Abstract
Infection of the central nervous system (CNS) by several viruses can lead to upregulation of proinflammatory cytokines and chemokines. In immunocompetent adults, these molecules induce prominent inflammatory infiltrates. However, with immunosuppressive retroviruses, such as human immunodeficiency virus (HIV), little CNS inflammation is observed yet proinflammatory cytokines and chemokines are still upregulated in some patients and may mediate pathogenesis. The present study examined expression of cytokines and chemokines in brain tissue of neonatal mice infected with virulent (Fr98) and avirulent (Fr54) polytropic murine retroviruses. While both viruses infect microglia and endothelia primarily in the white matter areas of the CNS, only Fr98 induces clinical CNS disease. The pathology consists of gliosis with minimal morphological changes and no inflammation, similar to HIV. In the present experiments, mice infected with Fr98 had increased cerebellar mRNA levels of proinflammatory cytokines tumor necrosis factor alpha (TNF-α), TNF-β, and interleukin-1α and chemokines macrophage inflammatory protein-1α (MIP-1α), MIP-1β, monocyte chemoattractant protein 1 (MCP-1), gamma-interferon-inducible protein 10 (IP-10), and RANTES compared to mice infected with Fr54 or mock-infected controls. The increased expression of these genes occurred prior to the development of clinical symptoms, suggesting that these cytokines and chemokines might be involved in induction of neuropathogenesis. Two separate regions of the Fr98 envelope gene are associated with neurovirulence. CNS disease associated with the N-terminal portion of the Fr98 env gene was preceded by upregulation of cytokines and chemokines. In contrast, disease associated with the central region of the Fr98 env gene showed no upregulation of cytokines or chemokines and thus did not require increased expression of these genes for disease induction.
Retrovirus infection of the central nervous system (CNS) can induce neurological diseases in both humans and animals (17, 28, 41). The development of neurological symptoms is often associated with major pathology, such as the marked mononuclear cell infiltration and in some cases demyelination observed after visna virus (13, 41), human T-cell lymphotropic virus type 1 (14), and simian immunodeficiency virus (5) infection or the spongiosis (20, 21) or intracerebral hemorrhages (25) found after infection with ecotropic murine leukemia viruses (MuLVs). In contrast, only minimal changes in histopathology are seen after CNS infection with human immunodeficiency virus (HIV), feline immunodeficiency virus, or polytropic MuLVs despite the development of severe neurological diseases (4, 17, 28). Increased expression of cytokines and chemokines in the CNS has been suggested as a possible mechanism of neurological disease induced by HIV (9). However, there was not a consistent correlation between increased expression of cytokine and chemokines and HIV dementia. For example, CNS expression of cytokine and chemokine mRNAs was not always upregulated in HIV dementia cases and, conversely, expression was increased in some HIV-infected patients lacking dementia (36, 42). Instead of being a mechanism of disease induction, the increase in cytokine and chemokine mRNA expression may simply be a host response to viral infection or pathogenesis that varies due to the genetic heterogeneity of the patients. Distinguishing among these possibilities in HIV dementia would be difficult due to the inability to analyze brain tissue prior to death. Therefore, in the present study we used the mouse model of polytropic MuLV infection, where cytokine and chemokine expression can be compared between avirulent and neurovirulent viruses over the course of neurological disease in genetically homogeneous hosts.
In neonatal mice, infection with the polytropic MuLV clone Fr98 leads to the development of a severe clinical disease characterized by ataxia, seizures, and death starting at 14 to 16 days postinfection (28). In contrast, mice infected with another closely related polytropic MuLV clone, Fr54, do not develop neurological disease (28). Fr54 differs from Fr98 only at the envelope gene and the 3′ end of the polymerase gene (12, 28). Therefore, this region is critical for the development of neurological disease in this model. Both Fr98 and Fr54 infect microglia and capillary endothelia located primarily in the white matter tracts of the cerebellum as well as the hippocampus, thalamus, and corpus callosum. The two viruses also induce similar pathology, characterized by astrogliosis and microgliosis with minimal degenerative changes (12, 28, 33). However, Fr98 is found at a twofold higher level than Fr54 in the brain (33), suggesting that virus load may be a factor in disease development. Fr98 does not infect neuronal cells, and no obvious morphological signs of neuronal damage are found in Fr98-infected mice (28). Thus, the mechanism of neurovirulence induced by Fr98 may be indirect, possibly involving activated astrocytes or infected endothelia or microglia. These infected and/or activated cells might lack appropriate support functions required for neuronal viability. Alternatively, these cells might injure neurons through the production of cytokines and chemokines or other toxic molecules (6, 23, 45). In the present experiments we analyzed the mRNA levels of various cytokines and chemokines in the brains of mice infected with either Fr98 or Fr54 using an RNase protection assay. A dramatic increase in the levels of several proinflammatory cytokines and chemokines was observed in Fr98-infected mice compared to Fr54-infected mice. Increased expression of some of these cytokines and chemokines was observed prior to the development of clinical symptoms, suggesting that these molecules might be involved in the pathogenesis of neurological disease induced by Fr98.
MATERIALS AND METHODS
Infection of mice.
Inbred Rocky Mountain White mice were bred and housed at the Rocky Mountain Laboratories animal facility. All animal experiments were carried out in accordance with the regulations of the Rocky Mountain Laboratories Animal Care and Use Committee and the guidelines of the National Institutes of Health. Within 24 to 48 h of birth, mice were injected intraperitoneally (i.p.) with 104 focus-forming units (FFU) of virus. Mice were observed daily for clinical signs of CNS disease. Initially mice showed signs of hyperactivity, which was followed by obvious ataxia and then seizures (30). The entire clinical course of the disease from ataxia to death was usually 1 to 5 days. The time of onset of symptoms varied with the different virus clones studied.
Viruses.
The construction of virus clones Fr98, Fr54, SE, EC, and EC-1 has been previously described (12, 28, 30). All clones were created by inserting polytropic envelope sequences into a nonneurovirulent ecotropic Friend virus clone, FB29. Virus stocks were prepared from the supernatants of confluently infected Mus dunni fibroblast cells (12). Virus titers were determined by focal infectivity assay using the envelope-specific monoclonal antibody 514 (33).
RNase protection assay.
Infected mice were exsanguinated by axillary incision under deep isoflurane anesthesia. Brains were removed from infected mice at the indicated times, and the cerebrum and midbrain were separated from the cerebellum and brain stem using a razor blade. The tissues were immediately frozen in liquid nitrogen and stored at −80°C. Total RNA from the cerebrum or cerebellum was prepared using Trizol reagent (Life Technologies, Rockville, Md.) according to the manufacturer's instructions. RNA was quantified by spectroscopy at 260 nm and diluted to equal concentration in hybridization buffer (PharMingen, San Diego, Calif.) and stored at −80°C until use. The RNA was then analyzed for specific cytokine and chemokine mRNA using the RiboQuant system (PharMingen). Approximately 8 to 10 μg of total RNA was hybridized overnight with [α-32P]UTP (NEN Life Sciences, Boston, Mass.)-labeled RNA probes (PharMingen). Samples were then treated with RNase (PharMingen) and precipitated, and protected cytokine RNA probes were resolved on precast Quick Point polyacrylamide gels (PharMingen). Bands were quantified using a STORM PhosphorImager (Molecular Dynamics) and Image Quant software. Data were expressed as a percentage of the protected cytokine RNA compared to protected RNA from L32 (housekeeping gene). mRNA from mice infected with the culture supernatant from uninfected Mus dunni cells (mock infected), were used as negative controls. As both Fr54 and Fr98 were created by inserting polytropic envelope sequences into the nonvirulent FB29 clone, mRNA was also analyzed from mice infected with FB29 as an additional negative control.
Statistics.
Statistical comparisons between groups were done using a one-way analysis of variance and the Newman-Keuls Multiple Comparison test. All data were expressed as the percentage of protected cytokine RNA compared to protected RNA from L32.
RESULTS
Cytokine and chemokine mRNA expression in Fr98- or Fr54-infected mice.
Although Fr98 and Fr54 have the same cell tropism and induce similar morphological changes as observed by histopathology, Fr98 induces a severe neurological disease while Fr54 does not (28, 33). One possible mechanism by which Fr98 may induce neurological disease is through the production of cytokines and/or chemokines (1, 23). To study the possible relationship between cytokine and/or chemokine expression and the development of Fr98-induced neurological disease, cytokine and chemokine mRNA levels in the cerebellum of mice infected with Fr98 or Fr54 were compared using an RNase protection assay. Cerebellar RNA was analyzed first because there is a high level of viral infection in the cerebellum and the main clinical symptom, ataxia, suggests an impairment of cerebellar functions (28). Cytokine mRNA expression was measured in Fr98-infected mice from 7 days postinfection until the development of severe clinical symptoms (days 14 to 16) and in Fr54-infected mice from day 7 until day 30.
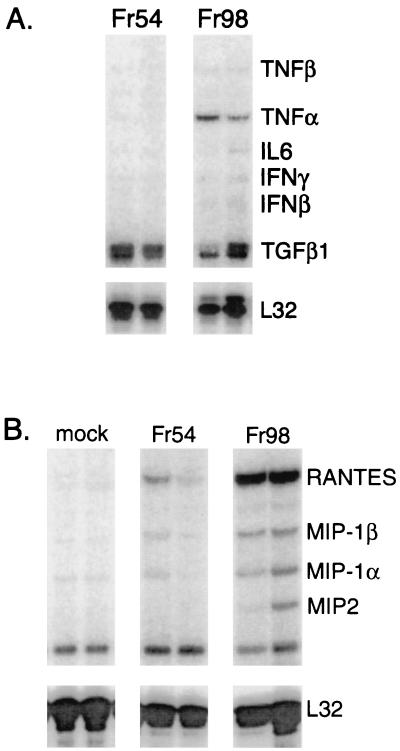
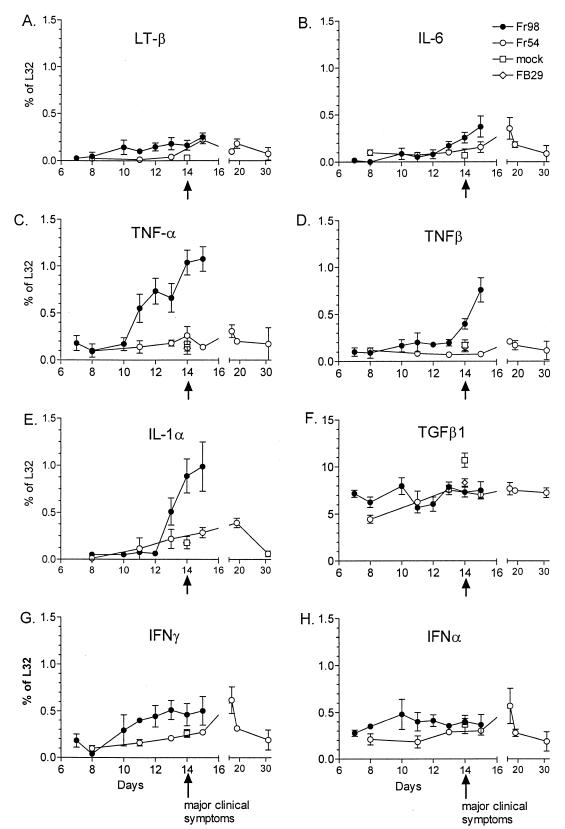
At 14 days postinfection, a significant increase in the mRNA expression of proinflammatory cytokine tumor necrosis factor alpha (TNF-α) was observed in Fr98-infected mice relative to Fr54- or control-infected mice (Fig. 1A). This increase in TNF-α mRNA expression in Fr98-infected mice was first observed at day 11 postinfection, prior to the development of clinical symptoms, and remained high throughout the duration of the illness until mice died between days 14 and 16 (Fig. 2C). An increase in TNF-β and interleukin-1α (IL-1α) mRNA expression occurred slightly later at days 13 to 15 postinfection, corresponding with the onset of clinical symptoms (Fig. 2D and E). In contrast, the mRNA expression levels of other cytokines measured, including lymphotoxin β, IL-6, transforming growth factor β, alpha interferon (IFN-α) (Fig. 2), IL-18, and IL-12 (data not shown) were similar between Fr98-infected mice and control mice. There was no increase in any T cell-related cytokine mRNA including IL-2, IL-15, IL-4, and IL-10, and no increase in mRNA expression was observed for T-cell markers CD4, CD8, or CD3 (data not shown). A slight increase in IFN-γ mRNA expression was observed in Fr98-infected mice, although this increase was not statistically significant (Fig. 2G). The lack of increased mRNA for T-cell cytokines or T-cell expression markers concurs with previous results demonstrating the absence of inflammatory infiltrate in the brains of Fr98-infected mice (28).
FIG. 1.
Cytokine and chemokine mRNA expression in the cerebellum of mice infected with Fr54 or Fr98. Mice were infected i.p. with 104 FFU of either Fr98 or Fr54 at 24 to 48 h after birth. Cerebellums were removed at 14 days postinfection. Total RNA was purified from the cerebellum and analyzed for cytokine mRNA expression using the RNase protection assay. RNase protection assay results from two mice from each infected group are shown.
FIG. 2.
Kinetics of cytokine mRNA expression in the cerebellum of mice infected with Fr54 or Fr98. Mice were infected i.p. with 104 FFU of either Fr98 or Fr54 at 24 to 48 h after birth. Cerebellums were removed at the times indicated postinfection. Total RNA was purified from the cerebellum and analyzed for cytokine mRNA expression using the RNase protection assay. Results are expressed as the ratio of cytokine RNA to L32 (housekeeping gene) RNA for each sample. Results are the average of three to six mice per data point. Mock- and FB29-infected mice were also analyzed as a control for cytokine expression at day 14 postinfection. The arrow at day 14 indicates time of onset of clinical ataxia in Fr98-infected mice.
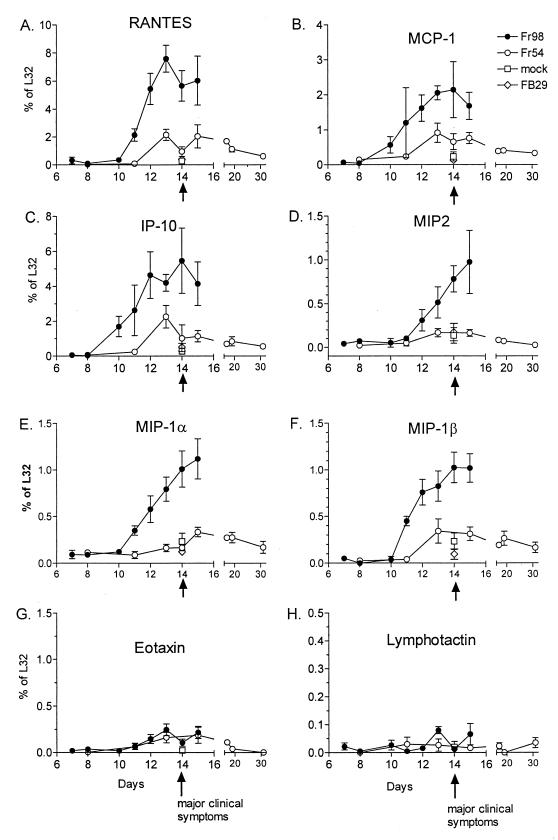
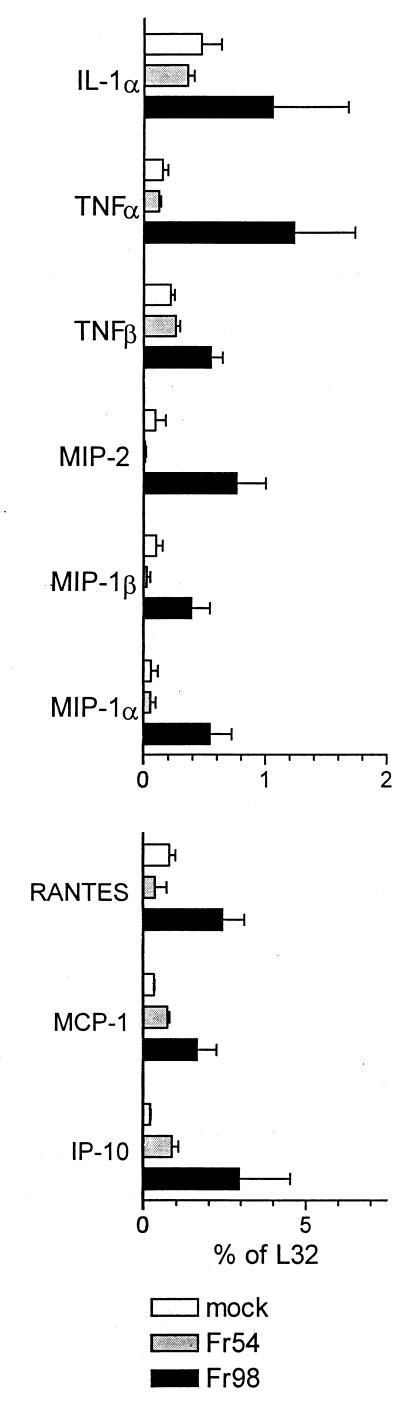
Both astrocytes and microglia have been reported to produce chemokines after in vitro activation by various stimuli including viruses and/or viral proteins (7, 16, 22, 26, 44). Therefore RNA from Fr98- and Fr54-infected mice was also analyzed for chemokine mRNA levels. mRNA expression of six chemokine genes, RANTES (regulated on activation, normal T cell expressed and secreted), monocyte chemoattractant protein 1 (MCP-1), IFN-γ-inducible protein 10 (IP-10), macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and MIP-2 was significantly higher in Fr98-infected mice than in either mock- or Fr54-infected mice (Fig. 1B). In kinetic studies, increased levels were first observed at 11 days postinfection and remained high throughout the duration of the illness (Fig. 3A, B, and D through F). In contrast, no differences were observed between the expression levels of lymphotactin or eotaxin mRNA in Fr98- or Fr54-infected mice (Fig. 3G and H). mRNA expression levels of the chemokine receptors, CC chemokine receptor 1 (CCR-1), CCR-2, and CCR-5 were similar in mock-, Fr98-, and Fr54-infected mice (data not shown). By being stained with antiviral antibody, Fr98-infected cells were found predominately in the cerebellum, but focal areas of infection were also observed in the hippocampus, corpus callosum, and thalamus sections of the cerebrum (28). Accordingly, at 14 days postinfection the same pattern of increased cytokines and chemokines was found in the cerebrum of mice infected with Fr98 as was seen in the cerebellum (compare Fig. 2 and 3 with Fig. 4).
FIG. 3.
Kinetics of chemokine mRNA expression in the cerebellum of mice infected with Fr54 or Fr98. Total cerebellar RNA was analyzed for chemokine expression using the RNase protection assay described in Fig. 1. The specificity of MCP-1 and IP-10 expression by the RNase protection assay (11) was confirmed by a probe set containing only MCP-1, L32, and GAPDH or IP-10, L32, and GAPDH. Results are the average for three to six mice per data point. Mock- and FB29-infected mice were also analyzed as a control for cytokine expression at day 14 postinfection.
FIG. 4.
Cytokine and chemokine mRNA expression in the cerebrum of mice infected with Fr98 or Fr54 or mock infected at 14 days postinfection. Total cerebrum RNA was analyzed for chemokine expression using the RNase protection assay described in Fig. 1. Results are the average for three mice per data point.
In the brain, cytokines have been reported both to contribute to neuronal apoptosis and to provide neuroprotective responses (19). However, mRNA expression levels of apoptosis genes, Fas, FasL, Fas-activated death domain, TNF-activated death domain, Bax, Bcl-x, and apoptosis inhibitory genes Bcl-w and Bcl-2 were similar in Fr98-, Fr54-, and mock-infected mice (data not shown). Although these genes can be regulated by posttranscriptional modification, these results are consistent with the absence of morphological signs of neuronal death in Fr98-infected mice (28).
Similar pattern of cytokine and chemokine mRNA expression observed in SE-infected mice.
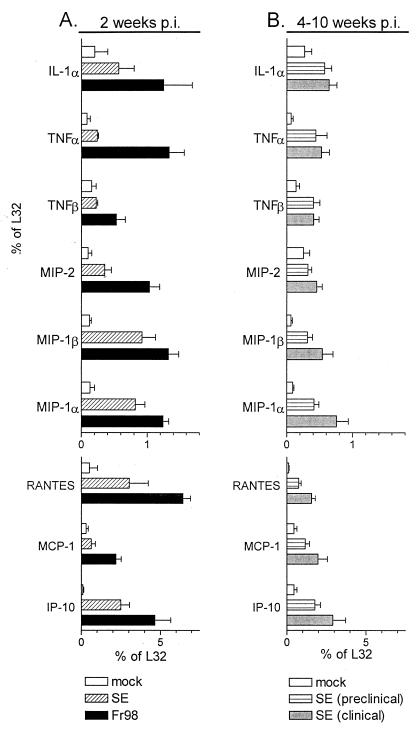
The recombinant virus clone SE contains the N-terminal one-third of the Fr98 env gene and the C-terminal two-thirds of the Fr54 env gene. Infection with SE induces neurological disease with clinical symptoms and pathology similar to those of Fr98, but the development of disease is slower with clinical symptoms not appearing until 4 to 10 weeks postinfection (12). At 2 weeks postinfection, a time point where Fr98-infected mice have clinical symptoms but SE-infected mice do not, expression of mRNAs for chemokines MIP-1α, MIP-1β, RANTES, and IP-10 was significantly higher in SE-infected mice than in mock-infected controls (Fig. 5A). However, in contrast to the results observed in Fr98-infected mice, mRNA levels of cytokines IL-1α, TNF-α, and TNF-β and chemokines MIP-2 and MCP-1 were not significantly upregulated at 2 weeks in SE-infected mice (Fig. 5A). These differences might correlate with the different times of clinical disease onset in SE- and Fr98-infected mice. Therefore, SE-infected mice were also examined at later times when clinical symptoms were apparent.
FIG. 5.
Cytokine and chemokine mRNA expression in the cerebellum of mice infected with SE. (A) Cerebellums were removed from Fr98-, SE-, or mock-infected mice at 14 days postinfection. At this time point, only Fr98-infected mice had clinical symptoms of disease. Total RNA was analyzed for cytokine and chemokine mRNA expression as described in the legend to Fig. 1. Results are the average for four to six mice per group. (B) Cerebellums were removed from SE-infected mice after the development of clinical symptoms and were compared to those of age-matched but nonsymptomatic SE-infected mice or mock-infected mice. Results are the average for four to eight mice per group.
The progression of neurological disease is more variable in SE-infected mice than in Fr98-infected mice. To ascertain if clinical symptoms in SE-infected mice coincided with increased cytokine and chemokine mRNA expression, at 4 to 10 weeks postinfection with SE, mice with either severe clinical symptoms (clinical) or no symptoms (preclinical) were analyzed. Surprisingly, no significant differences were observed between preclinical and clinical SE-infected mice (Fig. 5B). The expression of MIP-1α, MIP-1β, RANTES, and IP-10 chemokine mRNAs remained at high levels in all SE-infected mice at 4 to 10 weeks postinfection (Fig. 5B). In addition, mRNA levels of cytokines IL-1α, TNF-α, and TNF-β and the chemokine MCP-1 which were not upregulated at 2 weeks postinfection were upregulated at 4 to 10 weeks (Fig. 5B). In contrast, MIP-2 mRNA levels were not upregulated in SE-infected mice at these later time points.
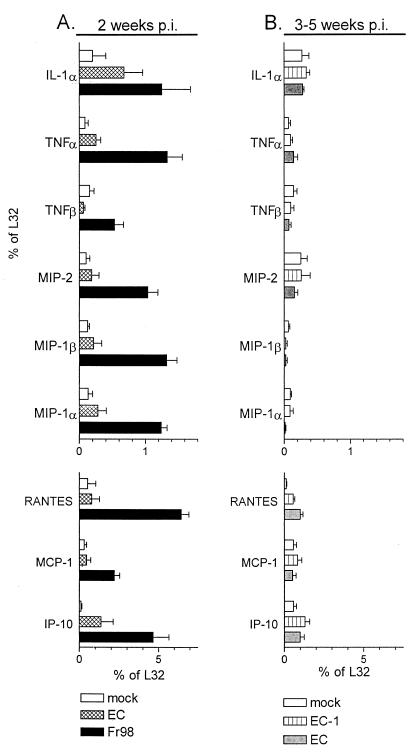
Lack of cytokine or chemokine mRNA upregulation after EC virus infection.
The chimeric virus clone EC contains a second neurovirulence determinant located in the EcoRI/AvrII region in the middle of the Fr98 envelope gene (12). The disease induced by EC develops at 3 to 5 weeks postinfection, and the pathology and clinical symptoms are similar to those induced by Fr98 and SE (12). At 2 weeks postinfection, prior to development of clinical signs, IP-10 was significantly upregulated in EC-infected mice compared to mock-infected controls (Fig. 6A). IL-1α also appeared slightly increased at this time but this increase was not statistically significant. Neither IP-10 nor IL-1α mRNA expression was increased in EC-infected mice at the time of clinical disease, 3 to 5 weeks postinfection (Fig. 6B). All of the other cytokines and chemokines analyzed were not increased in EC-infected mice at either time tested as compared to mock-infected controls or to mice infected with EC-1, which differs from EC by two amino acids but does not induce neurological disease. Thus, with the exception of IP-10 at 2 weeks postinfection, the development of neurological disease in EC-infected mice did not correlate with an increase in the cytokines or chemokines that were upregulated in mice infected by Fr98 and SE viruses.
FIG. 6.
Cytokine and chemokine mRNA expression in the cerebellum of mice infected with EC. (A) Cerebellums were removed from Fr98-, EC-, or mock-infected mice at 14 days postinfection. Only Fr98-infected mice had clinical symptoms of disease. Total RNA was analyzed for cytokine and chemokine mRNA expression as described in the legend to Fig. 1. Results are the average for four to six mice per group. Although the increase in IP-10 expression at 2 weeks postinfection in EC-infected mice is significant, this could be due to the very low values for the mock-infected control at this time point. (B) Cerebellums were removed from EC-infected mice after the development of clinical symptoms and compared to those of age-matched but nonsymptomatic mice infected with the nonvirulent virus EC-1, which differs from EC by two amino acids in the envelope gene. RNAs from the cerebellum of age-matched mock-infected mice were used as additional negative controls. Results are the average for four to eight mice per group.
DISCUSSION
The upregulation of cytokine and chemokine mRNA in the CNS after retroviral infection (1, 27, 36, 42) may be responsible for the induction of neurological disease by these viruses (9). Alternatively, the increased expression of these genes may not be a mechanism of viral pathogenesis but rather a host response to either retroviral infection of the CNS or virus-induced neuronal damage. In the current study with polytropic MuLVs, the increase in cytokine and chemokine mRNA expression was not due to a generic host response to viral infection, as avirulent viruses did not induce upregulation of gene expression (Fig. 1 to 3). Furthermore, the upregulation of cytokine and chemokine mRNA expression after neurovirulent retroviral infection did not appear to be a late response to viral pathogenesis, as the upregulation of these genes occurred prior to the development of clinical disease (Fig. 2, 3, and 5). Thus, the upregulation of cytokine and chemokine mRNA expression could be part of the disease process induced by neurovirulent retroviral infection. However, it cannot be ruled out that these genes were upregulated in response to an early pathogenic event and are an indicator, but not a mechanism, of disease progression.
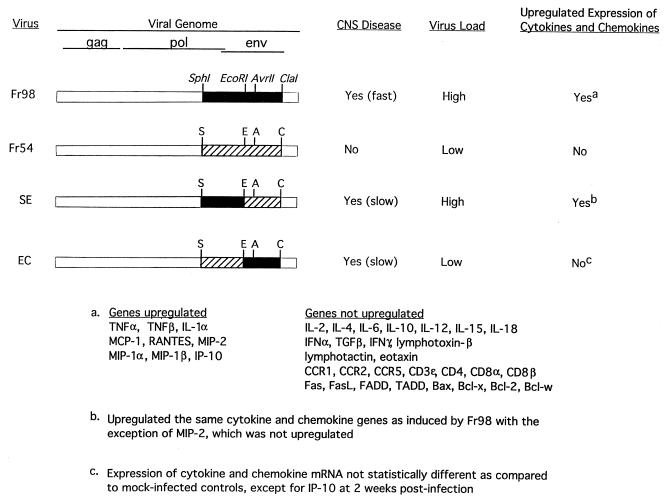
The upregulation of cytokines and chemokines by Fr98 but not Fr54 demonstrates that the SphI to ClaI region of Fr98 is responsible for the upregulation of these genes (Fig. 7). This region can be further restricted to the SphI to EcoRI segment, as virus clone SE, but not virus clone EC, induced the mRNA expression of many of the same cytokines and chemokines induced by Fr98 infection (Fig. 7). At the time of clinical disease, two- to threefold higher cerebellar virus levels were found in mice infected with Fr98 or SE than in age-matched mice infected with Fr54 or EC (28, 29). Thus, viral burden may influence the upregulation of cytokine and/or chemokine mRNA. The rapid progression of disease in Fr98-infected mice makes it difficult to determine the relationship between virus load and cytokine and chemokine upregulation. In contrast, disease progression is slower in SE-infected mice and viral burden increases from a level similar to that of Fr54 at 2 weeks postinfection to a higher level, similar to that of Fr98, at 4 to 10 weeks postinfection (33). In SE-infected mice, chemokine mRNA was upregulated at 2 weeks postinfection (Fig. 5A), prior to the increase in viral burden. Thus, chemokine mRNA upregulation did not appear to be dependent on high virus load in the cerebellum. In contrast, cytokine mRNA expression may be induced by a high virus load, as cytokine mRNA was not upregulated until 4 to 10 weeks postinfection (Fig. 5B). The high virus load in Fr98- and SE-infected mice may itself be induced by the increased expression of cytokines such as TNF-α, which has been shown to increase retroviral expression in vitro (8, 39).
FIG. 7.
Correlation between envelope sequences and cytokine and chemokine expression. The viral genomes of Fr98, Fr54, SE, and EC are shown. Black bars indicate Fr98 envelope gene sequences, hatched bars indicate Fr54 envelope gene sequences, and white bars indicate FB29 sequences. Development of clinical signs of ataxia, seizures, and death occurred at approximately 2 weeks in Fr98-infected mice, 4 to 10 weeks in SE-infected mice, and 3 to 5 weeks in EC-infected mice. High virus load indicates that viral p30 expression in the cerebellum at the time of clinical disease was two- to threefold higher than that in mice with low viral loads (30). Upregulated expression of cytokine and chemokine mRNA was determined by comparison to mock-infected controls as described in Fig. 1 through 5.
The ability of virus clone EC to induce clinical symptoms without increasing cytokine or chemokine mRNA expression demonstrates that the upregulation of these genes is not necessary for the development of all retrovirus-induced neurological disease. EC contains a different segment of the Fr98 envelope than SE (Fig. 7) (12) and induces neurological disease at a lower viral burden than SE (30, 33). Therefore, the neurovirulent regions encoded by EC and SE may influence different aspects of the disease process and, when combined, induce disease at an increased rate as observed during Fr98 infection. For example, the EC region of the envelope gene could possibly be directly neurotoxic, as has been postulated for envelope sequences of other viruses such as HIV and Sindbis (15, 18), and the SE region might influence the rate of virus spread into the brain (33).
The pathology induced by polytropic MuLVs consists mainly of gliosis with minimal neuronal degeneration and in this regard is similar to HIV dementia in humans. Although increased levels of cytokine and chemokine mRNA expression have been found in some patients with HIV dementia (36, 42), not all patients show upregulation even at the terminal stage of disease when brain samples are taken. In the current study with polytropic MuLVs, two clones, SE and EC, which contain different regions of the Fr98 envelope gene, differed in their capacity to induce cytokine and chemokine mRNA expression even though both clones caused clinically similar neurological diseases (Fig. 7). By analogy, the differences in cytokine and chemokine mRNA expression in some HIV dementia cases may be due to sequence differences in the envelope gene or other genes of HIV variants (31). Possibly, HIV may induce neurological disease by more than one pathway, which may or may not include cytokine and/or chemokine mRNA upregulation.
In contrast to the polytropic MuLVs studied here, the neurological disease induced by the ecotropic MuLV virus, FrCasE, is associated with extensive spongiform pathology (28). Fr98 and FrCasE infect the same cell types but in different regions of the brain and utilizing different receptors. Similar to Fr98, FrCasE infection did induce the upregulation of several chemokines and cytokines (1a), including TNF-α, RANTES, MIP-1α, MIP-1β, and MCP-1. However, only MIP-1α and MIP-1β were upregulated in FrCasE mice prior to the onset of severe clinical disease, and these chemokines appeared to associate with regions of spongiosis (1a). This more restricted response in FrCasE-infected mice is surprising, since FrCasE is present in the brain at two- to threefold higher levels than Fr98 (28). As Fr98 differs from FrCasE only at the envelope region, the differences in cytokine and chemokine responses and pathology induced by the two viruses appear to be encoded by the envelope sequences.
The most likely sources of the increased cytokine and chemokine mRNA expression found in Fr98- and SE-infected mice are astrocytes and/or microglia, both of which can produce cytokines and chemokines after in vitro stimulation (24, 26, 45). As all of the viruses in this study induce microgliosis and astrogliosis, activation of microglia or astrocytes alone is not responsible for the increase in cytokine and chemokine mRNA expression (30, 33). Astrocytes and microglia have unique proliferative and cytokine responses dependent upon the type of in vitro activation, demonstrating that these cells can respond differently to specific stimuli (16, 37, 44). Thus, the difference in cytokine and chemokine profiles observed after Fr98 and Fr54 infection may be due to different downstream signaling events or biochemical pathways in astrocytes and/or microglia induced by Fr98 and SE but not by Fr54 or EC.
The similar levels of mRNA expression for apoptosis genes in Fr98- and Fr54-infected mice suggest that neuronal apoptosis is not a primary mechanism of disease induction in this model. The lack of apoptosis gene upregulation is consistent with the absence of morphological signs of neuronal damage and the lack of TUNEL-positive neurons in Fr98-infected mice (data not shown) (28). Thus, the neurological disease induced by Fr98 may be the result of impaired neuronal function rather than the loss of neurons.
The upregulation of cytokines and chemokines like TNF-α, IL-1α, MIP-1α, and MCP-1 is normally associated with recruiting immune cells to a site of inflammation (1, 32, 38). However, no inflammatory infiltrate is associated with the neurological disease induced by Fr98 and SE (28). This lack of infiltrate may be due to T-cell tolerance induced by neonatal viral infection, preventing the recruitment of inflammatory cells to the brain (3, 34). A similar pattern of increased cytokines, no inflammation, but the development of neurological disease is also observed following neonatal infection with Borna disease virus in rats (35) and Sindbis virus in mice (40). In the absence of inflammation, there may be other pathological effects of increased cytokines and chemokines since, at physiological levels, chemokines are involved in neuronal development and signaling (2) and neurotoxic cytokines such as TNF-α (10, 43) may be detrimental to brain function.
REFERENCES
- 1.Asensio V C, Campbell I L. Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci. 1999;22:504–512. doi: 10.1016/s0166-2236(99)01453-8. [DOI] [PubMed] [Google Scholar]
- 1a.Askovic S, Favara C, McAtee F, Portis J L. Increased expression of MIP-1α and MIP-1β mRNAs in the brain correlates spatially and temporally with the spongiform neurodegeneration induced by a murine oncornavirus. J Virol. 2001;75:2665–2674. doi: 10.1128/JVI.75.6.2665-2674.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacon K B, Harrison J K. Chemokines and their receptors in neurobiology: perspectives in physiology and homeostasis. J Neuroimmunol. 2000;104:92–97. doi: 10.1016/s0165-5728(99)00266-0. [DOI] [PubMed] [Google Scholar]
- 3.Basch R S, Grausz D, Harris N, Mitchison N A. Murine leukemia virus-associated cell surface antigens in rats neonatally infected with Gross murine leukemia virus. JNCI. 1979;63:1485–1492. [PubMed] [Google Scholar]
- 4.Boche D, Hurtrel M, Gray F, Claessens-Maire M A, Ganiere J P, Montagnier L, Hurtrel B. Virus load and neuropathology in the FIV model. J Neurovirol. 1996;2:377–387. doi: 10.3109/13550289609146903. [DOI] [PubMed] [Google Scholar]
- 5.Boche D, Khatissian E, Gray F, Falanga P, Montagnier L, Hurtrel B. Viral load and neuropathology in the SIV model. J Neurovirol. 1999;5:232–240. doi: 10.3109/13550289909015809. [DOI] [PubMed] [Google Scholar]
- 6.Bonwetsch R, Croul S, Richardson M W, Lorenzana C, Valle L D, Sverstiuk A E, Amini S, Morgello S, Khalili K, Rappaport J. Role of HIV-1 Tat and CC chemokine MIP-1 alpha in the pathogenesis of HIV associated central nervous system disorders. J Neurovirol. 1999;5:685–694. doi: 10.3109/13550289909021297. [DOI] [PubMed] [Google Scholar]
- 7.Conant K, Garzino-Demo A, Nath A, McArthur J C, Halliday W, