed genes (DEGs) were annotated using BioMart (Smedley et al., 2015). The following comparisons were made: control eggs vs BaP eggs, and control M x control F 10 hpf embryos vs the three treated groups.
Differentially expressed genes (FDR <0.05) were classified based on gene ontology (molecular function) using a gProfiler package g:GOSt (Reimand et al., 2016). The up- and down-regulated datasets for each treatment were processed individually. The gene ontology (GO) terms were compared between the two (eggs) or four (10 hpf embryo) groups using the g:Cocoa, a package of gProfiler (Reimand et al., 2016). Further, REVIGO was used to remove redundant GO terms (Supek et al., 2011). Non-redundant pathways were visualized using circular gene ontology software (CirGO) (Kuznetsova et al., 2019). Only unique GO terms with a distinct set of genes were considered for further analysis. In addition, we conducted protein interaction analysis of differentially expressed genes using string data base (Szklarczyk et al., 2021).
2.8. Reduced representation bisulfite sequencing (RRBS)
RRBS library preparation and sequencing was done at Zymo Research Services. Genomic DNA (100 ng) was digested with 30 units of MspI (NEB). Fragments were ligated to pre-annealed adapters containing 5′-methyl-cytosine instead of cytosine according to Illumina’s specified guidelines. Adaptor-ligated fragments ≥50 bp in size were recovered using the DNA Clean & Concentrator™−5 (Cat#: D4003). The fragments were then bisulfite-treated using the EZ DNA Methylation-Lightning™ Kit (Cat#: D5030). Preparative-scale PCR was performed, and the resulting products were purified with DNA Clean & Concentrator™−5 (Cat#: D4003) for sequencing on an Illumina platform. Sequence reads from bisulfite-treated classic RRBS libraries were identified using standard Illumina base calling software and then raw FASTQ files were quality trimmed (removing adapter sequences and trimming reads based on Phred quality) using TrimGalore 0.6.4. FastQC 0.11.8 was used to assess the effect of trimming and overall quality distributions of the data. The following comparisons were made: control eggs vs BaP eggs, control sperm vs BaP sperm, and control M x control F 10 hpf embryos vs the three treated groups.
Bisulfite sequencing data were analyzed using the Bisulfite Analysis Toolkit (BAT; v.0.1) (Kretzmer et al., 2017), reads were aligned with segemehl (v0.2.0) to the zebrafish reference genome danRer10 using standard parameters of the module “BAT_calling”. The resulting vcf files were filtered using the module “BAT_filter_vcf” and summarized using “BAT_summarize”. The calling of differentially methylated regions (DMRs) was carried out with “BAT_DMRcalling” using metilene (v.0.2–8) (Jühling et al., 2016). The genomic regions enrichment of annotations tool (GREAT) was used (McLean et al., 2010) to annotate the differentially methylated regions (DMRs). The genome coordinates were converted from the latest version (GRCz11; used in methylation analysis) to older version (Zv9) using the liftOver tool in UCSC genome browser prior to analysis with GREAT.
2.9. Statistical analysis
All data were assessed for normality and homogeneity of variance using Shapiro-Wilk and Brown-Forsythe tests, respectively. The incidence of developmental deformities (%) was calculated and averaged per replicate tank (n = 5). Larval length, eye diameter, and eye area were recorded per fish and then averaged per replicate tank (n = 5). Differences between the groups were assessed either by t-test, p ≤ 0.05 (F0 control vs BaP exposed), or 1-way (or 2-way when adults [sex as a second factor]) analysis of variance (ANOVA), with Student-Newman-Keuls (SNK) post hoc test (the four crosses) p ≤ 0.05. If the data did not meet assumptions for ANOVA, an ANOVA on ranks with Dunn’s posthoc test p ≤ 0.05 was conducted. All graphs and statistical analyses were conducted using Sigmaplot 14.0 software.
3. Results
3.1. Effect of dietary BaP exposure on F0 generation
The 21-day dietary BaP exposure had no significant effect on F0 growth (length, weight, and liver somatic index, Table S1). There was also no significant effect on adult F0 fecundity (p > 0.05, Table S2) and open field behavior (Fig. S1). However, dietary exposure to BaP resulted in significantly lower (~62%) gonad weight and gonadal somatic index in males, but not in females (p ≤ 0.05, Table S1).
3.2. Effect of F0 dietary BaP exposure on F1 generation
There was no significant effect of parental BaP exposure on the survival or incidence of developmental abnormalities in the F1 generation. There was also no effect of parental BaP exposure on adult F1 offspring gonadal somatic index, liver somatic index (p > 0.05, Table S1), or fecundity (p > 0.05, Table S2). There was no effect of BaP exposure on the length or weight in the F1 offspring of Bm x Bf cross at any time-point measured (p > 0.05, Fig. S2–3). However, the Bm x Cf group was significantly smaller in length (6%) at 96 hpf. At 90 dpf, this group continued to be significantly smaller in length (8%) and weight (21%) for male and female fish (p ≤ 0.05, Fig. S2–3).
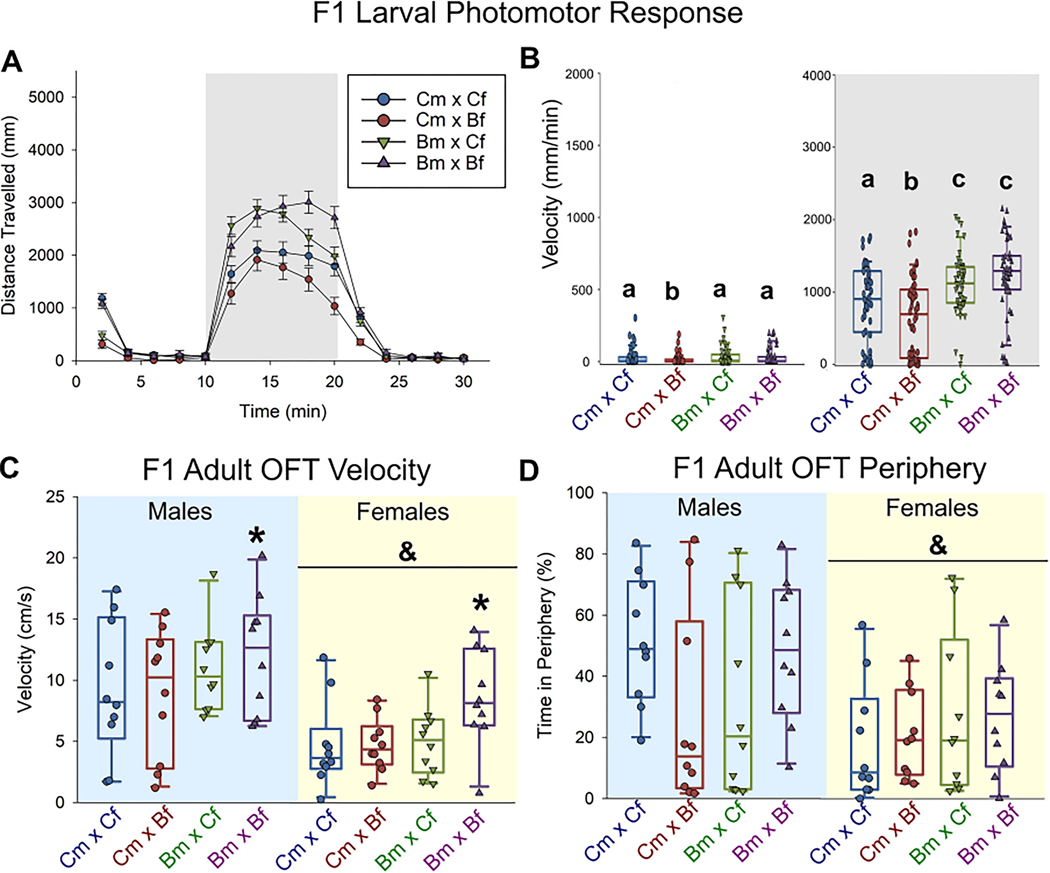
At 96 hpf, the F1 offspring of both the Bm x Cf and Bm x Bf crosses exhibited hyperactivity (28% and 42% increased total distance traveled, respectively) during the dark phase of the larval photomotor response (LPR) test (p ≤ 0.05, Fig. 2A,B).
