Abstract
Vaccines designed to control chronic infections by intracellular agents such as human immunodeficiency virus type 1 (HIV-1) require the induction of cell-mediated immune responses to rid the host of pathogen-infected cells. Listeria monocytogenes has characteristics that make it an attractive vaccine vector for use against such infections. Here we show that parenteral immunization with a new highly attenuated strain of this organism provided complete protection against systemic and mucosal challenges with a recombinant vaccinia virus expressing HIV-1 gag. Immunization also generated a strong, long-term memory cytotoxic-T-lymphocyte (CTL) response in spleen, mesenteric lymph nodes, and Peyer's patches directed against the gag protein. Oral immunization with this attenuated strain also produced complete, long-lasting protection against the recombinant virus but only against mucosal virus challenge. Curiously, oral immunization was associated with a transient CTL response in the three lymphoid tissues examined.
Vaccinology has made major contributions to the eradication or management of infectious diseases (19). Protective vaccines have usually been designed to induce humoral antibodies to neutralize viruses or promote the phagocytosis of bacteria or inactivation of their toxins. However, the control of diseases characterized by chronic infection of cells by intracellular infectious agents requires the induction of cell-mediated immune responses to rid the host of pathogen-infected cells. Growing evidence suggests that cell-mediated immunity plays an essential role in controlling human immunodeficiency virus (HIV) infection (12, 38), although efforts to develop an effective vaccine for HIV have been daunting. Since natural infections by HIV are initiated at mucosal sites, a vaccine that can also induce responses at these sites may be able to block early stages of infection and be particularly valuable.
To initiate cell-mediated immune responses, antigen must gain access to the cytoplasm of host cells, where it can be processed to peptides that are presented to the cellular immune system. Listeria monocytogenes is a gram-positive facultative intracellular microorganism that has long served as a model pathogen for the study of cell-mediated immunity (21). Subsequent to infection and uptake, this bacterium is able to escape the endocytic vacuoles and replicate in the host cell cytosol (43), where secreted bacterial proteins are delivered directly to the major histocompatibility complex class I pathway of antigen processing and presentation (4). Mice infected with a sublethal dose of the organism rapidly clear the pathogen and develop long-lasting immunity, mediated predominantly by CD8+ T cells (13, 21). This property of L. monocytogenes makes it attractive as a potential live vaccine vector, and recombinant L. monocytogenes expressing foreign antigens has successfully been used to protect mice against lymphocytic choriomeningitis (17, 39) and influenza (20) virus infections and against lethal tumor cell challenge (34, 35).
Because of the potential use of this organism as a vaccine vector, the safety of L. monocytogenes is of paramount concern, since the pathogen can cause fatal infections in humans (25). Consequently, some investigators have suggested use of mutated Listeria vectors with restricted intracellular movement or recombinant organisms that have become physiologically crippled, but these bacteria nevertheless possess full genetic potential for virulence. We have constructed a strain of L. monocytogenes that is genetically attenuated and can grow only if supplemented with d-alanine, a rare amino acid produced only by microbial organisms for cell wall synthesis (42). Here we demonstrate that appropriate immunization with an HIV gag recombinant of this novel attenuated strain of Listeria, in the presence of a small amount of d-alanine to initiate infection, can elicit a persistent systemic and mucosal anti-gag CD8+ cytotoxic-T-lymphocyte (CTL) response. This appears to be the first report of the induction of mucosal CTLs by a bacterial vector.
To test the efficacy of the immune response, we performed virus challenge experiments. Since HIV does not infect mice, we used as a surrogate virus recombinant vaccinia virus expressing HIV gag. We challenged animals by both mucosal and systemic routes. We found that orally immunized mice were protected against mucosal virus challenge and that systemically immunized mice were protected against virus challenge by either route of infection. In conjunction with the observation that the attenuated gag recombinant Listeria is efficient at stimulating gag-specific human blood CTLs in vitro (15), it would appear that these organisms may be a useful adjunct to the vaccine options available for HIV and other infectious diseases.
MATERIALS AND METHODS
Bacterial strains.
