Abstract
Kiwifruit (Actinidia spp.), celebrated for its unique flavor and rich nutritional content, is a globally popular fruit. This fruit requires post-harvest ripening before consumption. However, the unpredictable ripening pace significantly impacts consumer acceptance and sales, thereby hindering the commercial growth of kiwifruit. To address this, understanding the key molecular mechanisms and metabolites governing postharvest ripening and senescence could offer valuable insights for developing storage strategies and breeding techniques in yellow-fleshed kiwifruits. We constructed two models that integrated these findings with existing theories. The first model suggests that, unlike the T6P-sucrose regulatory mechanism observed in plant leaves, the separation of harvested kiwifruit from the mother plant leads to an insufficient supply of T6P, which activates the SnRK1 kinase. This, in turn, inhibits the TOR kinase signaling pathway, regulating starch metabolism. The T6P-SnRK1-TOR-starch metabolism pathway plays a regulatory role during postharvest ripening, limiting excessive starch degradation that could accelerate aging and decay in yellow-fleshed kiwifruit. The second model highlights the role of abscisic acid (ABA), cytokinins (CKs), and ethylene in regulating the process, inducing the activation of ERFs and cell wall-degrading enzymes, promoting fruit postharvest softening. These findings indicate that at least two models, the T6P-SnRK1-TOR-starch metabolism model and the ABA-CKs-ethylene-cell wall degradation model, regulate postharvest fruit ripening, offering new insights into the artificial regulation of yellow-fleshed kiwifruit ripening speed.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-05817-8.
Keywords: Kiwifruit, Hormones, T6P/SnRK, Crosstalk, Postharvest
Introduction
Kiwifruit, a nutritionally and economically significant fresh fruit crop, has garnered global attention [1]. This fruit, along with its derivatives such as juices and wine, is rich in vitamins, minerals, dietary fiber, and various health-promoting phytochemicals [2]. The center of origin for kiwifruit is the temperate forests of the mountains and hills of southwest China [3]. According to the Food and Agriculture Organization (FAO), the global cumulative harvested area of kiwifruit stands at approximately 287,000 hectares, producing 4.467 million tons annually, positioning it among the world's major fruits consumed (http://faostat.fao.org). Approximately 30% of the harvested fruits, however, undergo physiological deterioration, leading to losses and waste [4]. Thus, improving the agronomic traits of kiwifruit is essential for enhancing consumption and enhancing dietary quality. By reducing spoilage, substantial cost savings can be realized, thereby contributing to food security for undernourished populations globally [2].
Minimizing postharvest spoilage of fruits poses a significant challenge for plant biologists [5, 6]. In recent decades, extensive research has illuminated the intricate networks of endogenous signals, including sugars, calcium, plant hormones, and transcription factors, which regulate postharvest ripening and senescence in agricultural and horticultural crops [5–8]. Among these signals, ethylene plays a crucial role as a key inducer of fruit maturation, triggering senescence and increasing ABA content [9]. The involvement of auxin, CKs, and brassinosteroids in fruit ripening and senescence has also been reported [10–13]. Furthermore, the morphology, crystallinity, composition, size, and insoluble nature of starch granules collectively influence fruit respiration and metabolism, thereby directly impacting fruit firmness during postharvest storage [14]. The degradation of dense granules into soluble sugars leads to an increase in intercellular space, promoting fruit softening [15]. These signals interact with each other, forming a complex network that collectively regulates postharvest fruit ripening and senescence, which has not yet been clearly elucidated in yellow-fleshed kiwifruit.
In recent years, the kiwifruit industry in China has experienced rapid growth, with the country leading globally in both cultivation area and production volume, accounting for approximately 50% of the total. Despite this, China is viewed more as a major producer of kiwifruit rather than a dominant force, largely due to its low yield per unit area and high post-harvest loss rate. Moreover, like other fruits, the postharvest ripening process within the Actinidia genus exhibits botanical diversity, as noted by Garcia et al. [16]. Although numerous studies have delved into kiwifruit ripening, particularly in varieties with red hearts or green flesh, there has been a dearth of research into the regulatory mechanisms governing the ripening and senescence of yellow-fleshed kiwifruit [17, 18]. Thus, elucidating the key molecular factors and metabolites that regulate postharvest ripening and senescence in fruits is crucial. This study comprehensively investigates the potential roles of endogenous chemicals, physiological changes, and key gene regulatory networks during postharvest ripening. Such findings can offer valuable insights for the development of storage strategies and new breeding techniques, thereby extending the shelf life and enhancing the quality of yellow-fleshed kiwifruits.
Materials and methods
Plant materials
Kiwifruit (A.eriantha × A.chinensis cv. ‘Jinyan’) were harvested at 180 days after full bloom (DAFB) from Fengxin County Doctor Kiwifruit Base in Yichun City, China (E114°45’, N28°34’). The fruits were uniform in size and individually wrapped in polybags before being placed in cardboard boxes. They were stored in artificial incubators simulating room temperature conditions (21–25 °C, 60–70% RH, dark). Samples were taken at 1, 3, 5, 7, 9, 11, and 13 days after harvest (DAH). For each experiment in this study, three biological replicates were conducted, each comprising 15 fruits. Fruit samples, approximately 2–3 cm in diameter near the equatorial region, were collected from each replicate. The fruits were sliced into small pieces and mixed. After collection, all samples were flash-frozen in liquid nitrogen and stored at −80 °C. The preserved samples were used for further testing and analysis, including RNA extraction, measurement of phytohormone content, starch content, T6P content, enzyme activity, and soluble sugars. Titratable acidity content was assessed according to the standard method (GB/T8210-2011, China). Fruit firmness was determined using a GY-4 fruit firmness tester with a 7.9-mm plunger (AIPLI, Zhejiang, China), and soluble solid concentration (SSC) was determined using a digital handheld refractometer (Atago, Tokyo, Japan).
Measurement of T6P content
T6P was extracted using 30% (v/v) acetonitrile and analyzed according to a previously described method [19]. The quantitative analysis of T6P was conducted using an Agilent 1260 high-performance liquid chromatograph tandem 6420A mass spectrometer (HPLC–MS/MS).
Phytohormone assays
Plant hormones, including ABA, zeatin (tZ), jasmonic acid (JA), tryptophan (TRP), SA, indole acetic acid (IAA), isopentenyladenine (IP), isopentenyladenine-9-glucoside (ip9G), and abscisic acid glucose ester (ABA-GE), were extracted using 80% (v/v) methanol and quantified using a previously established method [20]. The sample extracts were detected using a UPLC-ESI–MS/MS system.
Ethylene was collected and measured according to a previously described method [21]. The fresh fruit samples were ground into powder in liquid nitrogen, and 0.1 g of each sample was collected in brown, sealed bottles to collect the gas at room temperature for 24 h. The gas was then injected into a gas chromatograph (7890,Agilent) to determine the ethylene content.
Enzyme activity, starch, and maltose assays
The activities of β-amylase, α-amylase, and starch phosphorylase, as well as the levels of starch and maltose, were quantified using kits obtained from Beijing Solarbio Science. All assays were performed in accordance with the manufacturer's instructions.
Analysis of RNA-Seq data
The total RNA was extracted from each replicate using the RNAprep Pure Plant Kit (Tiangen, Beijing, China), following the manufacturer's protocol. The libraries were sequenced on an Illumina NovaSeq platform at Metware Corporation (Wuhan, China). Gene expression levels were estimated using the FPKM method. Differential expression analysis between the two groups was conducted using DESeq2 v1.22.1 and edgeR v3.24.3, with the Benjamini & Hochberg method applied for P-value correction. Significance for differential expression was determined based on a corrected P-value < 0.05 and |log2foldchange|≥ 1. Enrichment analysis was conducted using the hypergeometric test, with KEGG pathways analyzed at the pathway level and GO terms analyzed based on the GO term.
Quantitative real-time reverse transcription PCR (qRT-PCR) analysis
To investigate the expression patterns of key genes in the T6P-SnRK1-TOR-starch metabolism and ABA-CKs-ethylene-cell wall degradation models during postharvest ripening in kiwifruit, 21 DEGs were selected for further qRT-PCR analysis. The experiment was conducted with three biological replicates, and the relative expression levels of the selected genes were calculated using the 2−ΔΔCtmethod [22]. qRT-PCR was performed using ChamQ Universal SYBR qPCR Master Mix on a CFX96™ real-time PCR system (Bio-Rad, CA, USA).AcActin and AcGAPDH were employed as reference genes in this study. The specific primers used for qRT-PCR are listed in Table S1.