Fig. 2.
Impact of parental BaP exposure on offspring behavior in F1 generation (A-D). Distance traveled over 30 min larval photomotor response test (A-B, 10 min light acclimation, 10 min dark, 10 min light) of 96 hpf F1 zebrafish (n = 50). Within dark phase, hyperactivity was measured in Bm x Bf and Bm x Cf larval F1 zebrafish. Letters that are not in common are significantly different (ANOVA, SNK post-hoc p ≤ 0.05). Open field behavior (OFT) of 120-day old (adult) F1 zebrafish (n = 10 per sex; C-D). Significantly increased velocity was detected when both parents were exposed (C). Females had significantly lower velocity and spent less time in periphery compared to males (C-D). *Indicates a significant difference from the control cross; &indicates a significant difference between males and females (two-way-ANOVA, SNK post-hoc p ≤ 0.05).
As adults, both male and female F1 offspring from the Bm x Bf cross were more active (p ≤ 0.05, Fig. 2C), but thigmotaxis was unaffected by parental BaP exposure (Fig. 2D). In general, female fish moved less and spent less time in the periphery compared to males (p ≤ 0.05, Fig. 2 C, D), and had significantly increased freezing duration (data not shown).
3.3. Effect of F0 dietary BaP exposure on F2 generation
There was no significant effect of parental BaP exposure on the survival or incidence of developmental abnormalities in the F2 generation for all exposed crosses. However, in the F2 generation the groups with exposed male parents were hyperactive in the light phase (p ≤ 0.05, Fig. S4).
3.4. BaP-induced transcriptional changes in F0 gametes and 10 hpf F1 embryos
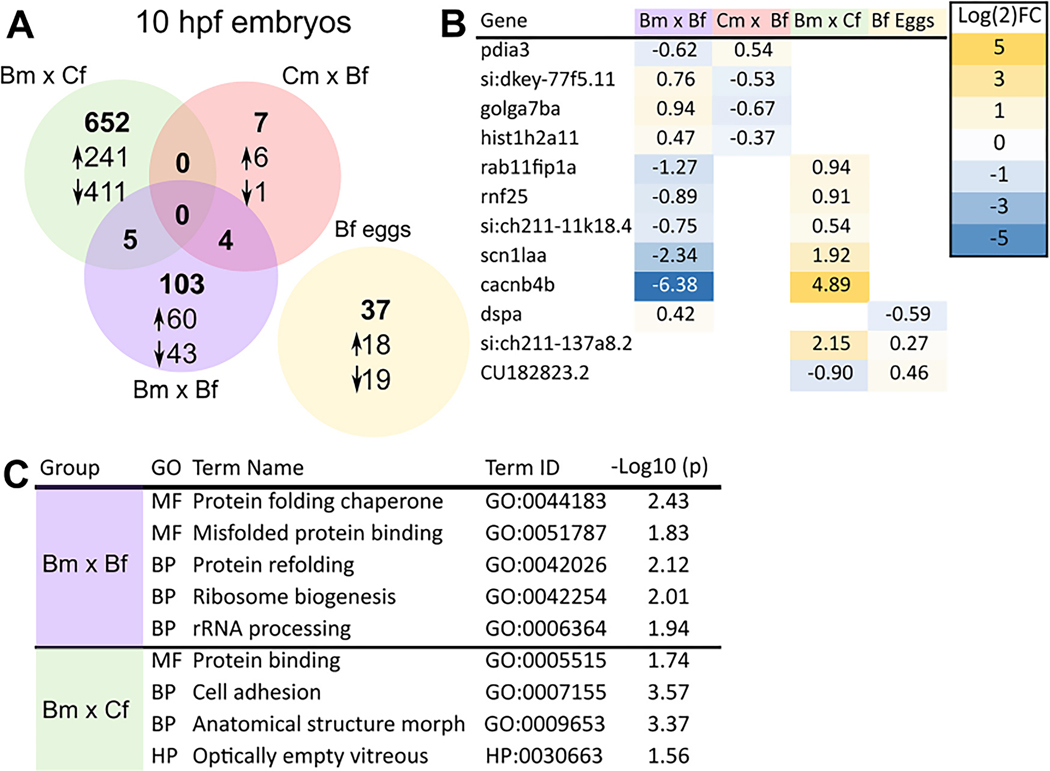
Transcriptional profiling of oocytes from BaP-exposed females (F0) revealed 37 differentially expressed genes (DEGs) with 18 and 19 up- and downregulated genes, respectively. For F0 sperm, there was not enough total RNA to sequence the transcriptome. For F1 generation 10 hpf embryos, the Bm x Cf cross showed differential expression of 657 genes in comparison to the control group. Among them, 245 and 412 genes were up and downregulated, respectively. In comparison, the Cm x Bf F1 embryos only had 11 DEGs (7 upregulated and 4 downregulated). When both parents are exposed to BaP (Bm x Bf), 112 genes were differentially expressed with 63 and 49 up- and downregulated genes, respectively (Fig. 3A).
Fig. 3.
Transcriptional profiles in response to BaP exposure. RNAseq was done on F0 germ cells and F1 generation 10 hpf embryos from all four parental crosses (A). Genes that overlap along with their fold change (B). GO term analysis results. GO terms enriched in the differentially expressed datasets from Bm x Cf and Bm x Bf exposures are shown (C).
There was no overlap in DEGs between the three treatment groups. Five genes were common between the paternal BaP exposure groups (Bm x Cf and Bm x Bf), and 4 genes were shared between Cm x Bf and Bm x Bf crosses (Fig. 3A). The genes common between different groups and their expression levels are shown in Fig. 3B.
GO term analysis for the two groups with the largest number of DEGs (Bm x Cf and Bm x Bf) showed enrichment of GO terms associated with protein folding, ribosome biogenesis, and cell adhesion (Fig. 3C). The entire list of DEG as well as their fold changes are provided in supplementary data (BaP_SI.xlsx).