L. monocytogenes strain 10403S (36), the wild-type organism used in these studies, was grown in brain heart infusion medium (Difco Laboratories). L. monocytogenes daldat (Lmdaldat) is a double-deletion mutant of L. monocytogenes in which 82% of the alanine racemase gene (dal) and 31% of the d-amino acid aminotransferase gene (dat) have been removed in frame (42). In laboratory media its growth requires 100 μg of d-alanine per ml. The 50% lethal dose (LD50) of wild-type L monocytogenes strain 10403 in female BALB/c mice following intravenous (i.v.) or intraperitoneal (i.p.) infection is approximately 1 × 104. The LD50 of the double mutant strain of L. monocytogenes was >8 × 108 bacteria or, when injected i.v. in the presence of 20 mg d-alanine, approximately 7 × 107 bacteria (42) or higher (unpublished). The LD50 following intragastric (i.g.) infection is unknown for either organism but is probably greater than 1010 bacteria. Recombinants of strain Lmdaldat were produced by a stable modification of its chromosomes using the shuttle vector pKSV7 and a modification of the protocol described by Camilli et al. (9), as described previously (14). In that way, strain Lmdaldat-gag, otherwise exactly analogous to L. monocytogenes expressing gag (14), was constructed.
Induction and assay of splenic, Peyer's patch, and mesenteric lymph node gag-specific CTLs.
Female BALB/c mice (H-2d), 8 to 10 weeks of age (Charles River Laboratories, Raleigh, N.C.), were immunized systemically by i.p., intramuscular (i.m.), or subcutaneous (s.c.) inoculation or orogastrically by i.g. intubation with a feeding tube with 0.1 to 0.8 LD50s of either strain Lmdaldat, Lmdaldat-gag, or the wild type expressing gag. The i.p. inoculum (200 μl) contained 10 mg of d-alanine to initiate the infection by attenuated strain Lmdaldat or Lmdaldat-gag. The i.m. or s.c. inocula (100 μl) contained 5 mg of d-alanine, and 30 to 60 min before infection the mice were injected i.p. with 40 mg of d-alanine so that some amino acid could diffuse to the vicinity of the infection. Intragastric infection was preceded either by supplying 20 mg of d-alanine per ml of water overnight or by i.p. injection of d-alanine as above. Some mice were boosted after 21 days with a second inoculation, and some were boosted with an additional inoculation 1 month later. After the last inoculation, cells were isolated at various times from spleen, Peyer's patches, and mesenteric lymph nodes of the immunized animals. Splenocyte suspensions were obtained by pressing the tissue through a nylon mesh screen. Cells of Peyer's patches and mesenteric lymph nodes were isolated by teasing the cells into suspension, syringing to dissociate clumps, and filtering through nylon mesh to remove cartilagenous debris. The medium for splenocyte isolation and in vitro stimulation cultures was RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 5 × 10−5 M 2-mercaptoethanol, 25 mM HEPES, 2 mM l-glutamine, and 50 μg of gentamicin per ml. The medium for mucosal tissue isolation included 2.5 μg of amphotericin B per ml. This medium was also used for in vitro stimulation of mucosal cells, except with the omission of amphotericin B and the addition of 10% concanavalin A-conditioned medium.
Antigen-presenting cells for all in vitro stimulation cultures were splenocytes from naive mice. They were loaded with 5 to 10 μM peptide for 60 min, irradiated with a cobalt source at 2,000 rads, and used at a ratio of three or four lymphocytes per cell. In most experiments, the peptide used was the HIV-1 gag Kd epitope, amino acids (aa) 197 to 205 (30). Splenocyte, Peyer's patch, and mesenteric lymph node cultures contained, respectively, 6 × 107 cells in 8 ml of medium, 6 × 106 cells in 3 ml of medium, or 1 × 107 cells in 5 ml of medium. After 5 to 6 days of in vitro stimulation at 37°C in 10% CO2, the cultures were assayed for the presence of CTL activity capable of recognizing peptide-labeled P815 cells (0.2 μM peptide) by previously published procedures (14, 44). Every determination of lytic activity was assayed in triplicate and corrected for spontaneous release from target cells (2 to 5%) and for activity on target cells not labeled with peptide. To compare results of independent experiments and samples assayed at different effector/target ratios, lytic units (16, 32) rather than percent lysis were plotted. Lytic units were calculated as the number of effector cells, in a total of 107 lymphocytes, required to produce 15% lysis of 2 × 104 target cells. In this low range of CTL activity the effector/target ratio is linear with CTL activity and is least affected by inhibitory constituents in the lymphocyte preparations (10).
Vaccinia virus protection.
Following immunization, mice were challenged systemically by i.p. infection with 0.5 × 107 to 2.5 × 107 PFU of vaccinia virus expressing HIV-1 gag (vVK1) or HIV-1 nef (vTFNef) in 100 μl of phosphate-buffered saline. Mucosal challenge was administered by intrarectal infection using a 6-cm 5-French umbilical vessel catheter (1). Six days after challenge, the mice were sacrificed and their ovaries were removed, homogenized, and assayed for virus content by plating serial dilutions on BSC-2 indicator cells and staining with 1% crystal violet.
RESULTS
An attenuated strain of L. monocytogenes that expresses the HIV-1 gag gene can induce a long-lasting systemic anti-gag CTL response.
The attenuated L. monocytogenes strain Lmdaldat has major deletions in two genes required for cell wall synthesis and is incapable of growth in the absence of supplemented d-alanine (42). Its immunogenicity requires that infection be initiated in the presence of a small amount of this rare amino acid. After initiation of infection and dissipation of the amino acid, the organism cannot survive; thus, death is the default state for this attenuated strain of Listeria. When 108 recombinants of this organism expressing HIV-1 gag were used to infect mice by the i.p. route, high CTL activity directed against the CD8 gag epitope, aa 197 to 205 (30), could be detected in in vitro-stimulated splenocyte cultures for at least 6 months after initial antigen exposure (Fig. 1).
FIG. 1.
Attenuated Lmdaldat-gag (Lmdd-gag) induced a long-term-memory CTL response following a single i.p. infection. BALB/c mice were infected i.p. with 108 Lmdaldat (Lmdd) or Lmdaldat-gag bacteria. Splenocytes were isolated at the indicated times, stimulated in a 6-day in vitro culture with irradiated naïve splenocytes decorated with HIV-1 gag peptide (aa 197 to 205), and assayed for CTL activity in a 4-h Cr51 release assay. In these assays, effector/target (E/T) ratios used were 90, 30, and 10. P < 0.0004 for Lmdaldat versus Lmdaldat-gag at an E/T ratio of 90.
The response was sufficiently strong 7 days after a boost to allow measurement of CTL activity directly ex vivo (Fig. 2A) without the usual 6-day in vitro stimulation (Fig. 2B). The response to strain Lmdaldat-gag was at least as strong as that induced by the wild-type-derived gag recombinant. The phenotype of the gag-specific CTLs was found by antibody depletion to be CD8+ (not shown). The attenuated strain lacking the gag gene failed to induce CTL activity against the gag epitope, indicating that the activity was specific for that peptide (Fig. 1). A further test of peptide-specific recognition using the CD4 gag epitope, aa 253 to 272 (29), to label target cells resulted in no CTL activity, confirming that the activity resided in CD8+ T cells (not shown). These observations indicated that foreign antigens introduced by i.p. infection with this recombinant attenuated strain of L. monocytogenes could elicit a robust, long-term-memory CTL response in mice despite the fact that the microorganisms survive for only 2 days under the conditions of immunization (42).
FIG. 2.
Ex vivo detection of CTL activities of splenocytes following i.p. infection and a boost 21 days later with either strain Lmdaldat (Lmdd), Lmdaldat-gag (Lmdd-gag), or the wild type expressing gag (Lm-gag). (A) Direct ex vivo assay of CTL activity of splenocytes 7 days after the boost; (B) CTL activity of the same splenocytes as in panel A except that the assay followed a 6-day in vitro culture in the presence of the gag peptide. See the legend to Fig. 1 for details.
Memory cells were found in gut-associated lymphoid tissues as well as in spleen following two or more systemic immunizations.
The mucosal immune system is believed to be partially independent and regulated differently than peripheral lymphoid tissues (8). It was therefore anticipated that systemic immunization with attenuated Listeria would not elicit a CTL response in gut-associated tissues. At 7 days after a single i.p. infection with 108 Lmdaldat-gag bacteria, CTL activity was seen in splenocytes but low activities could also be detected in Peyer's patches and mesenteric lymph nodes (Fig. 3). However, a boost after 21 days with 108 bacteria led to much higher CTL activities in all these tissues, as early as 3 days postinfection. This contrasts with the expected result at 3 days after a primary immunization, when little CTL activity would be anticipated in any tissue (6). This abrupt increase in mucosal activity suggested a recall response by T cells. By 60 days, there was some decline of activity in the mucosal tissues. Following a third immunization 1 month later (Fig. 3), there were again early, high activities in spleen, mesenteric lymph nodes, and Peyer's patches. After the initial burst of effector activity, a memory response again stabilized in the three tissues. The gag-specific CTLs in Peyer's patches and mesenteric lymph nodes, like those of the spleen, were predominantly CD8+ T cells (not shown).
FIG. 3.
The CTL response in mucosal lymphoid tissues and spleen after one or more systemic (i.p.) infections. Groups of mice were infected with Lmdaldat-gag either once, twice, or three times by the i.p. route. At various times after the last infection, lymphocytes were isolated and cultured for 6 days with gag peptide-labeled antigen-presenting cells. In order to compare multiple independent experiments, the data are presented in lytic units, which is the number of effector cells per 107 total cells that can lyse 15% of 2 × 104 target cells (see Materials and Methods). The data are means with standard errors of the means for the number (n) of independent experiments indicated in parentheses.
Oral administration of the attenuated gag recombinant Listeria produced a gag-specific immune response in Peyer's patches and mesenteric lymph nodes as well as in spleen after priming and a boost, but CTLs did not persist in these organs.
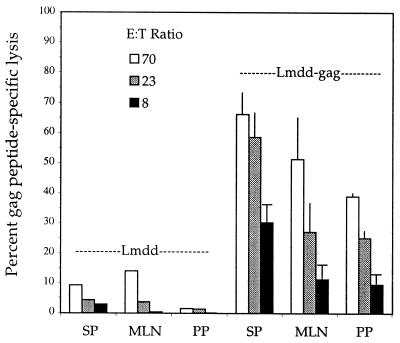
The natural route of infection by Listeria is through the gut, following ingestion of contaminated samples of water, milk, vegetation, or meat products. Whether CTLs are elicited in mucosal lymphoid tissues following infection by Listeria has not previously been reported. We therefore determined whether a CTL response could be detected after i.g. infection with 108 bacteria, either in the spleen or in two gut-associated tissues, Peyer's patches and mesenteric lymph nodes. Seven days after oral administration of either wild-type or attenuated gag recombinant Listeria, splenocytes showed only a weak CTL response against either the foreign gag antigen or the homologous Listeria antigen, LLO peptide 91–99. Lymphocytes from Peyer's patches and mesenteric lymph nodes showed no activity against either antigen. However, following a boost with 108 bacteria by the oral route, high anti-gag CTL activities were detected in all three tissues (Fig. 4). The active cells in these tissues were also CD8+ T cells. The responses to gag and a Listeria peptide, LLO, were equivalent in magnitude (data not shown). To explore the strength and duration of this immune response following oral immunization, mice that had been primed and boosted were sampled at several time points following the second infection. Within 2 weeks the response was severely diminished (Fig. 5).
FIG. 4.
Induction of gag-specific CTLs in spleen (SP), mesenteric lymph nodes (MLN), and Peyer's patches (PP) by i.g. infection with 108 strain Lmdaldat (Lmdd) or Lmdaldat-gag (Lmdd-gag) bacteria (priming and a boost 21 days later). At 7 days after the boost, cells were stimulated in culture and assayed against gag-labeled target cells, as for Fig. 1. Effector/target (E/T) ratios used were 70, 23, and 8. P < 0.017 for strain Lmdaldat versus Lmdaldat-gag at an E/T ratio of 70. The data are means with standard errors of the means for three independent experiments.
FIG. 5.
The lytic activities of cells isolated from spleen, Peyer's patches, and mesenteric lymph nodes did not persist after infection with strain Lmdaldat-gag by the i.g. route. Samples were taken for the CTL assay at various times after priming and a boost 21 days later. To compare independent experiments, the data are in lytic units per 107 effector cells (see the legend to Fig. 4 and Materials and Methods). The data are means with standard errors of the means for the number (n) of independent experiments indicated in parentheses.
Protection against systemic or mucosal viral challenge.
Because mice are not susceptible to HIV-1 infection, it is not possible to assess directly the protection produced by immunization with attenuated Listeria-gag. However, mice can be infected by a vaccinia gag recombinant virus, which can serve here as an HIV-1 surrogate (1, 31). Using this model, we found a 6-log reduction of virus titer in mouse ovaries at the peak of a systemic virus challenge in i.p.-immunized mice (Fig. 6A). The same result was seen with the wild-type Listeria expressing gag. Mice immunized with the nonrecombinant attenuated Listeria showed virtually no protection. A 2-log reduction of virus titer followed oral immunization. Surprisingly, mucosal challenge of mice immunized orally showed a 6-log reduction of virus titer (Fig. 6B). This protection was seen despite the absence of CTL activity in the three tissues previously examined (Fig. 5). Mice immunized by the i.p. route were also fully protected against mucosal virus challenge. A single systemic boost following the oral immunizations generated good protection, regardless of whether the challenge route was systemic or mucosal (Fig. 6). As expected, no protection against challenge with vaccinia virus expressing nef was observed in mice immunized with Lmdaldat-gag.
FIG. 6.
Immunization with strain Lmdaldat-gag (Lmddgag) protected mice against subsequent systemic and/or mucosal challenge with an HIV-1 gag recombinant vaccinia virus (vac-gag). (A) Systemic challenge. Mice immunized by various routes were challenged 21 days later (i.p. immunization) or 15 days later (i.g. immunization) with 1 × 107 to 2.5 × 107 PFU of gag recombinant vaccinia virus injected i.p. (B) Mucosal challenge. Mice immunized by various routes were challenged 21 days later (i.p. immunization) or 43 days later (i.g. immunization) with 1 × 107 to 2.5 × 107 PFU of gag recombinant vaccinia virus deposited intrarectally. The data are titers of vaccinia virus in mouse ovaries, the preferred organ of replication, at 6 days after virus infection. The mean of each set of data (four to seven mice per group) is indicated by a bar. Mice protected against vac-gag replication by i.p. immunization with Lmdd gag allowed full replication of vac-nef (V-nef) (not shown). Lmdd, strain Lmdaldat; Lmgag, wild-type Listeria expressing gag.
DISCUSSION
The response of a host to intracellular infectious agents like HIV-1 and L. monocytogenes is to induce pathogen-specific CD4+ and CD8+ T cells. CD8+ CTLs can eliminate infected cells through the action of cytokines as well as by direct killing of the cells through perforin-mediated or Fas-mediated pathways (3), providing a powerful host response to infection. We initiated the present study to determine whether a new hyperattenuated strain of L. monocytogenes that produced a protective immune response against the homologous organism (42) could also elicit a CD8+ T-cell response to HIV antigens. We found that a single i.p. infection of mice with an HIV gag recombinant of the attenuated strain elicited an anti-gag CTL memory response that persisted for at least 6 months after initial antigen exposure (Fig. 1). The response was especially strong after a boost and could then be detected by a direct ex vivo CTL assay (Fig. 2). Anti-gag CTLs could also be detected following i.m. and s.c. immunizations.
While a single systemic infection generated low CTL activities in Peyer's patches or mesenteric lymph nodes, a systemic boost produced a rapid and vigorous burst of mucosal CTL activity within 3 days, indicating a recall response in these tissues (Fig. 3). A second or third boost produced somewhat stronger responses, and a significant fraction of this activity was stable during the following 2 months or longer in the spleen and mesenteric lymph nodes, though often not in Peyer's patches (Fig. 3). These long-lasting responses were evoked despite the fact that the organism is eliminated from the host within a few days following infection (42).
The CTL activity seen in mucosa-associated lymphoid tissues after systemic infection must originate in recirculating memory T cells, and their abundance at these sites may in part reflect the increased traffic in animals reexposed to antigen. Also, the large bolus of Listeria introduced systemically results in its dissemination throughout the entire lymph system and blood, accumulating predominantly in spleen and liver (28), but also in the mesenteric lymph nodes and Peyer's patches (27, 28). This is true for the attenuated strain as well (unpublished). The presence of antigen at these mucosal sites may help explain their retention of memory T cells (7). Although high levels of cytolytic T cells are seen in spleen, mesenteric lymph nodes, and Peyer's patches, it is likely that the bulk of the anti-gag CD8+ T cells induced by Lmdaldat gag are occupied with immune surveillance of mucosal surfaces in the gut and other nonlymphoid effector sites (26). A prominent role in these lymphocyte movements may be played by α4β7 integrin (24) and l-selectin (41). Systemic immunization resulted in protection of these animals against systemic and mucosal challenge by recombinant vaccinia virus expressing gag (Fig. 6). The presence of CTLs at the various mucosal sites might explain protection against mucosal challenge by the virus.
Natural infection by L. monocytogenes is initiated at the intestinal surface. While there is considerable information regarding the induction and properties of T cells following systemic infection of mice with Listeria (5, 13), little is known about induction, memory, and trafficking of mucosal T lymphocytes after intestinal infection. After a single oral infection with wild-type or attenuated gag recombinant bacteria, we found no detectable CTL activity in either mesenteric lymph nodes or Peyer's patches and little in the spleen, directed against either the gag protein or LLO, the major protective antigen of L. monocytogenes (18). However, a boost resulted in CD8+ cytolytic activities in all three tissues (Fig. 4). Surprisingly, this response did not persist, and little activity was seen after 2 weeks (Fig. 5). These results suggested that either the oral immunization of mice with attenuated Listeria failed to induce a lasting response or the CTLs we initially saw had relocated to sites not yet examined. That the latter is a possibility was suggested by virus challenge experiments. Despite the loss of CTL activity from spleen, Peyer's patches, and mesenteric lymph nodes after oral immunization, these mice were completely protected against mucosal challenge by vaccinia virus expressing the HIV-1 gag gene (Fig. 6B). This suggests the possible migration of immune cells to sites such as lamina propria. However, while CD8+ effector T cells are the key mediators for clearance of both cytopathic and noncytopathic viruses, CD4+ T cells can also play a role in viral protection by providing help for B cells and CTL responses and directly by elaboration of cytokines (45). This clearly can be the case for vaccinia virus infection (40). At present we do not know what cells were responsible for the protection that was seen. However, in preliminary experiments (unpublished), we found that long-term antiviral protection could be conferred to naïve mice by transfer of cells from spleen, mesenteric lymph nodes, or Peyer's patches of immunized mice following their in vitro expansion in the presence of the class I gag epitope.
While priming and one or more boosts by the oral route did not appear to generate long-term-memory CTLs in spleen or mucosal lymphoid tissues, a subsequent boost by the systemic route did result in mucosal and splenic memory T cells that persisted for several months (not shown). Mice immunized by such a regimen showed high levels of protection against virus challenge by either route (Fig. 6).
The generation of mucosal CTLs following bacterial infection has not previously been reported, although there have been numerous reports of mucosal CTL activity following virus infection and peptide immunization (1, 2, 16, 22, 33). In the case of infection of mice with attenuated L. monocytogenes, we found that antigen-specific CTLs were indeed elicited in mucosal lymphoid tissues, with differing results following systemic and oral infections. A strong and durable immune response is likely to occur in humans following oral infection, since humans may be more susceptible than mice to infection with L. monocytogenes by this route (11, 23, 37). The HIV gag recombinant of the hyperattenuated strain of Listeria used in this study has been shown to infect human monocytes and efficiently stimulate gag-specific human CTLs in vitro (15). Therefore, our results argue that this strain may provide a safe vector for human use with potential for the induction of strong, long-lasting cell-mediated immunity and protection in both systemic and mucosal compartments. The rapid recall response that was seen at mucosal sites may provide important T-cell protection against viral challenge at these sites.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI-42509.
We thank J. Cebra, P. Offit, C. Moser, I. Belyakov, and J. Berzofsky for introducing us to some of the techniques used here.
REFERENCES
- 1.Belyakov I M, Derby M A, Ahlers J D, Kelsall B L, Earl P, Moss B, Strober W, Berzofsky J A. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc Natl Acad Sci USA. 1998;95:1709–1714. doi: 10.1073/pnas.95.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennink J, Effros R B, Doherty P C. Influenzal pneumonia: early appearance of cross-reactive T cells in lungs of mice primed with heterologous type A viruses. Immunology. 1978;35:503–509. [PMC free article] [PubMed] [Google Scholar]
- 3.Berke G. The binding and lysis of target cells by cytotoxic lymphocytes: molecular and cellular aspects. Annu Rev Immunol. 1994;12:735–773. doi: 10.1146/annurev.iy.12.040194.003511. [DOI] [PubMed] [Google Scholar]
- 4.Braciale T J, Morrison L A, Sweetser M T, Sambrook J, Gething M J, Braciale V L. Antigen presentation pathways to class I and class II MHC-restricted T lymphocytes. Immunol Rev. 1987;98:95–114. doi: 10.1111/j.1600-065x.1987.tb00521.x. [DOI] [PubMed] [Google Scholar]
- 5.Busch D H, Pamer E G. T lymphocyte dynamics during Listeria monocytogenes infection. Immunol Lett. 1999;65:93–98. doi: 10.1016/s0165-2478(98)00130-8. [DOI] [PubMed] [Google Scholar]
- 6.Busch D H, Pilip I M, Vijh S, Pamer E G. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity. 1998;8:353–362. doi: 10.1016/s1074-7613(00)80540-3. [DOI] [PubMed] [Google Scholar]
- 7.Butcher E C. The regulation of lymphocyte traffic. Curr Top Microbiol Immunol. 1986;128:85–122. doi: 10.1007/978-3-642-71272-2_3. [DOI] [PubMed] [Google Scholar]
- 8.Cahill R N, Poskitt D C, Frost D C, Trnka Z. Two distinct pools of recirculating T lymphocytes: migratory characteristics of nodal and intestinal T lymphocytes. J Exp Med. 1977;145:420–428. doi: 10.1084/jem.145.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camilli A, Tilney L G, Portnoy D A. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark D A, Phillips R A, Miller R G. Characterization of cells that suppress the cytotoxic activity of T lymphocytes. I. Quantitative measurement of inhibitor cells. J Immunol. 1976;116:1020–1029. [PubMed] [Google Scholar]
- 11.Dalton C B, Austin C C, Sobel J, Hayes P S, Bibb W F, Graves L M, Swaminathan B, Proctor M E, Griffin P M. An outbreak of gastroenteritis and fever due to Listeria monocytogenes in milk. N Engl J Med. 1997;336:100–105. doi: 10.1056/NEJM199701093360204. [DOI] [PubMed] [Google Scholar]
- 12.Evans D T, O'Connor D H, Jing P, Dzuris J L, Sidney J, da Silva J, Allen T M, Horton H, Venham J E, Rudersdorf R A, Vogel T, Pauza C D, Bontrop R E, DeMars R, Sette A, Hughes A L, Watkins D I. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat Med. 1999;5:1270–1276. doi: 10.1038/15224. [DOI] [PubMed] [Google Scholar]
- 13.Finelli A, Kerksiek K M, Allen S E, Marshall N, Mercado R, Pilip I, Busch D H, Pamer E G. MHC class I restricted T cell responses to Listeria monocytogenes, an intracellular bacterial pathogen. Immunol Res. 1999;19:211–223. doi: 10.1007/BF02786489. [DOI] [PubMed] [Google Scholar]
- 14.Frankel F R, Hedge S, Lieberman J, Paterson Y. Induction of cell-mediated immune responses to human immunodeficiency virus type 1 Gag protein by using Listeria monocytogenes as a live vaccine vector. J Immunol. 1995;155:4775–4782. [PubMed] [Google Scholar]
- 15.Friedman R S, Frankel F R, Xu Z, Lieberman J. Induction of human immunodeficiency virus (HIV)-specific CD8 T-cell responses by Listeria monocytogenes and a hyperattenuated Listeria strain engineered to express HIV antigens. J Virol. 2000;74:9987–9993. doi: 10.1128/jvi.74.21.9987-9993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallichan W S, Rosenthal K L. Long-lived cytotoxic T-lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J Exp Med. 1996;184:1879–1890. doi: 10.1084/jem.184.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goossens P L, Milon G, Cossart P, Saron M-F. Attenuated Listeria monocytogenes as a live vector for induction of CD8+ T cells in vivo: a study with the nucleoprotein of the lymphocytic choriomeningitis virus. Int Immunol. 1995;7:797–802. doi: 10.1093/intimm/7.5.797. [DOI] [PubMed] [Google Scholar]
- 18.Harty J T, Bevan M J. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J Exp Med. 1992;175:1531–1538. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilleman M R. A simplified vaccinologists' vaccinology and the pursuit of a vaccine against AIDS. Vaccine. 1998;16:778–793. doi: 10.1016/s0264-410x(97)00272-7. [DOI] [PubMed] [Google Scholar]
- 20.Ikonomidis G, Portnoy D, Gerhard W, Paterson Y. Influenza-specific immunity induced by recombinant Listeria monocytogenes vaccines. Vaccine. 1997;15:433–440. doi: 10.1016/s0264-410x(96)00188-0. [DOI] [PubMed] [Google Scholar]
- 21.Kaufmann S H E. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 22.Klavinskis L S, Bergmeier L A, Gao L, Mitchell E, Ward R G, Layton G, Brookes R, Meyers N J, Lehner T. Mucosal or targeted lymph node immunization of macaques with a particulate SIVp27 protein elicits virus-specific CTL in the genito-rectal mucosa and draining lymph nodes. J Immunol. 1996;157:2521–2527. [PubMed] [Google Scholar]
- 23.Lecuit M, Dramsi S, Gottardi C, Fedor-Chaiken M, Gumbiner B, Cossart P. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 1999;18:3956–3963. doi: 10.1093/emboj/18.14.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefrancois L, Parker C M, Olson S, Muller W, Wagner N, Schon M P, Puddington L. The role of beta7 integrins in CD8 T cell trafficking during an antiviral immune response. J Exp Med. 1999;189:1631–1638. doi: 10.1084/jem.189.10.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorber B. Listeriosis. Clin Infect Dis. 1997;24:1–9. doi: 10.1093/clinids/24.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Mackay C R, Marston W L, Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990;171:801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marco A J, Domingo M, Prats M, Briones V, Pumarola M, Dominguez L. Pathogenesis of lymphoid lesions in murine experimental listeriosis. J Comp Pathol. 1991;105:1–15. doi: 10.1016/s0021-9975(08)80057-6. [DOI] [PubMed] [Google Scholar]
- 28.Marco A J, Domingo M, Ruberte J, Carretero A, Briones V, Dominguez L. Lymphatic drainage of Listeria monocytogenes and Indian ink inoculated in the peritoneal cavity of the mouse. Lab Anim. 1992;26:200–205. doi: 10.1258/002367792780740549. [DOI] [PubMed] [Google Scholar]
- 29.Mata M, Paterson Y. Th1 T cell responses to HIV-1 Gag protein delivered by a Listeria monocytogenes vaccine are similar to those induced by endogenous listerial antigens. J Immunol. 1999;163:1449–1456. [PubMed] [Google Scholar]
- 30.Mata M, Travers P J, Liu Q, Frankel F R, Paterson Y. The MHC class I-restricted immune response to HIV-gag in BALB/c mice selects a single epitope that does not have a predictable MHC-binding motif and binds to Kd through interactions between a glutamine at P3 and pocket D. J Immunol. 1998;161:2985–2993. [PubMed] [Google Scholar]
- 31.Mata, M., Z. Yao, A. Zubair, K. Syres, and Y. Paterson. Evaluation of a recombinant Listeria monocytogenes expressing an HIV protein that protects mice against viral challenge. Vaccine, in press. [DOI] [PubMed]
- 32.Murphey-Corb M, Wilson L A, Trichel A M, Roberts D E, Xu K, Ohkawa S, Woodson B, Bohm R, Blanchard J. Selective induction of protective MHC class I-restricted CTL in the intestinal lamina propria of rhesus monkeys by transient SIV infection of the colonic mucosa. J Immunol. 1999;162:540–549. [PubMed] [Google Scholar]
- 33.Offit P A, Dudzik K I. Rotavirus-specific cytotoxic T lymphocytes appear at the intestinal mucosal surface after rotavirus infection. J Virol. 1989;63:3507–3512. doi: 10.1128/jvi.63.8.3507-3512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan Z-K, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nat Med. 1995;1:471–477. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 35.Paterson Y, Ikonomidis G. Recombinant Listeria monocytogenes cancer vaccines. Curr Opin Immunol. 1996;8:664–669. doi: 10.1016/s0952-7915(96)80083-5. [DOI] [PubMed] [Google Scholar]
- 36.Portnoy D A, Jacks P S, Hinrichs D J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlech W F. Listeria gastroenteritis—old syndrome, new pathogen. N Engl J Med. 1997;336:130–132. doi: 10.1056/NEJM199701093360211. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 39.Shen H, Slifka M K, Matloubian M, Jensen E R, Ahmed R, Miller J F. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc Natl Acad Sci USA. 1995;92:3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spriggs M K, Koller B H, Sato T, Morrissey P J, Fanslow W C, Smithies O, Voice R F, Widmer M B, Maliszewski C R. Beta 2-microglobulin-, CD8+ T-cell-deficient mice survive inoculation with high doses of vaccinia virus and exhibit altered IgG responses. Proc Natl Acad Sci USA. 1992;89:6070–6074. doi: 10.1073/pnas.89.13.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steeber D A, Tang M L, Zhang X Q, Muller W, Wagner N, Tedder T F. Efficient lymphocyte migration across high endothelial venules of mouse Peyer's patches requires overlapping expression of l-selectin and beta7 integrin. J Immunol. 1998;161:6638–6647. [PubMed] [Google Scholar]
- 42.Thompson R J, Bouwer H G A, Portnoy D A, Frankel F R. Pathogenicity and immunogenicity of a Listeria monocytogenes strain that requires d-alanine for growth. Infect Immun. 1998;66:3552–3561. doi: 10.1128/iai.66.8.3552-3561.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wipke B T, Jameson S C, Bevan M J, Pamer E G. Variable binding affinities of listeriolysin O peptides for the H-2Kd class I molecule. Eur J Immunol. 1993;23:2005–2010. doi: 10.1002/eji.1830230842. [DOI] [PubMed] [Google Scholar]
- 45.Zinkernagel R M. Immunology taught by viruses. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]