Results
Characteristics of fruit ripening and senescence in kiwifruit
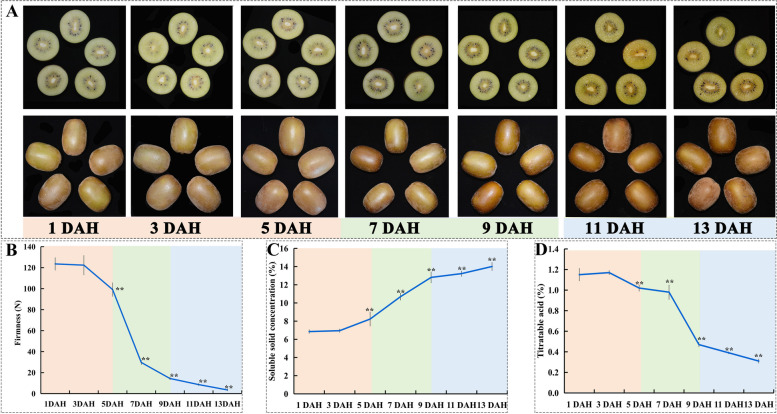
To assess the alterations in natural postharvest ripening of kiwifruit, the dynamic changes in fruit morphology, hardness, and physiological indicators were monitored. The postharvest maturity of kiwifruit can be categorized into three stages based on the rate of softening (Fig. 1). The first stage spans from the 1st to the 5th day after harvest, with a softening rate of 6.1 N/d. The second stage, from the 5th to the 9th day, exhibits a significantly accelerated softening rate of 21.19 N/d, which is 3.47 times faster than the first stage. The third stage also displays a slow softening trend, with the softening rate dropping to its lowest point, 2.72 N/d. According to the reference of ‘Jinyan’ kiwifruit's edible period by Mei Yan et al. [23], the fruit enters the edible state at 11 days after harvest (DAH), characterized by a hardness less than 10 N and a soluble solid concentration-acid ratio greater than 33.92. As illustrated in Fig. 1, the flesh color gradually transitions from green to a more yellowish hue with increased storage duration. The soluble solid concentration in the fruit increases with storage time, reaching a peak of 14.01% on the 13th DAH, while the titratable acid content of the pulp reaches its lowest point, at 0.31%.
Fig. 1.
Changes in fruit characteristics during kiwifruit ripening and senescence under room temperature. Changes in fruit color (A), fruit firmness (B), soluble solid concentration (C), and titratable acid (D). Bars indicate SD. Asterisk (**) indicates significant differences with Duncan’s test at p < 0.01
Plant hormones analysis
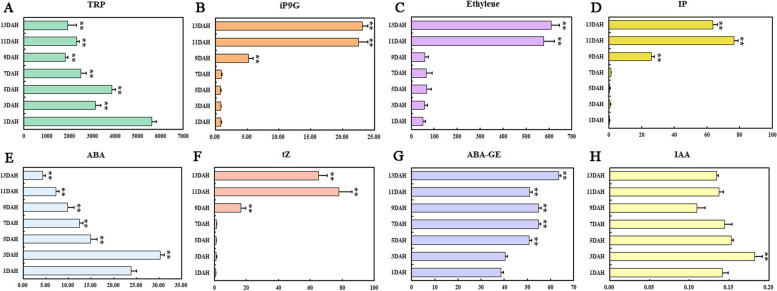
Plant hormones play a crucial role in regulating fruit ripening and quality development, as evidenced by previous studies [8]. Understanding the interactions among different plant hormones and the formation of signal transduction networks is essential for comprehending the regulation of fruit ripening. In this study, the concentrations of major phytohormones, including auxin (IAA, TRP), gibberellin (GA3), CKs (tZ, IP, ip9G), JA, ABA, and ABA-GE, were determined in postharvest kiwi fruits using an UPLC-ESI–MS/MS system (Figs. 2). The ethylene content was quantified using gas chromatography. Kiwi fruits exhibited a relatively high basal level of TRP, with a peak value of 5610.87 ng/g at 1 DAH (Fig. 2A). The TRP content decreased compared to the level at 1 DAH as the duration of postharvest ripening extended. During the post-harvest ripening period of kiwifruit, the ABA content initially increased and then decreased, reaching its maximum value at 3 DAH and subsequently decreasing to the lowest level (4.36 ng/g) at 13 DAH. In contrast, the content of ABA-GE (one of the most abundant forms of ABA conjugates) was significantly higher than ABA and continuously accumulated, reaching its maximum value at 13 DAH, which was 14.54 times higher than ABA (Fig. 2E).
Fig. 2.
Analysis of plant hormone accumulation in kiwifruit. A TRP; B iP9G; C Ethylene; D IP; E ABA; F tZ; G ABA-GE; H IAA. Values are the mean ± SD. Bars indicate SD. Names are abbreviated as follows: TRP: tryptophan; ABA: abscisic acid; tZ: trans-Zeatin; ABA-GE: abscisic acid glucose ester; JA: jasmonic acid. Bars indicate SD. Asterisk (**) indicates significant differences with Duncan’s test at p < 0.01
As two key hormones in fruit ripening, ethylene and ABA exhibit contrasting trends. As illustrated in Fig. 2C, ethylene levels remained stable until a substantial increase was observed from 9 to 13 DAH, with a 10.24-fold rise (p < 0.05)..
Interestingly, the accumulation patterns of CKs (tZ, IP, and iP9G) mirrored ethylene, showing minimal levels (≤ 1 ng/g) from 1 to 7 DAH before a significant rise starting at 7 DAH, peaking at 11 or 13 DAH. For example, tZ content in 'Jinyan' at 11 DAH was 97.02 times higher than at 1 DAH (Fig. 2F). Throughout the post-harvest ripening of kiwifruit, GA3 and IAA levels remained below 1 ng/g and showed no significant changes. Additionally, JA levels experienced a sharp increase at 3 DAH (4.66-fold, p < 0.05), followed by a recovery to a similar baseline level at 5 DAH.
Identification of DEGs during postharvest ripening
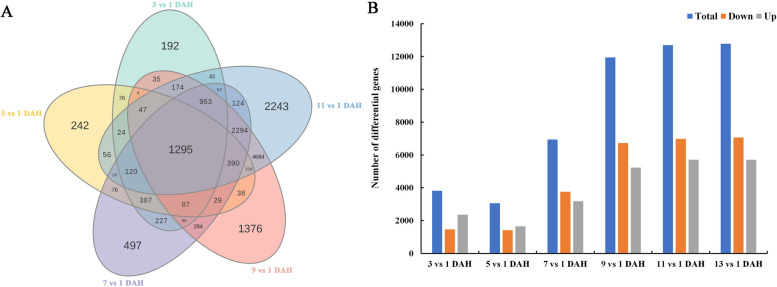
Further transcriptome comparative analysis was conducted by sampling fruits at 1, 3, 5, 7, 9, 11, and 13 DAH, elucidating the potential molecular regulatory mechanisms underlying post-harvest ripening in ‘Jinyan’. A total of 21 samples were divided into six groups for analysis (3 vs 1 DAH, 5 vs 1 DAH, 7 vs 1 DAH, 9 vs 1 DAH, 11 vs 1 DAH, 13 vs 1 DAH; Table S2). Based on the fruit reaching edible maturity at 11 DAH, a Venn diagram was used to analyze the comparative groups of the first five groups (Fig. 3). The comparative analysis showed that 1,295 DEGs overlapped among the 3 vs 1 DAH, 5 vs 1 DAH, 7 vs 1 DAH, 9 vs 1 DAH, and 11 vs 1 DAH groups, respectively. The number of DEGs in the 9 vs 1 DAH group was twice that of the 7 vs 1 DAH group, which had twice as many DEGs as the 5 vs 1 DAH group, while the difference in the number of DEGs between the remaining adjacent comparison groups was not significant. These findings suggest that 7 DAH and 9 DAH may represent crucial periods for molecular regulation of postharvest ripening in 'Jinyan' fruits.
Fig. 3.
Venn diagram comparing numbers of DEGs among the various combinations (A) and column chart of DEGs number in each group (B)
Starting from 3 DAH, the number of enriched GO terms gradually increased, reaching a peak of 558 in the 9 vs 1 DAH group, and then decreased until 13 DAH (Table S3). In all six groups, DEGs were significantly enriched in 54 functional groups across molecular function (MF, 10 subcategories), biological process (BP, 40 subcategories), and cellular component (CC, 4 subcategories), including processes such as glucan metabolic process, regulation of stomatal movement, beta-amylase activity, and photosystem (Table S4).Furthermore, all DEGs with significant matches were mapped to 60 KEGG pathways across five categories: cellular process, environmental information processing, genetic information processing, metabolism, and organismal systems. Starting from 3 DAH, the number of enriched KEGG pathways gradually increased, reaching a maximum in the 13 vs 1 DAH group with a total of 40 pathways. The 9 vs 1 DAH group had 11 more enriched KEGG pathways compared to the 7 vs 1 DAH group, representing the largest difference in pathway enrichment among adjacent groups. Interestingly, a relatively high percentage of the DEGs in all these groups were significantly enriched in pathways such as starch and sucrose metabolism (ko00500), MAPK signaling pathway–plant (ko04016), plant hormone signal transduction (ko04075), and porphyrin metabolism (ko00860) (Table S5). Therefore, DEGs involved in starch metabolism, hormone signaling, MAPK signaling pathway, and other metabolic and environmental information processing events play important roles in the postharvest ripening process of 'Jinyan' fruits (Table S5).
DEGs involved in controlling starch degradation rate
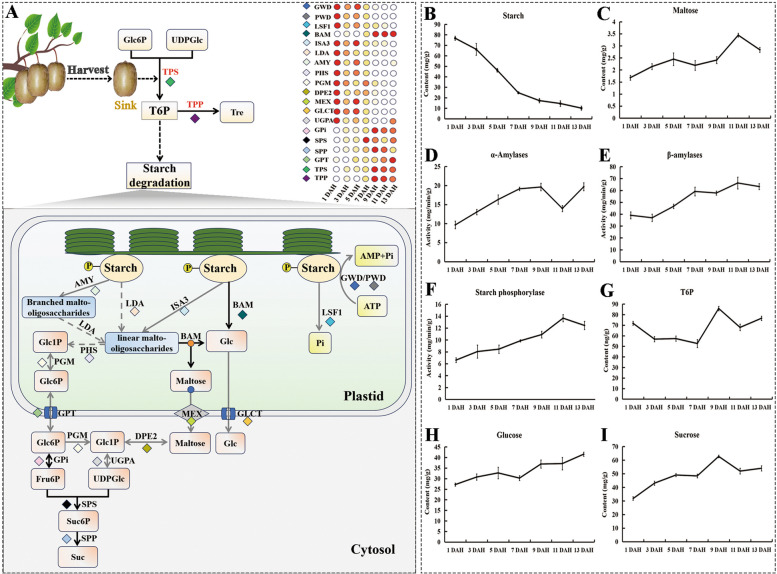
After fruit harvest, the supply of nutrients and water from the tree ceases, and the fruit's respiration relies on its stored nutrients, particularly starch. As illustrated in Fig. 4, the trend of starch degradation closely parallels the decline in fruit hardness. Initially, starch degradation occurs rapidly, followed by a slower phase, which corresponds to the near-edible stage of the fruit.
Fig. 4.
The T6P-SnRK1-TOR-starch metabolism model during postharvest ripening in yellow-fleshed kiwifruit (A), the content of Starch (B), Maltose (C), T6P (G), Glucose (H), Sucrose (I), the activity of α-amylases (D) and β-amylases (E)
Starch content and degradation rate significantly influence the hardness and quality of fruits. To explore the role of starch anabolism in the postharvest ripening of kiwifruit, the expression patterns of seven DEGs encoding 1,4-alpha-glucan-branching enzyme and starch synthase were examined. These genes, including AcGBEs (AcGBE1, AcGBE3.1, AcGBE3.2), AcSSYs (AcSSY3.1, AcSSY3.2, AcSSY4.1) and AcSSG1, exhibited similar expression patterns, with a continuous downregulation of expression levels during the postharvest ripening period of kiwifruit (Table S6). Isoamylase 1 (ISA1) and isoamylase 2 (ISA2), debranching enzymes involved in starch biosynthesis, showed down-regulated expression levels that reached their lowest point at 13 DAH.
In the context of starch and sucrose metabolism, the processes of starch phosphorylation and hydrolysis are crucial for regulating starch metabolism, enabling plants to efficiently store and mobilize energy reserves. In the current study, six AcBAM genes (AcBAM1, AcBAM3.1-AcBAM3.4, AcBAM9), encoding β-amylases, were found to be significantly upregulated when extending the duration of postharvest ripening in kiwifruit (Fig. 4). The qRT-PCR results indicated that the relative expression of AcBAM3.1 and AcBAM3.2 increased more than 150-fold 9 DAH to 13 DAH, compared to 1 DAH. Concomitantly, β-amylase activity mirrored the expression pattern of the AcBAM genes. β-Amylase activity was activated starting from 7 DAH and showed fluctuating upregulation, reaching its peak at 13 DAH, which was 1.62 times higher than that at 1 DAH (Fig. 4).
It has been reported that β-amylase hydrolyzes linear polyglucans to release β-maltose units, which are transported into the cytoplasm via the maltose transporter (MEX) [24]. As one of the downstream products of β-amylase, maltose maintains relatively low levels (below 4 mg/g) but continues to accumulate over postharvest ripening, reaching its highest level at 11 DAH. As shown in Fig. 4, the expression levels of AcMEX1.1 and AcMEX1.2 were significantly downregulated during postharvest ripening.
The activity of α-amylase was notably lower than that of β-amylase, ranging from 0.2 to 0.3 times throughout the post-harvest ripening period of kiwifruit. The enzymes responsible for starch degradation exhibited a gradual, oscillating increase in activity, reaching 203% of the initial level at 9 and 13 DAH (Fig. 4). However, qRT-PCR analysis indicated that the expression of AcAMY3.1 and AcAMY3.2 was progressively down-regulated, extending the duration of postharvest ripening in kiwifruit, with exceptions. Furthermore, isoamylase 3 (ISA3) and limit dextrinase (LDA), which hydrolyze α (1, 6) glucosidic linkages, are crucial for starch degradation. Similar to AcISA1 and AcISA2, the expression levels of the four AcISA3 isoforms (AcISA3.1-AcISA3.4) and AcLDA significantly decreased during postharvest ripening, reaching their lowest point at 13 DAH.
Phosphorylation is a unique covalent modification of starch, influencing its crystallinity, surface charge, hydrophilicity, and chemical stability. This modification plays a crucial role in regulating the timing and spatial aspects of starch degradation, thereby controlling its mobilization and utilization. Four genes encoding glucan water dikinase, namely AcGWD1.1-AcGWD1.3 and AcGWD3, along with one AcPWD gene (phosphoglucan water dikinase), one AcLSF1 gene (phosphoglucan phosphatase-like sex four 2), and four AcPHS genes (alpha-glucan phosphorylase), AcPHS1 and AcPHS2.1-AcPHS2.3, were found to be downregulated during postharvest ripening in kiwifruit. Specifically, AcPHS2.1, which showed a more than 120-fold decrease in expression from 1 to 13 DAH, was chosen for qRT-PCR analysis. The glucose produced by the disproportionating enzyme (DPE) is transported into the cytoplasm via the glucose transporter (GlcT). In this study, similar to the starch phosphorylation and dephosphorylation genes, one AcDEP2 and three AcpGlcT4 genes (AcpGlcT4.1-AcpGlcT4.3) exhibited a consistent downregulation pattern during postharvest ripening.
Research has shown that the SnRK/T6P pathway is crucial for regulating carbon allocation in plant development. The aim of this study was to uncover the mechanisms governing the regulation of the SnRK/T6P pathway during postharvest fruit ripening in kiwifruit. HPLC–MS/MS analysis revealed that the concentration of T6P in kiwifruit fruits decreased gradually over the first seven days after harvest, followed by a significant increase of 17.49 ng/g on the ninth day, which was then followed by a substantial decrease (Fig. 4). As illustrated in Fig. 4 and Fig.S1, the RNA-seq results indicated that one AcSnRK1.1, two AcTPPFs, and three AcTPSs (AcTPS9, AcTPS10, AcTPS11) were upregulated during postharvest ripening in kiwifruit, which was further validated by qRT-PCR. Among these genes, three AcTPSs exhibited slightly different expression patterns, starting their upregulation specifically from 9 DAH rather than at the initial stages. Conversely, the expression levels of two genes encoding Serine/threonine-protein kinase TOR (AcTOR) were downregulated. Overall, the expression profiles of these genes and substances suggest a well-balanced regulatory mechanism in controlling the rate of starch degradation during postharvest ripening in kiwifruit.
DEGs involved in the ABA-CKs-ethylene crosstalk regulatory mechanism
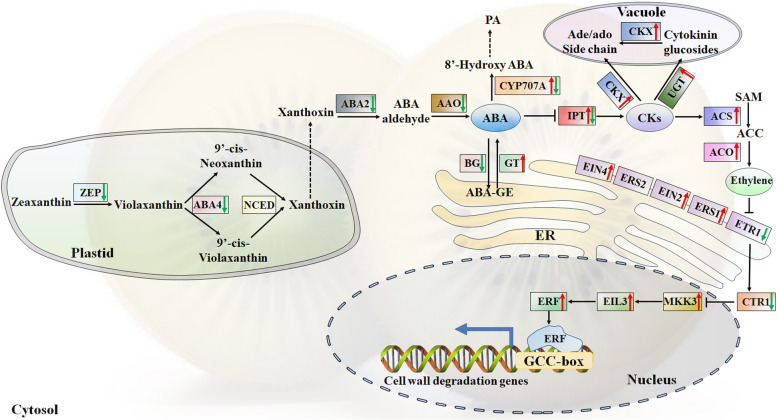
Understanding the influence of phytohormones, including ABA, CKs, and ethylene, on postharvest ripening and their crosstalk is crucial for analyzing the chemical constituents that affect the ripening rate in plants [25]. The primary objective of this study was to explore the mechanisms underlying the changes in ABA during postharvest ripening in kiwifruit. In the ABA synthesis pathway, all six DEGs were identified across the six groups (Fig. 5 and Table S7). Specifically, one zeaxanthin epoxidase (AcZEP), one xanthoxin dehydrogenase (AcABA2), one protein ABA deficient 4 (AcABA4), and one abscisic-aldehyde oxidase (AcAAO3) were down-regulated during postharvest ripening. In contrast, the key ABA biosynthesis genes, the AcNCEDs, exhibited very low expression levels, with an average expression level around 1. In the ABA catabolism pathway, CYP707A, which encodes ABA 8’-hydroxylase, plays a key role in oxidative catabolism and influences the dynamic equilibrium of ABA content [26]. The expression patterns of the twoAcCYP707A genes exhibited a similar trend to that of ABA levels. Specifically, the expression level of AcCYP707A.2was significantly increased during the first 3 DAH, reaching a 13.56-fold increase, followed by a return to basal levels comparable to the control at 13 DAH. Furthermore, the regulation of cellular ABA levels involves the ABA-glucose conjugation pathway, where ABA is conjugated by cytosolic UDP-glucosyltransferases (GTs) or released by β-glucosidases (BGs) [27]. These processes, facilitated by intracellular or intertissue transport, contribute to the modulation of ABA homeostasis. In the present study, the expression level ofAcGT29 was significantly increased, reaching more than 1266-fold from 9 to 13 DAH compared to 1 DAH. Conversely, two AcBG genes (AcBG11 and AcBG47) were downregulated when the duration of postharvest ripening was extended.
Fig. 5.
The ABA-CKs-ethylene-cell wall degradation model during postharvest ripening in yellow-fleshed kiwifruit. Red and green arrows denote an increase and decrease, respectively. The annotation of these genes is provided in Table S7. The expression patterns of key genes, as determined by qRT-PCR analysis, are illustrated in Fig. S1. Enzyme and chemical names are abbreviated as follows; BG: beta-glucosidase; GT: UDP-glucosyltransferase; IPT: adenylate isopentenyltransferase; ZEP: zeaxanthin epoxidase; CKX: cytokinin dehydrogenase; NCED: 9-cis-epoxycarotenoid dioxygenase; AAO: indole-3-acetaldehyde oxidase; CYP707A: abscisic acid 8'-hydroxylase; ACS: 1-amino-cyclopropane-1-carboxylate synthases; EIN: ethylene-insensitive; EIL: ethylene insensitive; ACO: aminocyclopropanecarboxylate oxidase; CTR: serine/threonine protein kinase; ETR: ethylene receptor; ERF: ethylene response factors; ERS: ethylene response sensor; MKK: mitogen-activated protein kinase kinase
CKs are pivotal in various aspects of plant growth and development [28]. The homeostasis of CKs in plant cells is tightly regulated through the synergistic actions of isopentenyltransferase (IPT) and CK oxidase/dehydrogenase (CKX) enzymes, which are influenced by the plant's developmental stage and environmental conditions [29]. In this study, the concentration of CKs begins to increase from the 7 DAH and is positively correlated with the postharvest ripening of kiwifruit. This finding contrasts with a previous study on banana, where exogenous CKs were found to delay the transition from green to yellow by regulating CKs oxidase and antioxidant activity [30]. Additionally, the expression of AcIPT was significantly increased during the first 9 DAH, subsequently returning to basal levels similar to 1 DAH at 13 DAH. The qRT-PCR results showed that the relative expression ofAcIPT was 3.97-fold higher compared with the control at 9 DAH (Fig. S1). During the first 7 DAH, the expression levels of AcCKX6.1 and AcCKX6.2 were lower than or equal to 0.1, but significantly increased from 9 DAH and reached a maximum level at 11 DAH or 13 DAH. Among these two genes, the expression of AcCKX6.2was increased 197-fold compared with the control at 13 DAH during fruit postharvest ripening in kiwifruit by qRT-PCR (Fig. S1). Upon CK perception, the autophosphorylation of AHKs occurs, leading to the subsequent transfer of the phosphoryl group to the acceptor site of the AHKs. Subsequently, AHPs receive the phosphoryl groups from AHKs and facilitate their transfer to nuclear type B ARRs. These type B ARRs, in turn, play a crucial role in regulating the expression of downstream target genes, including type A ARRs [31]. In the present study, the expression level of twoAcAHP1 genes was decreased, whereas AcAHK3 was significantly up-regulated at 9 DAH. In this study, three AcARRs genes, including AcARR6, AcARR9, and AcARR17, were up-regulated during postharvest ripening in kiwifruit. By contrast, AcARR2 and AcARR4 presented a different pattern and were down-regulated when extending the duration of ripening at first 11 DAH. As a CK response factor, the expression level of AcCRF5 was increased gradually and reached the highest level at 13 DAH (threefold, P < 0.05), whereas AcCRF2was up-regulated at first 5 DAH and then returned to basal levels similar to the level of 1 DAH. Additionally, CKs are mostly present as glycosides in plants, and their glycosylation modifications are catalyzed by family 1 glycosyltransferases in plants [32]. In the present study, oneAcUGT73C4 gene encoding CK glycosyltransferase was significantly up-regulated, reaching 1297% of the 13 DAH (Table S7).
CK has been reported to stimulate ethylene production by modulating the stability of ACS proteins, thereby enhancing ethylene production in plants [33, 34]. As a key hormone that triggers various aspects of fruit ripening, ethylene plays a significant role in the postharvest ripening of kiwifruit. To investigate this role, the expression patterns of DEGs encoding key enzymes in ethylene biosynthesis and metabolism were analyzed. The expression patterns of DEGs encoding ethylene biosynthesis enzymes were identified and analyzed. As illustrated in Fig. 5, three AcACS genes and seven AcACO genes were significantly upregulated. The expression patterns of AcACS and AcACO genes showed a similar trend of gradual upregulation, reaching their peak at 11 or 13 DAH (Fig. 5). The qRT-PCR results indicated that the relative expression of AcACS3 and AcACO5 was 3409-fold and 463-fold higher, respectively, compared to the control at 13 DAH (Fig. S1). In the ethylene signaling pathway, 12 DEGs were identified across the six groups. As depicted in Fig. 5 and Table S7, one AcEIN2, one AcEIN4, one AcEIL3, one AcMKK3, and three AcERS1 genes were upregulated from 3 DAH. Conversely, the primary negative regulators of ethylene signaling, AcCTR1 and AcETR1, were downregulated from 3 to 13 DAH, with the lowest expression levels observed at 13 DAH (more than 0.17-fold downregulated compared to the control). In climacteric fruits, several ERFs have been implicated in cell wall degradation and softening. The expression levels of AcERF3, AcERF8, and AcERF71 were upregulated during postharvest ripening in kiwifruit. Due to their higher expression levels compared to 1 DAH, AcERF8 was selected for qRT-PCR analysis. As shown in Fig. S1, AcERF8 exhibited more than a tenfold upregulation at 9 DAH compared to 7 DAH.
Softening in kiwifruit is primarily attributed to the degradation of cell walls and a reduction in intercellular adhesion, processes that are catalyzed by a suite of enzymes, including pectin methylesterase (PME), polygalacturonase (PG), and pectate lyase (PL) [35]. As indicated in Table S7, several genes were differentially expressed during the postharvest ripening process. Specifically, twoAcPG, one gene encoding a beta-D-xylosidase 1 (AcBXL1), one gene encoding an expansin-like B1 (AcEXLB1), two genes encoding beta-galactosidase (AcBGA), five genes encoding xyloglucan endotransglucosylase/hydrolase (AcXTH), two genes encoding beta-galactosidase (AcBGAL), and four genes encoding alpha-expansin (AcEXPA) were upregulated. Among these, AcBGA16.1 and AcBGA16.2 exhibited more than a 700-fold increase in expression during postharvest ripening in kiwifruit. Based on the RNA-seq data, genes such as AcPG1, AcPG2, AcBGAL.2, AcEXPA4, and AcEXPA6, which showed higher expression levels during the later stages of postharvest ripening in kiwifruit, were selected for further validation using qRT-PCR. The results of qRT-PCR confirmed the expression patterns observed in the RNA-seq data. Overall, these genes are likely to play crucial roles in the regulation of postharvest ripening in yellow-fleshed kiwifruit.
Discussion
Kiwifruit, a globally consumed fruit, undergoes a post-harvest ripening process to develop its distinctive flavor, rendering it edible. However, the uncontrollable ripening pace and high loss rate in many kiwifruit varieties pose significant challenges to the industry, reducing consumer purchasing interest. Previous research has identified various factors influencing the post-harvest ripening of kiwifruit, particularly in varieties with red hearts or green flesh. Nonetheless, the regulatory mechanisms governing the post-harvest ripening of yellow-fleshed kiwifruit, involving plant hormones, sugars, key genes, and other factors, remain elusive. This study elucidated significant variations in endogenous plant hormones (ABA, ethylene, cytokinins, etc.), physiological and biochemical indicators (enzyme activities, starch, sugars, etc.), and the expression patterns of key genes throughout the post-harvest ripening of kiwifruit. These findings highlight the potential roles of the T6P-starch metabolism and ABA-CKs-ethylene-cell wall degradation models in the post-harvest ripening of yellow-fleshed kiwifruit, as discussed in the following sections.
In the context of postharvest considerations, the presence of starch in climacteric-ripening fruits is crucial as an energy source, sustaining biological processes and facilitating the synthesis of quality-related metabolites, thereby minimizing loss and waste [14]. Kiwifruit, as a transitory-storage starch fruit, undergoes distinct processes of net starch synthesis before harvest and degradation after harvest [36]. In the starch metabolism pathway, it has been reported that phosphorylation, catalyzed by glucan water dikinase and phosphoglucan water dikinase, is the sole natural covalent modification of starch that influences its crystallinity, surface charge, hydrophilicity, and chemical susceptibility, enabling starch to undergo subsequent hydrolysis (Mahlow et al. [37]). Bananas, as a representative climacteric fruit, exhibit a progressive increase in the activities of starch-hydrolyzing enzymes, including AMY, BAM, and AGL, which closely correlate with starch degradation during the ripening process. Conversely, the activities of starch phosphorylating enzymes, such as ISA, PU, and PHS, remain relatively constant at low levels in bananas [38, 39]. However, in the present study, the expression level of genes encoding phosphorylase (AcGWDs, AcPWD, AcPHSs) was significantly higher during the early stage of postharvest ripening compared to the later stage. The starch phosphorylase activity was significantly activated from 3 DAH, indicating that the high expression of genes during the early stage promotes the enhancement of enzyme activity. Consistent with starch phosphorylase activity, six DEGs encoding β-amylase were up-regulated in kiwifruit during postharvest ripening. However, in this study, the regulation of α-amylase is similar to that of starch phosphorylase, wherein the gene expression levels are higher in the early stage of postharvest ripening of kiwifruit, while the enzyme activity is higher in the later stage. Additionally, the activity of β-amylase is significantly higher than that of α-amylase, indicating that postharvest starch hydrolysis in kiwifruit primarily depends on β-amylase. Among these AcBAM genes, the expression levels of AcBAM3s and AcBAM9s are hundreds of times higher than AcBAM1, suggesting that the former genes play a more crucial role in starch degradation during postharvest ripening of kiwifruit.
During the postharvest ripening, there is a significant increase in the levels of soluble sugars such as sucrose, glucose, and maltose in this study. It has been reported that the increase in glucose content in the starch degradation pathway is primarily regulated by the DEP1gene [40]. However, none of theAcDEP1 genes were identified from the differentially expressed genes, indicating that the detected glucose in kiwifruit may be derived from the catalytic action of β-amylase rather than through DEP1. Overall, these results suggest that during the postharvest ripening process of yellow-fleshed kiwifruit, starch remobilization initially relies on phosphorylase-mediated covalent modification, followed by a starch hydrolysis process primarily regulated by the AcBAM3 genes.
In plants, the starch pools synthesized in photosynthetic tissues, including leaves, and non-storage organs such as developing seeds, tubers, and fruits, are critical for driving growth and development [41]. Starch serves as an energy reservoir, mobilized and transported to support various metabolic processes and growth requirements in different plant organs [42]. It provides a readily available energy source for the crucial stages of growth and development in non-storage sinks [42, 43]. The activity of T6P/SnRK1 is considered a key regulator of assimilate allocation between source and sink organs in plants, maintaining carbon partitioning balance through the regulation of starch metabolism (including accelerated synthesis or remobilization) [44, 45]. In the present study, as the harvested fruit ripening, there is a continuous reduction in starch content and an accumulation of sucrose. However, the T6P levels also gradually decrease, contrary to the T6P-sucrose regulatory mechanism observed in plant leaves. This suggests that the carbon flux within the harvested fruit may no longer adhere to the source-sink interaction regulated by T6P. In kiwifruit, the harvested fruit severs its source, resulting in inadequate T6P supply, which activates the SnRK1 kinase. Consequently, the TOR kinase signaling pathway, regulating energy-consuming biological processes such as plant growth, is suppressed to ensure the maintenance of energy reserves. However, the harvested fruit must utilize stored carbohydrates, such as starch or sugars, through respiration to undergo a series of physiological and biochemical changes, generating energy to sustain life [46]. Therefore, the T6P-SnRK1-TOR pathway plays a regulatory role in yellow-fleshed kiwifruits during postharvest ripening, limiting excessive starch degradation that could lead to accelerated aging and decay.
It is widely accepted that CKs act antagonistically to ABA in various growth and physiological processes. Recent research has illuminated the complex interactions and crosstalk between CKs and ABA, as well as their signaling pathways 47, 30]. The biosynthesis and metabolism of CKs begin with the activity of IPT, which is inhibited by ABA [48]. In this study, the decrease in ABA content from 3 to 13 DAH resulted in a reduced inhibition of IPT, leading to an increased accumulation of CKs within the fruit. In nature, CKs predominantly exist as glycosides, serving as an important mechanism for regulating the metabolic balance of CKs within plants [49, 50]. Glycosylation plays a significant role in various plant processes, including CK transport, normal growth, and development [49]. Family 1 glycosyltransferases (GT1) act as the primary catalysts for CK glycosylation and are conserved across different plant species [51]. In the present study, the expression levels of oneAcUGT73C4 and two AcCKX6 genes were significantly upregulated, suggesting that the synthesis of CKs during the postharvest ripening process of kiwifruit relies on IPT, while their dynamic balance is determined by glycosylation mediated by CKX and GT.
ABA has been demonstrated to regulate ethylene production in apples, tomatoes, and various plant tissues [52, 53]. In tomatoes, ABA treatment significantly elevates ethylene levels and upregulatesLeACS2 expression, whereas the application of NDGA, an ABA biosynthesis inhibitor, suppresses LeACS2expression [53, 54]. Studies on CKs have shown that they influence the stability of ACS proteins, thereby promoting ethylene production in plants [34, 55]. Genetic investigations have confirmed that the CK-mediated stabilization of ACS proteins is dependent on a functional CK signaling pathway [34]. In this study, the upregulation of CKs content precedes the period of increasedAcACS gene expression and ethylene accumulation. Unlike Arabidopsis, the differentially expressed ACS genes were primarily AcACS1 and AcACS3, suggesting that AcACS1 and AcACS3, rather than AcACS5, play important roles in the CK-mediated ACS process in kiwifruit postharvest ripening.
In climacteric fruits, ethylene acts as the primary regulator of the ripening process [56]. In this study, ethylene levels increased significantly from 9 DAH and reached a peak at 13 DAH, approximately 12.08 times higher than the levels observed during the first 9 days of postharvest ripening in kiwifruit. In contrast, the peak in ethylene production was significantly delayed by 6 days compared to ABA. ABA reached its maximum level at 3 DAH, which was 5.86 times higher than the edible period, suggesting that ABA might promote ethylene production in yellow-fleshed kiwifruit. ERF transcription factors, essential components of the ethylene signaling pathway, play critical roles in ripening processes, including ethylene biosynthesis, color change, and fruit postharvest softening, across various fleshy fruit species [57]. MdERF3 has been identified as a regulator of fruit ripening and ethylene biosynthesis through modulation of MdACS1transcription in apple [58]. However, in the current study, unlike apple, the expression pattern ofAcERF3 was consistent with AcACS1, suggesting that ethylene production mediated by AcACSs may not be regulated by AcERFs.
Several ERF transcription factors have been identified to play crucial roles in the process of cell wall degradation and fruit softening in various climacteric fruits [57, 59]. For instance, in tomato, the enhanced expression of the dominantSlERF.B3 variant has been shown to accelerate fruit softening and upregulate the expression level of SlPG2a, highlighting its significant involvement in fruit ripening and softening [60]. Similarly, in persimmon, the ERF transcription factors DkERF8/19 have been found to play an important role in fruit softening by directly binding to the DkXTH9 promoter through recognizing the DRE motif [61]. Additionally, it has been observed that DkERF8/19 can interact with DkNAC9 to activate DkEGase1, ultimately promoting the softening process [62]. In the present study, the expression levels of theAcPGs, AcXTHs, AcBGAs, AcBGALs, and AcEXPAs genes were significantly up-regulated, with the highest levels reached during the late stage of postharvest ripening in yellow-fleshed kiwifruit. Overall, the crosstalk regulation between ABA-CKs-ethylene pathways promotes cell wall degradation and ultimately accelerates the postharvest ripening of yellow-fleshed kiwifruit.
Conclusions
This study offers critical insights into the hormonal and molecular mechanisms underlying postharvest ripening in yellow-fleshed kiwifruit. It comprehensively investigates the potential roles of endogenous hormones, enzyme activities, physiological changes, and gene expression models during postharvest ripening. During the postharvest ripening process of yellow-fleshed kiwifruit, starch remobilization initially relies on phosphorylase-mediated covalent modification, followed by a starch hydrolysis process primarily regulated by the AcBAM3 genes. Transcriptome analyses suggest that the T6P-SnRK1-TOR pathway regulates the rate of starch degradation to prevent excessive degradation, which could potentially lead to postharvest aging and loss of flavor in fruits. Additionally, the results indicate that the ripening process is positively correlated with increased levels of endogenous ethylene, CKs, and ABA-GE, while showing a negative correlation with TRP in yellow-fleshed kiwifruit. ABA accumulates to its highest level at 3 DAH and subsequently decreases in a regulated manner. During postharvest ripening and softening in yellow-fleshed kiwifruit, there is crosstalk among the ABA-CKs-ethylene signaling pathways, which induces the expression of cell wall degradation genes, activates the activity of degrading enzymes, and ultimately promotes the postharvest ripening of yellow-fleshed kiwifruit. Further research should explore the functions of key genes in these models and investigate the potential of CKs, ABA, TRP, and other factors to identify efficient and environmentally friendly treatments to artificially regulate the postharvest softening time of kiwifruit, thereby enhancing fruit quality.
Supplementary Information
Acknowledgements
Not applicable.
Authors’ contributions
Mengfei Lin: Writing original draft, software, data curation, funding acquisition. Xiaoling Wang: Conceptualization, revise manuscript, validation, supervision. Zhu Gao: Software, supervision. Jipeng Mao and Liuyi Pan: Resources, investigation, methodology. Xuchen Gong, Dongliang Yao, and Huiqi Zhong: Investigation, formal analysis. Heqiang Huo: Validation, Supervision.
Funding
This work is supported by Jiangxi Talent Team Plan [20232BCJ23045], Jiangxi Natural Science Foundation [20232BAB215039], Scientific Research Project of Jiangxi Academy of Sciences [2023YSBG50002, 2022YSBG22004, 2022YYB21].
Data availability
We have uploaded the raw transcriptome data to the National Genomics Data center, the number is PRJCA028378.
Declarations
Ethics approval and consent to participate
The authors declare that the experimental research conducted on the plants described in this paper adheres to institutional, national, and international guidelines. The collection of fruits was carried out with full permission from the Jiangxi Academy of Sciences. Sampling was conducted at the Fengxin County Doctor Kiwifruit Base in Yichun City, China (114°45’ E, 28°34’ N), which is managed by the Jiangxi Academy of Sciences, with all necessary authorizations in place.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gearry, R., Fukudo, S., Barbara, G., Kuhn-Sherlock, B., Ansell, J., Blatchford, P., Eady, S., Wallace, A., Butts, C., Cremon, C., Barbaro, M.R., Pagano, I., Okawa, Y., Muratubaki, T., Okamoto, T., Fuda, M., Endo, Y., Kano, M., Kanazawa, M., Nakaya, N., Nakaya, K., Drummond, L., 2023. Consumption of 2 Green Kiwifruits Daily Improves Constipation and Abdominal Comfort-Results of an International Multicenter Randomized Controlled Trial. Am J Gastroenterol. 118 (6), 1058–1068. 10.14309/ajg.0000000000002124 [DOI] [PMC free article] [PubMed]
- 2.Lin M, Gao Z, Wang X, Huo H, Mao J, Gong X, Chen L, Ma S, Cao Y. Eco-friendly managements and molecular mechanisms for improving postharvest quality and extending shelf life of kiwifruit: A review. Int J Biol Macromol. 2024;257(1): 128450. 10.1016/j.ijbiomac.2023.128450. [DOI] [PubMed] [Google Scholar]
- 3.Li D, Liu Y. Gene Introgression from Wild Relatives. Cham: The Kiwifruit Genome. Springer International Publishing; 2016. p. 237–48. [Google Scholar]
- 4.Sharma K, Kumar R, Kumar A. Himalayan Horticulture Produce Supply Chain Disruptions and Sustainable Business Solution—A Case Study on Kiwi Fruit in Uttarakhand. Horticulturae. 2022;8(11):1018. 10.3390/horticulturae8111018. [Google Scholar]
- 5.Zhang H, Serwah Boateng NA, Ngolong Ngea GL, Shi Y, Lin H, Yang Q, Wang K, Zhang X, Zhao L, Droby S. Unravelling the fruit microbiome: The key for developing effective biological control strategies for postharvest diseases. Compr Rev Food Sci Food Saf. 2021;20(5):4906–30. 10.1111/1541-4337.12783. [DOI] [PubMed] [Google Scholar]
- 6.Zhang W, Jiang H, Cao J, Jiang W. Advances in biochemical mechanisms and control technologies to treat chilling injury in postharvest fruits and vegetables. Trends Food Sci Tech. 2021;113:355–65. 10.1016/j.tifs.2021.05.009. [Google Scholar]
- 7.Nath P, Sane AP, Trivedi PK, Sane VA, Asif MH. Role of transcription factors in regulating ripening, senescence and organ abscission in plants. Stewart Postharvest Review. 2007;3(2):1–14. 10.2212/spr.2007.2.6. [Google Scholar]
- 8.Xiang W, Wang H-W, Sun D-W. Phytohormones in postharvest storage of fruit and vegetables: mechanisms and applications. Crit Rev Food Sci. 2021;61(18):2969–83. 10.1080/10408398.2020.1864280. [DOI] [PubMed] [Google Scholar]
- 9.Gupta K, Wani SH, Razzaq A, Skalicky M, Samantara K, Gupta S, Pandita D, Goel S, Grewal S, Hejnak V, Shiv A, El-Sabrout AM, Elansary HO, Alaklabi A, Brestic M. Abscisic Acid: Role in Fruit Development and Ripening. Front Plant Sci. 2022;13: 817500. 10.3389/fpls.2022.817500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aremu AO, Fawole OA, Makunga NP, Masondo NA, Moyo M, Buthelezi NMD, Amoo SO, Spíchal L, Doležal K. Applications of Cytokinins in Horticultural Fruit Crops: Trends and Future Prospects. Biomol. 2020;10(9):1222. 10.3390/biom10091222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji Y, Qu Y, Jiang Z, Yan J, Chu J, Xu M, Su X, Yuan H, Wang A. The mechanism for brassinosteroids suppressing climacteric fruit ripening. Plant Physiol. 2021;185(4):1875–93. 10.1093/plphys/kiab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trainotti L, Tadiello A, Casadoro G. The involvement of auxin in the ripening of climacteric fruits comes of age: the hormone plays a role of its own and has an intense interplay with ethylene in ripening peaches. J Exp Bot. 2007;58(12):3299–308. 10.1093/jxb/erm178. [DOI] [PubMed] [Google Scholar]
- 13.Xiong T, Tan Q, Li S, Mazars C, Galaud J-P, Zhu X. Interactions between calcium and ABA signaling pathways in the regulation of fruit ripening. J Plant Physiol. 2021;256: 153309. 10.1016/j.jplph.2020.153309. [DOI] [PubMed] [Google Scholar]
- 14.Yu J, Tseng Y, Pham K, Liu M, Beckles DM. Starch and sugars as determinants of postharvest shelf life and quality: some new and surprising roles. Curr Opin Biotech. 2022;78: 102844. 10.1016/j.copbio.2022.102844. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Wang J, Mujumdar AS, Jin X, Liu Z-L, Zhang Y, Xiao H-W. Effects of postharvest ripening on physicochemical properties, microstructure, cell wall polysaccharides contents (pectin, hemicellulose, cellulose) and nanostructure of kiwifruit (Actinidia deliciosa). Food Hydrocolloid. 2021;118: 106808. 10.1016/j.foodhyd.2021.106808. [Google Scholar]
- 16.Garcia CV, Quek S-Y, Stevenson RJ, Winz RA. Kiwifruit flavour: A review. Trends Food Sci Tech. 2012;24(2):82–91. 10.1016/j.tifs.2011.08.012. [Google Scholar]
- 17.Gao Y, Ping H, Li B, Li Y, Zhao F, Ma Z. Characterization of free, conjugated, and bound phenolics in early and late ripening kiwifruit cultivars. J Sci Food Agr. 2021;101(11):4743–50. 10.1002/jsfa.11120. [DOI] [PubMed] [Google Scholar]
- 18.Tian S, Qu M, Xu H. Establishment of evaluation criterion based on starch dyeing method and implementation of optical and acoustic techniques for postharvest determination of “HongYang” kiwifruit ripeness. Eur J Agron. 2023;142: 126682. 10.1016/j.eja.2022.126682. [Google Scholar]
- 19.Schluepmann H, Pellny T, van Dijken A, Smeekens S, Paul M. Trehalose 6-phosphate is indispensable for carbohydrate utilization and growth in Arabidopsis thaliana. P Natl A Sci. 2003;100(11):6849–54. 10.1073/pnas.1132018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urano K, Maruyama K, Jikumaru Y, Kamiya Y, Yamaguchi-Shinozaki K, Shinozaki K. Analysis of plant hormone profiles in response to moderate dehydration stress. Plant J. 2017;90:17–36. 10.1111/tpj.13460. [DOI] [PubMed]
- 21.Lin, M., Wang, S., Liu, Y., Li, J., Zhong, H., Zou, F., Yuan, D., 2022. Hydrogen cyanamide enhances flowering time in tea oil camellia (Camellia oleifera Abel.). Ind. Crop. Prod. 176, 114313. 10.1016/j.indcrop.2021.114313
- 22.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Mei Yan C, Ting Ting Z, Xiao Li L, Fei H, Peng Z, Cai Hong Z. Factor analysis and comprehensive evaluation of fruit quality of ‘Jinyan’kiwifruit (Actinidia eriantha× Actinidia chinensis). Plant Sci J. 2021;39(1):85–92. 10.11913/PSJ.2095-0837.2021.10085.
- 24.Santelia D, Trost P, Sparla F. New insights into redox control of starch degradation. Curr Opin Plant Biol. 2015. 10.1016/j.pbi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Fenn MA, Giovannoni JJ. Phytohormones in fruit development and maturation. Plant J. 2021;105(2):446–58. 10.1111/tpj.15112. [DOI] [PubMed] [Google Scholar]
- 26.Kushiro T, Okamoto M, Nakabayashi K, Yamagishi K, Kitamura S, Asami T, Hirai N, Koshiba T, Kamiya Y, Nambara E. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: key enzymes in ABA catabolism. EMBO J. 2004;23(7):1647–56. 10.1038/sj.emboj.7600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan W, Liang J, Sui J, Li J, Liu C, Xin Y, Zhang Y, Wang S, Zhao Y, Zhang J, Yi M, Gazzarrini S, Wu J. ABA and Bud Dormancy in Perennials: Current Knowledge and Future Perspective. Genes. 2021;12(10):1635. 10.3390/genes12101635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zubo YO, Blakley IC, Yamburenko MV, Worthen JM, Street IH, Franco-Zorrilla JM, Zhang W, Hill K, Raines T, Solano R, Kieber JJ, Loraine AE, Schaller GE. Cytokinin induces genome-wide binding of the type-B response regulator ARR10 to regulate growth and development in Arabidopsis. P Natl A Sci. 2017;114(29):E5995–6004. 10.1073/pnas.1620749114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakakibara, H., 2010. Cytokinin Biosynthesis and Metabolism, Plant Hormones: Biosynthesis, Signal Transduction, Action! Springer Netherlands, Dordrecht. 95–114. 10.1007/978-1-4020-2686-7_5
- 30.Huang X, Hou L, Meng J, You H, Li Z, Gong Z, Yang S, Shi Y. The Antagonistic Action of Abscisic Acid and Cytokinin Signaling Mediates Drought Stress Response in Arabidopsis. Mol Plant. 2018;11(7):970–82. 10.1016/j.molp.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Li T, Jiang Z, Zhang L, Tan D, Wei Y, Yuan H, Li T, Wang A. Apple (Malus domestica) MdERF2 negatively affects ethylene biosynthesis during fruit ripening by suppressing MdACS1 transcription. Plant J. 2016;88(5):735–48. 10.1111/tpj.13289. [DOI] [PubMed] [Google Scholar]
- 32.Koeslin-Findeklee, F., Becker, M. A., van der Graaff, E., Roitsch, T., & Horst, W. J. (2015). Differences between winter oilseed rape (Brassica napus L.) cultivars in nitrogen starvation-induced leaf senescence are governed by leaf-inherent rather than root-derived signals. J. Exp. Bot. 66(13), 3669–3681. 10.1093/jxb/erv170 [DOI] [PMC free article] [PubMed]
- 33.Chae HS, Faure F, Kieber JJ. The eto1, eto2, and eto3 Mutations and Cytokinin Treatment Increase Ethylene Biosynthesis in Arabidopsis by Increasing the Stability of ACS Protein. Plant Cell. 2003;15(2):545–59. 10.1105/tpc.006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen M, Chae HS, Kieber JJ. Regulation of ACS protein stability by cytokinin and brassinosteroid. Plant J. 2009;57(4):606–14. 10.1111/j.1365-313X.2008.03711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tucker G, Yin X, Zhang A, Wang M, Zhu Q, Liu X, Xie X, Chen K, Grierson D. Ethylene and fruit softening. Food Qual Saf. 2017;1(4):253–67. 10.1093/fqsafe/fyx024. [Google Scholar]
- 36.Luengwilai K, Beckles DM. Starch Granules in Tomato Fruit Show a Complex Pattern of Degradation. J Agric Food Chem. 2009;57(8):8480–7. 10.1021/jf901593m. [DOI] [PubMed] [Google Scholar]
- 37.Mahlow S, Orzechowski S, Fettke J. Starch phosphorylation: insights and perspectives. Cell Mol Life Sci. 2016;73(14):2753–64. 10.1007/s00018-016-2248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bassinello PZ, Cordenunsi BR, Lajolo FM. Amylolytic Activity in Fruits: Comparison of Different Substrates and Methods Using Banana as Model. J Agric Food Chem. 2002;50(21):5781–6. 10.1021/jf011370p. [DOI] [PubMed] [Google Scholar]
- 39.Da Mota R, Cordenunsi B, Do Nascimento J, Purgatto E, Rosseto M, Lajolo F. Activity and expression of banana starch phosphorylases during fruit development and ripening. Planta. 2002;216(2):325–33. 10.1007/s00425-002-0858-6. [DOI] [PubMed] [Google Scholar]
- 40.Zhao, Mingzhu, Zhao, Minghui, Gu, S., Sun, J., Ma, Z., Wang, L., Zheng, W., Xu, Z., 2019. DEP1 is involved in regulating the carbon–nitrogen metabolic balance to affect grain yield and quality in rice (Oriza sativa L.). PLOS ONE. 14 (3), e0213504. 10.1371/journal.pone.0213504. [DOI] [PMC free article] [PubMed]
- 41.Sulpice R, Pyl E-T, Ishihara H, Trenkamp S, Steinfath M, Witucka-Wall H, Gibon Y, Usadel B, Poree F, Piques MC, Von Korff M, Steinhauser MC, Keurentjes JJB, Guenther M, Hoehne M, Selbig J, Fernie AR, Altmann T, Stitt M. Starch as a major integrator in the regulation of plant growth. P Natl A Sci. 2009;106(25):10348–53. 10.1073/pnas.0903478106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacNeill GJ, Mehrpouyan S, Minow MAA, Patterson JA, Tetlow IJ, Emes MJ. Starch as a source, starch as a sink: the bifunctional role of starch in carbon allocation. J Exp Bot. 2017;68(16):4433–53. 10.1093/jxb/erx291. [DOI] [PubMed] [Google Scholar]
- 43.Sharma G, Sharma P. Chitosan nanofertilizer boost source activity in plant. J Plant Nutr. 2021;44(16):2486–99. 10.1080/01904167.2021.1918159. [Google Scholar]
- 44.Liu H, Si X, Wang Z, Cao L, Gao L, Zhou X, Wang W, Wang K, Jiao C, Zhuang L, Liu Y, Hou J, Li T, Hao C, Guo W, Liu J, Zhang X. TaTPP-7A positively feedback regulates grain filling and wheat grain yield through T6P-SnRK1 signalling pathway and sugar–ABA interaction. Plant Biotechnol J. 2023;21(6):1159–75. 10.1111/pbi.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu S-M, Lo S-F, Ho T-HD. Source-Sink Communication: Regulated by Hormone, Nutrient, and Stress Cross-Signaling. Trends Plant Sci. 2015;20(12):844–57. 10.1016/j.tplants.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Yang F, Zhao R, Suo J, Ding Y, Tan J, Zhu Q, Ma Y. Understanding quality differences between kiwifruit varieties during softening. Food Chem. 2024;430: 136983. 10.1016/j.foodchem.2023.136983. [DOI] [PubMed] [Google Scholar]
- 47.Hai NN, Chuong NN, Tu NHC, Kisiala A, Hoang XLT, Thao NP. Role and Regulation of Cytokinins in Plant Response to Drought Stress. Plants. 2020;9(4):422. 10.3390/plants9040422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rivero RM, Gimeno J, Van Deynze A, Walia H, Blumwald E. Enhanced Cytokinin Synthesis in Tobacco Plants Expressing PSARK::IPT Prevents the Degradation of Photosynthetic Protein Complexes During Drought. Plant Cell Physiol. 2010;51(11):1929–41. 10.1093/pcp/pcq143. [DOI] [PubMed] [Google Scholar]
- 49.Chen L, Zhao J, Song J, Jameson PE. Cytokinin glucosyl transferases, key regulators of cytokinin homeostasis, have potential value for wheat improvement. Plant Biotechnol J. 2021;19(5):878–96. 10.1111/pbi.13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Šmehilová M, Dobrůšková J, Novák O, Takáč T, Galuszka P. Cytokinin-Specific Glycosyltransferases Possess Different Roles in Cytokinin Homeostasis Maintenance. Front Plant Sci. 2016;7:1264. 10.3389/fpls.2016.01264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo, L., 2024. Glycosylation of plant secondary metabolites: the regulation from chaos to harmony. Authorea Preprints.
- 52.Fernández-Cancelo P, Muñoz P, Echeverría G, Larrigaudière C, Teixidó N, Munné-Bosch S, Giné-Bordonaba J. Ethylene and abscisic acid play a key role in modulating apple ripening after harvest and after cold-storage. Postharvest Biol Tec. 2022;188: 111902. 10.1016/j.postharvbio.2022.111902. [Google Scholar]
- 53.Zhang M, Yuan B, Leng P. The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J Exp Bot. 2009;60(6):1579–88. 10.1093/jxb/erp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mou W, Li D, Bu J, Jiang Y, Khan ZU, Luo Z, Mao L, Ying T. Comprehensive Analysis of ABA Effects on Ethylene Biosynthesis and Signaling during Tomato Fruit Ripening. PLoS ONE. 2016;11(4): e0154072. 10.1371/journal.pone.0154072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamoune A, Zdarska M, Depaepe T, Korytarova A, Skalak J, Berendzen KW, Mira-Rodado V, Tarr P, Spackova E, Badurova L, Parizkova B, Franczyk A, Kovacova I, Pernisova M, Novak O, Meyerowitz E, Harter K, Straeten DVD, Hejatko J. Cytokinins regulate spatially-specific ethylene production to control root growth in Arabidopsis. Plant Com. 2023. 10.1101/2023.01.07.522790. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Tang M, Liu M, Su D, Chen J, Gao Y, Bouzayen M, Li Z. The Molecular Regulation of Ethylene in Fruit Ripening. Small Methods. 2020;4(8):1900485. 10.1002/smtd.201900485. [Google Scholar]
- 57.Gao J, Zhang Y, Li Z, Liu M. Role of ethylene response factors (ERFs) in fruit ripening. Food Qual Saf. 2020;4(1):15–20. 10.1093/fqsafe/fyz042. [Google Scholar]
- 58.Li W, Herrera-Estrella L, Tran L-SP. The Yin-Yang of Cytokinin Homeostasis and Drought Acclimation/Adaptation. Trends Plant Sci. 2016;21(7):548–50. 10.1016/j.tplants.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Wang X, Zeng W, Ding Y, Wang Y, Niu L, Yao J-L, Pan L, Lu Z, Cui G, Li G, Wang Z. Peach ethylene response factor PpeERF2 represses the expression of ABA biosynthesis and cell wall degradation genes during fruit ripening. Plant Sci. 2019;283:116–26. 10.1016/j.plantsci.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 60.Liu, M., Diretto, G., Pirrello, J., Roustan, J.-P., Li, Z., Giuliano, G., Regad, F., Bouzayen, M., 2014. The chimeric repressor version of an Ethylene Response Factor (ERF) family member, Sl-ERF.B3, shows contrasting effects on tomato fruit ripening. New Phytol. 203 (1), 206–218. 10.1111/nph.12771 [DOI] [PubMed]
- 61.Wang M, Zhu Q, Deng C, Luo Z, Sun N, Grierson D, Yin X, Chen K. Hypoxia-responsive ERFs involved in postdeastringency softening of persimmon fruit. Plant Biotechnol J. 2017;15(11):1409–19. 10.1111/pbi.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu Y, Li X, Zhang J, Zhao H, Tan S, Xu W, Pan J, Yang F, Pi E. ERF subfamily transcription factors and their function in plant responses to abiotic stresses. Front Plant Sci. 2022;13:1042084. 10.3389/fpls.2022.1042084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We have uploaded the raw transcriptome data to the National Genomics Data center, the number is PRJCA028378.