3.5. DNA methylation profiling of F0 gametes and F1 embryos
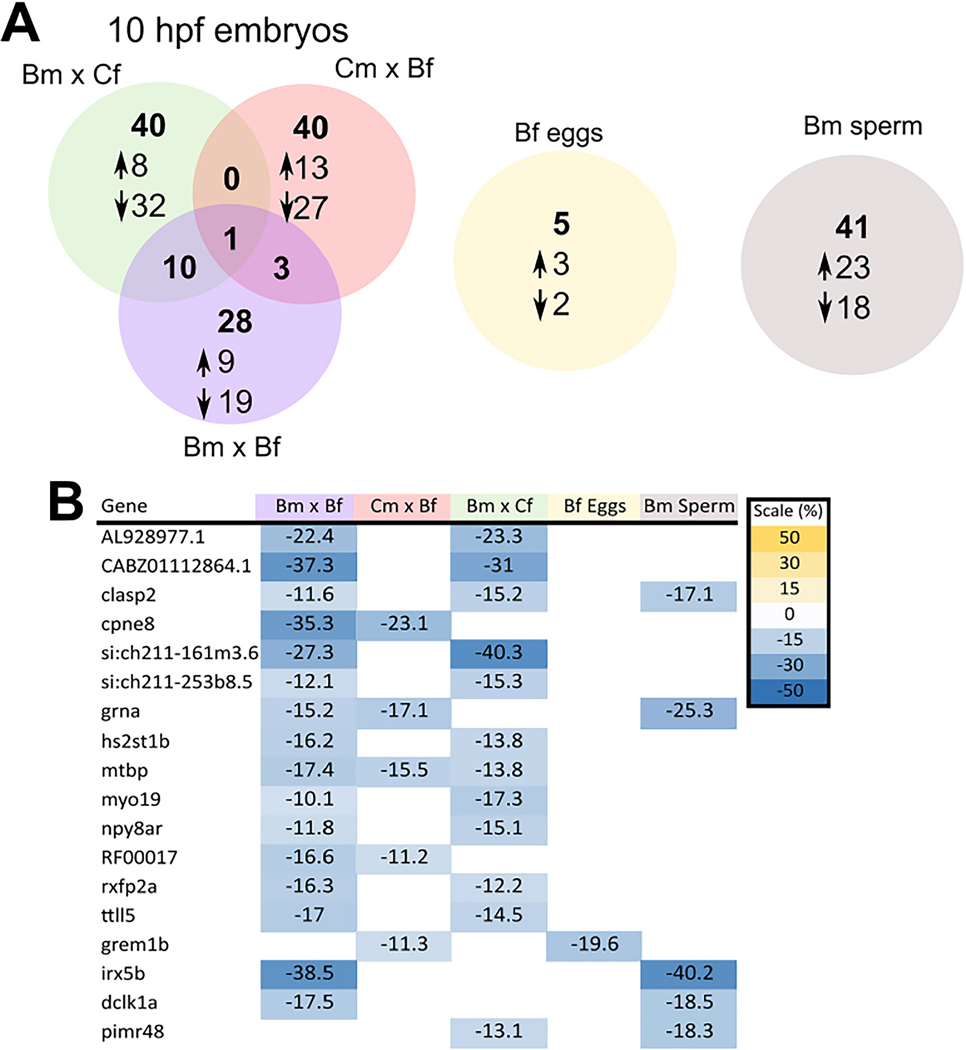
DNA methylation profiling of BaP-exposed F0 showed 41 DMRs in sperm and 5 DMRs in the oocytes. In 10 hpf F1 embryos DNA methylation profiling of Bm x Cf and Cm x Bf crosses revealed 51 and 44 DMRs, respectively (Fig. 4A). When both parents were exposed, 42 DMRs were detected in F1 embryos.
Fig. 4.
RRBS analysis (n = 4) of eggs, sperm, and of 10 hpf embryos with significant genes hyper (up) or hypomethylated (down) (A). Differentially methylated regions (DMRs) that overlapped between the different groups, all regions that overlapped were hypomethylated relative to their respective controls (B).
Overall, more DMRs were hypomethylated relative to controls and located in the intergenic regions (50–500 kb from the transcription start site). Among the three treatment groups, only one DMR was common to all treatments. This genomic region was predicted to be associated with the mtbp gene (MDM2 binding protein), a key player in tumor suppression and regulation protein ubiquitination (Iwakuma and Agarwal, 2012). Ten DMRs were shared between the Bm x Cf and Bm x Bf groups, and 3 DMRs were common between Cm x Bf and Bm x Bf groups. The genes with predicted associations with DMRs that are shared between different treatment groups and tissues and their mean methylation difference are shown in Fig. 4B. The entire list and information of genes associated with DMRs are provided in the supplementary data (BaP_SI.xlsx).
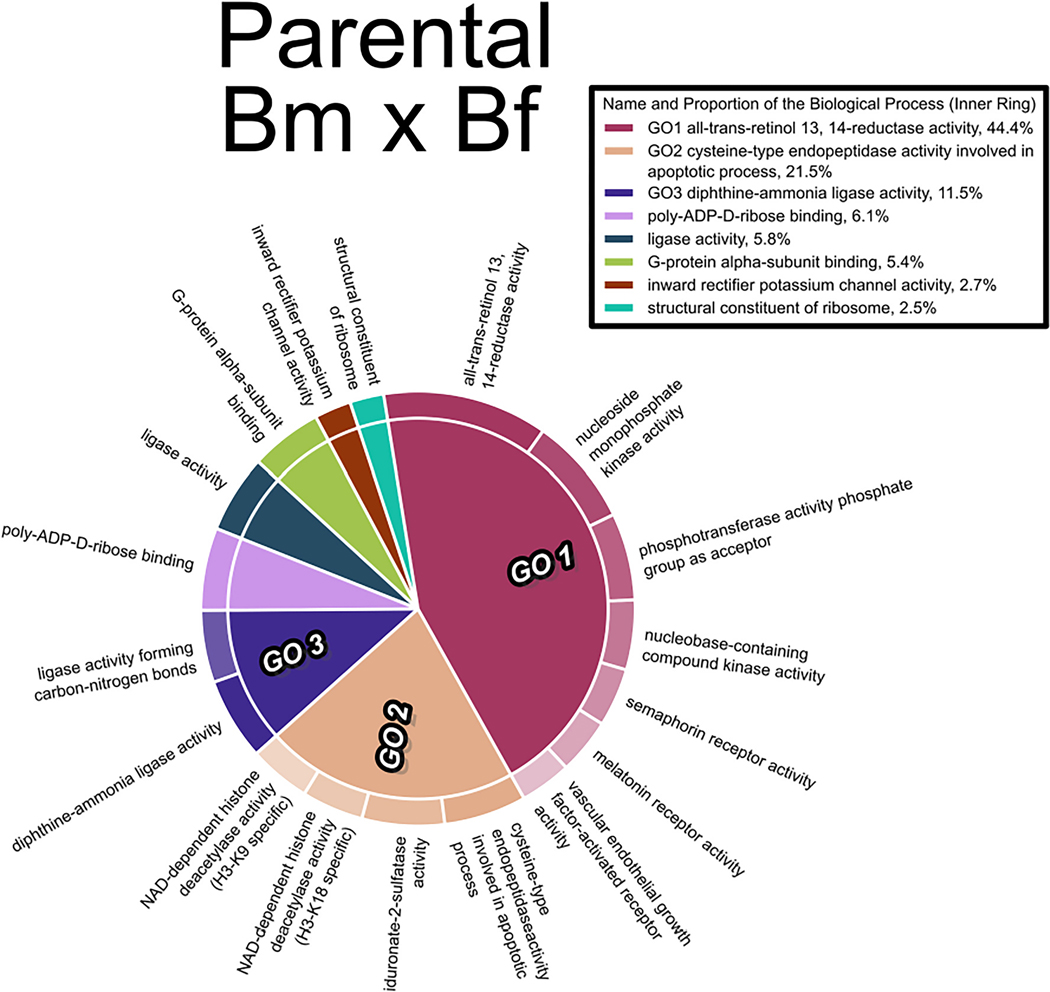
GO analysis of genes associated with DMRs revealed enrichment of GO biological process terms related to retinol metabolism and histone deacetylase activity in all three treatment groups of F1 embryos. A representative GO plot of the Bm x Bf group is shown in Fig. 5. The GO plots of remaining treatments are provided in the supplementary information (Fig. S5–6).
Fig. 5.
Gene Ontology analysis (molecular function pathways) of genes associated with DMRs in the F1 generation 10 hpf embryos from the Bm x Bf cross. The size of each GO term is determined by its statistical significance (p-value) and the different child terms under each parental GO term are represented by color gradient along the circumference of the plot.
RNAseq and RRBS raw files have been deposited in the NCBI Gene Expression Omnibus (GEO) database (GSE203631).
4. Discussion
This study demonstrated sex-dependent differences in BaP exposure-induced multigenerational effects. We found prenatal paternal exposure to BaP causes distinct changes in larval behavioral responses, gene expression, and DNA methylation. These observations concur with previous studies with diverse group of toxicants showing sex-specific differences in toxicant effects as well as paternal exposure-induced reproductive outcomes and persistent effects on subsequent generations (Soubry, 2018).
Significant hyperactivity was measured in the F1 offspring of the Bm x Cf and Bm x Bf crosses (which persisted into adulthood), suggesting the effect of BaP on behavior is carried through the male germline. The significant opposite effect (hypoactivity) detected in F1’s from the Cm x Bf cross further highlights the complicated nature of parental exposure. The effect of BaP on hyperactivity in the Bm x Cf and Bm x Bf crosses persisted into the F2 generation, though it was in the light phase rather than dark. The multigenerational hyperactive adverse outcome was also noted following an early life stage waterborne BaP exposure in zebrafish (Knecht et al., 2017). Similarly, increased anxiety-related behaviors, but not motor activity or impaired learning/memory, were noted in adult male rats following prenatal dietary maternal PAH exposure (Crépeaux et al., 2012). Epidemiological studies also found an increased prevalence of attention deficit/hyperactivity disorder in humans prenatally exposed to air pollution and PAHs (reviewed in: (Myhre et al., 2018)).
While the exact mechanism of how BaP exposure causes neurobehavioral effects is not known, it is likely to be mediated by aryl hydrocarbon (AHR) receptor (Knecht et al., 2017) because AHR is important in various neurodevelopmental processes (Crépeaux et al., 2012; Juricek and Coumoul, 2018). Furthermore, epigenetic mechanisms such as histone modification (Zhang et al., 2022), and DNA methylation (Gao et al., 2017) are implicated in AHR agonist-induced multigenerational effects. In this study, genes associated with neurological effects that were most up- and down-regulated in F1 embryos from the Bm x Cf and Bm x Bf crosses were related to epilepsy, including cacnb4b, scn1laa, and cux2b. There is an association between air pollution and epilepsy (Fernandes et al., 2019), and these data support that preconceptional exposure to pollutants such as BaP may play a role in the development of neurological defects in offspring.
Paternal exposure to BaP (Bm x Cf) caused differential expression of hundreds of genes in the F1 generation embryos and many of these genes are associated with AHR function. We identified three clusters (Supplemental Fig. S7–9) based on protein interaction networks. The genes representing these clusters are associated with developmental processes such as cell proliferation and cell adhesion, key functions of AHR during development (Gialitakis et al., 2017; Zablon et al., 2021). For instance, genes encoding ephrin receptors and ephrin ligands that are pivotal for directing coordinated cell migration in development were differentially expressed (Rozbesky and Jones, 2020), and the role of AHR in ephrin-dependent cellular processes has been demonstrated (Carvajal-Gonzalez et al., 2009). It remains to be determined how these processes affect tissue organization in preconceptionally exposed embryos.
Overall, very few DEGs (37) were observed in the F0 eggs despite the 21-day dietary exposure of adult females to BaP. However, a large number of DEGs were observed in the F1 generation (10 hpf), and these effects were most influenced by paternal exposure. Many of the pathways enriched in these embryos represent processes critical during early development including protein binding/folding and rRNA processing.
The importance of germ cell methylation and the potential impacts of environmental exposures on epigenetic signaling and multigenerational outcomes is an area of intense research. DNA methylation in oocytes, while not required for oocyte development or maturation, is critical in embryonic development and genomic imprinting (Sendžikaitė and Kelsey, 2019). A recent systematic review of studies considering toxicant effects on sperm DNA methylation highlighted associations between human exposures and altered DNA methylation, but signatures were inconsistent between studies and influences on subsequent offspring health unknown (Åsenius et al., 2020). Ma and coworkers found associations between urinary hydroxyPAH concentrations and methylation status of imprinted genes (e.g. H19 and PEG3) (Ma et al., 2019). In our study, there were only five DMRs in eggs from BaP-exposed F0, but in sperm more DMRs (41) were discovered with 23 DMRs hypermethylated and 18 hypomethylated. Our analysis revealed similar numbers of DMRs in the three F1 crosses, regardless of which parent was exposed. Offspring of BaP males had the most overlap in DMR genes and all were hypomethylated. This supports the claim that zebrafish sperm may be the mediator of epigenetic consequences (Jiang et al., 2013) including BaP toxicity because the embryonic profiles were similar to that of sperm. Clasp2, grna, irx5b and pimr48 were hypomethylated in both sperm and 10 hpf offspring from BaP exposed males, whereas only grem1b was hypomethylated in eggs and F1 of exposed females. DNA hypomethylation as a result of BaP exposure also occurred in zebrafish embryos (Corrales et al., 2014a; Fang et al., 2013b; Knecht et al., 2017), in mice blood and liver (Zhao et al., 2015), and rat sperm (Zhang et al., 2019).
Other AHR agonists, like 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), are also associated with reproductive adverse outcomes, specifically implicating the male germline. The transgenerational effects of TCDD on male gonad histology, fertility, and transcription were attributed to hypomethylation in the male germ line (Akemann et al., 2019, 2020; Baker et al., 2016; Baker et al., 2014). Despite the significant decrease in male GSI after dietary BaP exposure, we did not find a significant effect on fecundity. It is possible that these changes to the male gonad size did not measurably affect fecundity at this early life stage (4 months) but could result in significant changes to fecundity by the time the fish are fully grown (6 months).
DNA methylation was the epigenetic modification that was the focus of this study, but histone modifications (Xia et al., 2016; Zhang et al., 2022) and microRNA dysregulation (Brevik et al., 2012) also occur following BaP exposure. Likewise, TCDD was linked to altered DMRs of genes associated with histone modification and dysregulation of histone deacetylase (HDAC) activity (Akemann et al., 2020). We found significant effects on genes associated with epigenetic processes including differential methylation near anp32e and significant differential expression of mark3a and kdm6ba (supplemental file BaP_SI.xlsx). It is possible that some of the differentially hypomethylated genes that we detected are a result of HDAC activity. Furthermore, some DMRs modified by BaP exposure were associated with genes encoding chromatin modifying enzymes, suggesting regulation of chromatin conformation by DNA methylation. Dysregulation of histone deacetylase activity is associated with many types of disease (Delcuve et al., 2013) including persistent impaired cognitive function after prenatal BaP exposure (Zhang et al., 2022). Further work is needed to confirm the relationship between hypomethylation of histone modifying enzymes, their protein expression and activity, and what role HDACs play in BaP’s epigenetic effects.
Our transcriptional and DNA methylation results suggest that BaP exposure altered the expression of genes associated with diverse signaling pathways including ones not related to previously established mechanisms of BaP-induced AHR activation. For example, we did not observe significant upregulation of cyp1a in either the exposed adult germ cells or their 10 hpf offspring. BaP is quickly metabolized by fish (Hornung et al., 2007; Zhu et al., 2008), and the transcriptional responses observed could be related to BaP metabolites (e.g., 3-hydroxybenzo(a)pyrene, benzo(a)pyrene-9,10-dihydrodiol and benzo(a) pyrene-7,8-dihydrodiol 9,10-epoxide). These metabolites in turn can affect several metabolic pathways (Souza et al., 2016), activate many other transcription factors (van Delft et al., 2010), and induce oxidative stress, mitogenic signaling and formation of DNA adducts (Miller and Ramos, 2001). Preconceptional exposure to BaP could therefore affect diverse physiological pathways via BaP metabolites.
In a related dietary BaP study, Alsop et al. (2007) exposed adult zebrafish (strain unspecified) to 30 or 150 μg BaP/g diet (nominal) for 260 days. They demonstrated that long-term exposure to BaP did not statistically significantly impact growth or reproduction, however, exposure to BaP caused significant depletion of retinoids, retinyl esters, and retinal (Alsop et al., 2007). We found effects on retinol metabolism by dietary exposure to BaP in the parents may be passed on to their offspring through DNA methylation because significant enrichment of pathways related to retinol metabolism were observed in the F1 generation. Alsop et al. (2007) unfortunately was not able to measure effects on the F1 generation, due to complete mortality of all F1 embryos within a few days of fertilization (including controls).
Overall, these findings highlight that preconceptional exposure to BaP causes persistent behavioral hyperactivity. While the most serious and long-term effects were dependent on both parents being exposed, the highest number of differentially expressed genes depended on male parent exposure. Sperm compared to eggs contained significantly more differentially methylated regions with hypomethylation of certain genes conserved into the F1 embryos. Molecular analyses revealed significant effects on genes associated with epigenetic processes and processes critical during early development. These findings have important implications for assessing risk to offspring following prenatal parental exposure.
Supplementary Material
Acknowledgements
The authors would like to thank Arika Gardner, Daniel Decker, Kennedy Dickson, Kelle Thigpen, Bailey Westling, and Megha Patel for helping with the exposures, data collection, and sampling. We would also like to thank Dr. Robyn Tanguay (Oregon State University) for kindly providing the 5D zebrafish used in this study. The graphical abstract and Fig. 1 were created in part with BioRender.com.
Funding
This work was supported by the National Institutes of Health, National Institute of Environmental Health Sciences grant number 1R21ES030154.
Footnotes
Credit author statement
Z. Pandelides: Conceptualization, Investigation, Analysis, Validation, Writing; M. C. Sturgis: Methodology, Analysis, Visualization, Editing; C. Thornton: Methodology, Analysis, Validation, Editing; N. Aluru: Analysis, Data Curation, Editing, Visualization; K.L. Willett: Project administration, Funding Acquisition, Supervision, Editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Kristine L Willett reports financial support was provided by University of Mississippi School of Pharmacy and by National Institutes of Health.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.taap.2023.116545.
Data availability
RNAseq and RRBS raw files have been deposited in the NCBI Gene Expression Omnibus (GEO) database (GSE203631)
References
- Akemann C, Meyer DN, Gurdziel K, Baker TR, 2019. Developmental dioxin exposure alters the Methylome of adult male zebrafish gonads. Front. Genet 9 (7), 1–12. 10.3389/fgene.2018.00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akemann C, Meyer DN, Gurdziel K, Baker TR, 2020. TCDD-induced multi- and transgenerational changes in the methylome of male zebrafish gonads. Environmental Epigenetics 6 (1), 1–15. 10.1093/eep/dvaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop D, Brown S, Van Der Kraak G, 2007. The effects of copper and benzo[a]pyrene on retinoids and reproduction in zebrafish. Aquat. Toxicol 82 (4), 281–295. 10.1016/j.aquatox.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Ambeskovic M, Roseboom TJ, Metz GAS, 2020. Transgenerational effects of early environmental insults on aging and disease incidence. Neurosci. Biobehav. Rev 117, 297–316. 10.1016/j.neubiorev.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Andrews S, 2010. A Quality Control Tool for High throughput Sequence Data. URL. http://www.bioinformatics.babraham.ac.uk/projects/fastqc.
- Åsenius F, Danson AF, Marzi SJ, 2020. DNA methylation in human sperm: A systematic review. Hum. Reprod. Update 26 (6), 841–873. 10.1093/humupd/dmaa025. [DOI] [PubMed] [Google Scholar]
- Baker TR, King-Heiden TC, Peterson RE, Heideman W, 2014. Dioxin induction of transgenerational inheritance of disease in zebrafish. Mol. Cell. Endocrinol 398 (1–2), 36–41. 10.1016/j.mce.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BB, Yee JS, Meyer DN, Yang D, Baker TR, 2016. Histological and transcriptomic changes in male zebrafish testes due to early life exposure to low level 2,3,7,8-Tetrachlorodibenzo- p -dioxin. Zebrafish 13 (5), 413–423. 10.1089/zeb.2016.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffetta P, Trédaniel J, Greco A, 2000. Risk of childhood cancer and adult lung cancer after childhood exposure to passive smoke: A meta-analysis. Environ. Health Perspect 108 (1), 73–82. 10.2307/3454298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanova EA, Barsova EV, Shagina IA, Scheglov A, Anisimova V, Vagner LL, Lukyanov SA, Shagin DA, 2011. Normalization of full-length-enriched cDNA. Methods Mol. Biol. (Clifton) N.J.), 729, 85–98. 10.1007/978-1-61779-065-2_6. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B, 2014. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30 (15), 2114–2120. 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Messerlian C, Hauser R, 2017. Fathers matter: why It’s time to consider the impact of paternal environmental exposures on Children’s health. Curr. Epidemiol. Rep 4 (1), 46–55. 10.1007/s40471-017-0098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevik A, Lindeman B, Brunborg G, Duale N, 2012. Paternal benzo[a]pyrene exposure modulates microRNA expression patterns in the developing mouse embryo. Int. J. Cell Biol 2012 10.1155/2012/407431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowska B, Mokra K, Michałowicz J, 2022. Benzo[a]pyrene—environmental occurrence, human exposure, and mechanisms of toxicity. Int. J. Mol. Sci 23 (11), 6348. 10.3390/ijms23116348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Li J, Cheng L, Deng Y, Li Y, Yan Z, Duan L, Yang J, Niu Q, Perera F, Nie J, Tang D, 2020. The associations between prenatal exposure to polycyclic aromatic hydrocarbon metabolites, umbilical cord blood mitochondrial DNA copy number, and children’s neurobehavioral development. Environ. Pollut 265, 114594 10.1016/j.envpol.2020.114594. [DOI] [PubMed] [Google Scholar]
- Carvajal-Gonzalez JM, Mulero-Navarro S, Roman AC, Sauzeau V, Merino JM, Bustelo XR, Fernandez-Salguero PM, 2009. The dioxin receptor regulates the constitutive expression of the Vav3 proto-oncogene and modulates cell shape and adhesion. Mol. Biol. Cell 20, 1715–1727. 10.1091/mbc.E08-05-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne DL, Hoefnagels CCM, de Kloet RE, Richardson MK, 2010. Translating rodent behavioral repertoire to zebrafish (Danio rerio): relevance for stress research. Behav. Brain Res 214 (2), 332–342. 10.1016/j.bbr.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Clark TS, Pandolfo LM, Marshall CM, Mitra AK, Schech JM, 2018. Body condition scoring for adult zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci 57 (6), 698–702. 10.30802/AALAS-JAALAS-18-000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordier S, Monfort C, Filippini G, Preston-Martin S, Lubin F, Mueller BA, Holly EA, Peris-Bonet R, McCredie M, Choi W, Little J, Arslan A, 2004. Parental exposure to polycyclic aromatic hydrocarbons and the risk of childhood brain tumors: the SEARCH international childhood brain tumor study. Am. J. Epidemiol 159 (12), 1109–1116. 10.1093/aje/kwh154. [DOI] [PubMed] [Google Scholar]
- Corrales J, Fang X, Thornton C, Mei W, Barbazuk WB, Duke M, Schef BE, Willett KL, 2014a. Effects on specific promoter DNA methylation in zebrafish embryos and larvae following benzo [a] pyrene exposure, 163, 37–46. 10.1016/j.cbpc.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales Jone, Thornton C, White M, Willett KL, 2014b. Multigenerational effects of benzo[a]pyrene exposure on survival and developmental deformities in zebrafish larvae. Aquat. Toxicol 148, 16–26. 10.1016/j.aquatox.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crépeaux G, Bouillaud-Kremarik P, Sikhayeva N, Rychen G, Soulimani R, Schroeder H, 2012. Late effects of a perinatal exposure to a 16 PAH mixture: increase of anxiety-related behaviours and decrease of regional brain metabolism in adult male rats. Toxicol. Lett 211 (2), 105–113. 10.1016/j.toxlet.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Delcuve GP, Khan DH, Davie JR, 2013. Roles of histone deacetylases in epigenetic regulation: emerging paradigms from studies with inhibitors. In: Epigenetics and Pathology: Exploring Connections between Genetic Mechanisms and Disease Expression, 143–171. 10.1201/b16304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Corrales J, Thornton C, Scheffler BE, Willett KL, 2013a. Global and gene specific DNA methylation changes during zebrafish development. Comp. Biochem. Physiol. B Biochem. Mol. Biol 166 (1), 99–108. 10.1016/j.cbpb.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Thornton C, Scheffler BE, Willett KL, 2013b. Benzo[a]pyrene decreases global and gene specific DNA methylation during zebrafish development. Environ. Toxicol. Pharmacol 36 (1), 40–50. 10.1016/j.etap.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson-Smith AC, Patti ME, 2011. You are what your dad ate. Cell Metab. 13 (2), 115–117. 10.1016/j.cmet.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Fernandes MJS, Carletti CO, Sierra de Araújo LF, Santos RC, Reis J, 2019. Respiratory gases, air pollution and epilepsy. Rev. Neurol 175 (10), 604–613. 10.1016/j.neurol.2019.07.013. [DOI] [PubMed] [Google Scholar]
- Gao D, Wang C, Xi Z, Zhou Y, Wang Y, Zuo Z, 2017. Early-life benzo[a]pyrene exposure causes neurodegenerative syndromes in adult zebrafish (Danio rerio) and the mechanism involved. Toxicol. Sci 157 (1), 74–84. 10.1093/toxsci/kfx028. [DOI] [PubMed] [Google Scholar]
- Gialitakis M, Tolaini M, Li Y, Pardo M, Yu L, Toribio A, Choudhary JS, Niakan K, Papayannopoulos V, Stockinger B, 2017. Activation of the aryl hydrocarbon receptor interferes with early embryonic development. Stem Cell Reports 9 (5), 1377–1386. 10.1016/j.stemcr.2017.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Roig MD, Pascal R, Cahuana MJ, Garciá-Algar O, Sebastiani G, Andreu-Fernández V, Martínez L, Rodríguez G, Iglesia I, Ortiz-Arrabal O, Mesa MD, Cabero MJ, Guerra L, Llurba E, Domínguez C, Zanini MJ, Foraster M, Larqué E, Cabañas F, Vento M, 2021. Environmental exposure during pregnancy: influence on prenatal development and early life: a comprehensive review. Fetal Diagn. Ther 48 (4), 245–257. 10.1159/000514884. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Barouki R, Bellinger DC, Casteleyn L, Chadwick LH, Cordier S, Etzel RA, Gray KA, Ha EH, Junien C, Karagas M, Kawamoto T, Lawrence BP, Perera FP, Prins GS, Puga A, Rosenfeld CS, Sherr DH, Sly PD, Heindel JJ, 2015. Life-long implications of developmental exposure to environmental stressors: new perspectives. Endocrinol. (United States) 156 (10), 3408–3415. 10.1210/EN.2015-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Vandenberg LN, 2015. Developmental origins of health and disease: A paradigm for understanding disease cause and prevention. Curr. Opin. Pediatr 27 (2), 248–253. 10.1097/MOP.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S-M, Cheong A, Adgent MA, Veevers J, Suen AA, Tam NNC, Leung Y-K, Jefferson WN, Williams CJ, 2017. Environmental factors, epigenetics, and developmental origin of reproductive disorders. Reprod. Toxicol 68, 85–104. 10.1016/j.reprotox.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung MW, Cook PM, Fitzsimmons PN, Kuehl DW, Nichols JW, 2007. Tissue distribution and metabolism of benzo[a]pyrene in embryonic and larval medaka (Oryzias latipes). Toxicol. Sci 100 (2), 393–405. 10.1093/toxsci/kfm231. [DOI] [PubMed] [Google Scholar]
- Iwakuma T, Agarwal N, 2012. MDM2 binding protein, a novel metastasis suppressor. Cancer Metastasis Rev. 31 (3–4), 633–640. 10.1007/s10555-012-9364-x. [DOI] [PubMed] [Google Scholar]
- Jedrychowski WA, Perera FP, Camann D, Spengler J, Butscher M, Mroz E, Majewska R, Flak E, Jacek R, Sowa A, 2015. Prenatal exposure to polycyclic aromatic hydrocarbons and cognitive dysfunction in children. Environ. Sci. Pollut. Res 22 (5), 3631–3639. 10.1007/s11356-014-3627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji BT, Shu XO, Linet MS, Zheng W, Wacholder S, Gao YT, Ying DM, Jin F, 1997. Paternal cigarette smoking and the risk of childhood cancer among offspring of nonsmoking mothers. J. Natl. Cancer Inst 89 (3), 238–244. 10.1093/jnci/89.3.238. [DOI] [PubMed] [Google Scholar]
- Jiang L, Zhang J, Wang JJ, Wang L, Zhang L, Li G, Yang X, Ma X, Sun X, Cai J, Zhang J, Huang X, Yu M, Wang X, Liu F, Wu CI, He C, Zhang B, Ci W, Liu J, 2013. Sperm, but not oocyte, DNA methylome is inherited by zebrafish early embryos. Cell 153 (4), 773–784. 10.1016/j.cell.2013.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge BC, Reis ACC, Stein J, da Balin PS, Sterde ÉT, Barbosa MG, de Aquino AM, Kassuya CAL, Arena AC, 2021. Parental exposure to benzo(a) pyrene in the peripubertal period impacts reproductive aspects of the F1 generation in rats. Reprod. Toxicol 100 (October 2020), 126–136. 10.1016/j.reprotox.2021.01.011. [DOI] [PubMed] [Google Scholar]
- Jühling F, Kretzmer H, Bernhart SH, Otto C, Stadler PF, Hoffmann S, 2016. Metilene: fast and sensitive calling of differentially methylated regions from bisulfite sequencing data. Genome Res. 26 (2), 256–262. 10.1101/gr.196394.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juricek L, Coumoul X, 2018. The aryl hydrocarbon receptor and the nervous system. Int. J. Mol. Sci 19 (9) 10.3390/ijms19092504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirla KT, Groh KJ, Steuer AE, Poetzsch M, Banote RK, Stadnicka-Michalak J, Eggen RIL, Schirmer K, Kraemer T, 2016. Zebrafish larvae are insensitive to stimulation by cocaine: importance of exposure route and toxicokinetics. Toxicol. Sci 154 (1), 183–193. 10.1093/toxsci/kfw156. [DOI] [PubMed] [Google Scholar]
- Knecht AL, Truong L, Marvel SW, Reif DM, Garcia A, Lu C, Simonich MT, Teeguarden JG, Tanguay RL, 2017. Transgenerational inheritance of neurobehavioral and physiological deficits from developmental exposure to benzo [a]pyrene in zebrafish. Toxicol. Appl. Pharmacol 329, 148–157. 10.1016/j.taap.2017.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzmer H, Otto C, Hoffmann S, 2017. BAT: bisulfite analysis toolkit. F1000Research 6 (May), 1490. 10.12688/f1000research.12302.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova I, Lugmayr A, Siira SJ, Rackham O, Filipovska A, 2019. CirGO: An alternative circular way of visualising gene ontology terms. BMC Bioinform. 20 (1), 1–7. 10.1186/s12859-019-2671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois PH, Hoyt AT, Desrosiers TA, Lupo PJ, Lawson CC, Waters MA, Rocheleau CM, Shaw GM, Romitti PA, Gilboa SM, Malik S, 2014. Maternal occupational exposure to polycyclic aromatic hydrocarbons and small for gestational age offspring. Occup. Environ. Med 71 (8), 529–535. 10.1136/oemed-2013-101833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S, 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15 (12), 1–21. 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Lu Z, Wang L, Qiang M, 2019. Correlation of internal exposure levels of polycyclic aromatic hydrocarbons to methylation of imprinting genes of sperm DNA. Int. J. Environ. Res. Public Health 16 (14). 10.3390/ijerph16142606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CY, Bristor D, Hiller M, Clarke SL, Schaar BT, Lowe CB, Wenger AM, Bejerano G, 2010. GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol 28 (5), 495–501. 10.1038/nbt.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KP, Ramos KS, 2001. Impact of cellular metabolism on the biological effects of benzo[a]pyrene and related hydrocarbons †. Drug Metab. Rev 33 (1), 1–35. 10.1081/DMR-100000138. [DOI] [PubMed] [Google Scholar]
- Mohamed ESA, Song WH, Oh SA, Park YJ, You YA, Lee S, Choi JY, Kim YJ, Jo I, Pang MG, 2010. The transgenerational impact of benzo(a)pyrene on murine male fertility. Hum. Reprod 25 (10), 2427–2433. 10.1093/humrep/deq205. [DOI] [PubMed] [Google Scholar]
- Myhre O, Låg M, Villanger GD, Oftedal B, Øvrevik J, Holme JA, Aase H, Paulsen RE, Bal-Price A, Dirven H, 2018. Early life exposure to air pollution particulate matter (PM) as risk factor for attention deficit/hyperactivity disorder (ADHD): need for novel strategies for mechanisms and causalities. Toxicol. Appl. Pharmacol 354 (October 2017), 196–214. 10.1016/j.taap.2018.03.015. [DOI] [PubMed] [Google Scholar]
- Pandelides Z, Thornton C, Faruque AS, Whitehead AP, Willett KL, Ashpole NM, 2020. Developmental exposure to cannabidiol (CBD) alters longevity and health span of zebrafish (Danio rerio). GeroScience 42 (2), 785–800. 10.1007/s11357-020-00182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C, 2017. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14 (4), 417–419. 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai W-Y, Tang D, Diaz D, Hoepner L, Barr D, Tu Y-H, Camann D, Kinney P, 2006. Effect of prenatal exposure to airborne polycyclic aromatic Hydrocarbonson neurodevelopment in the first 3 years of life among Inner-City children. Environ. Health Perspect 114 (8), 1287–1292. 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Li Z, Whyatt R, Hoepner L, Wang S, Camann D, Rauh V, 2009. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics 124 (2), e195–e202. 10.1542/peds.2008-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Tang D, Wang S, Vishnevetsky J, Zhang B, Diaz D, Camann D, Rauh V, 2012. Prenatal polycyclic aromatic hydrocarbon (PAH) exposure and child behavior at age 6–7 years. Environ. Health Perspect 120 (6), 921–926. 10.1289/ehp.1104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MF, Lehner B, 2019. Intergenerational and transgenerational epigenetic inheritance in animals. Nat. Cell Biol 21 (2), 143–151. 10.1038/s41556-018-0242-9. [DOI] [PubMed] [Google Scholar]
- Reimand J, Arak T, Adler P, Kolberg L, Reisberg S, Peterson H, Vilo J, 2016. G: profiler-a web server for functional interpretation of gene lists (2016 update). Nucleic Acids Res. 44 (W1), W83–W89. 10.1093/nar/gkw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren A, Qiu X, Jin L, Ma J, Li Z, Zhang L, Zhu H, Finnell RH, Zhu T, 2011. Association of selected persistent organic pollutants in the placenta with the risk of neural tube defects. Proc. Natl. Acad. Sci 108 (31), 12770–12775. 10.1073/pnas.1105209108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozbesky D, Jones EY, 2020. Cell guidance ligands, receptors and complexes – orchestrating signalling in time and space. Curr. Opin. Struct. Biol 61, 79–85. 10.1016/j.sbi.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CW, 2018. Chips off the old block: how a father’s preconception exposures might affect the health of his children. Environ. Health Perspect 126 (2), 1–6. 10.1289/EHP2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendžikaitė G, Kelsey G, 2019. The role and mechanisms of DNA methylation in the ˙ oocyte. Essays Biochem. 63 (6), 691–705. 10.1042/EBC20190043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui AR, Gold EB, Yang X, Lee K, Brown KH, Bhutta ZA, 2008. Prenatal exposure to wood fuel smoke and low birth weight. Environ. Health Perspect 116 (4), 543–549. 10.1289/ehp.10782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sireeni J, Bakker N, Jaikumar G, Obdam D, Slabbekoorn H, Tudorache C, Schaaf M, 2020. Profound effects of glucocorticoid resistance on anxiety-related behavior in zebrafish adults but not in larvae. Gen. Comp. Endocrinol 292 (March) 10.1016/j.ygcen.2020.113461. [DOI] [PubMed] [Google Scholar]
- Smedley D, Haider S, Durinck S, Pandini L, Provero P, Allen J, Arnaiz O, Awedh MH, Baldock R, Barbiera G, Bardou P, Beck T, Blake A, Bonierbale M, Brookes AJ, Bucci G, Buetti I, Burge S, Cabau C, Kasprzyk A, 2015. The BioMart community portal: an innovative alternative to large, centralized data repositories. Nucleic Acids Res. 43 (W1), W589–W598. 10.1093/nar/gkv350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubry A, 2018. POHaD: Why we should study future fathers. Environmental Epigenetics 4 (2), 1–7. 10.1093/eep/dvy007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza T, Jennen D, van Delft J, van Herwijnen M, Kyrtoupolos S, Kleinjans J, 2016. New insights into BaP-induced toxicity: role of major metabolites in transcriptomics and contribution to hepatocarcinogenesis. Arch. Toxicol 90 (6), 1449–1458. 10.1007/s00204-015-1572-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen PJ, Richardson MK, Champagne DL, 2011. Patterns of avoidance behaviours in the light/dark preference test in young juvenile zebrafish: A pharmacological study. Behav. Brain Res 222 (1), 15–25. 10.1016/j.bbr.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Supek F, Bošnjak M, Škunca N, Šmuc T, 2011. Revigo summarizes and visualizes long lists of gene ontology terms. PLoS One 6 (7). 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, Doncheva NT, Legeay M, Fang T, Bork P, Jensen LJ, von Mering C, 2021. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 49 (18), 10800. 10.1093/nar/gkab835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delft JHM, Mathijs K, Staal YCM, van Herwijnen MHM, Brauers KJJ, Boorsma A, Kleinjans JCS, 2010. Time series analysis of benzo[a]pyrene-induced transcriptome changes suggests that a network of transcription factors regulates the effects on functional gene sets. Toxicol. Sci 117 (2), 381–392. 10.1093/toxsci/kfq214. [DOI] [PubMed] [Google Scholar]
- Xia B, Yang LQ, Huang HY, Pang L, Yang XF, Yi YJ, Ren XH, Li J, Zhuang ZX, Liu JJ, 2016. Repression of biotin-related proteins by benzo[a]pyrene-induced epigenetic modifications in human bronchial epithelial cells. Int. J. Toxicol 35 (3), 336–343. 10.1177/1091581816637071. [DOI] [PubMed] [Google Scholar]
- Yin X, Liu Y, Zeb R, Chen F, Chen H, Wang KJ, 2020. The intergenerational toxic effects on offspring of medaka fish Oryzias melastigma from parental benzo[a] pyrene exposure via interference of the circadian rhythm. Environ. Pollut 267, 115437 10.1016/j.envpol.2020.115437. [DOI] [PubMed] [Google Scholar]
- Zablon HA, Ko CI, Puga A, 2021. Converging roles of the aryl hydrocarbon receptor in early embryonic development, maintenance of Stemness, and tissue repair. Toxicol. Sci 182 (1), 1–9. 10.1093/toxsci/kfab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CM, Sun ZX, Wang ZL, Chen JS, Chang Z, Wang Z, Zhu L, Ma ZH, Peng YJ, Xu ZA, Wang SQ, 2019. Abnormal methylation of spermatozoa induced by benzo(a)pyrene in rats. Hum. Exp. Toxicol 38 (7), 846–856. 10.1177/0960327119836230. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Du L, Yan J, Bai Q, Niu Q, Mo Y, Zhang Q, Nie J, 2022. Prenatal benzo[a]pyrene exposure impairs hippocampal synaptic plasticity and cognitive function in SD rat offspring during adolescence and adulthood via HDAC2-mediated histone deacetylation. Ecotoxicol. Environ. Saf 246 (June), 114180 10.1016/j.ecoenv.2022.114180. [DOI] [PubMed] [Google Scholar]
- Zhao L, Zhang S, An X, Tan W, Pang D, Ouyang H, 2015. Toxicological effects of benzo[a]pyrene on DNA methylation of whole genome in ICR mice. Cell. Mol. Biol 61 (5), 115–119. 10.14715/cmb/2015.61.5.19. [DOI] [PubMed] [Google Scholar]
- Zhu S, Li L, Thornton C, Carvalho P, Avery BA, Willett KL, 2008. Simultaneous determination of benzo[a]pyrene and eight of its metabolites in Fundulus heteroclitus bile using ultra-performance liquid chromatography with mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci 863 (1), 141–149. 10.1016/j.jchromb.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNAseq and RRBS raw files have been deposited in the NCBI Gene Expression Omnibus (GEO) database (GSE203631)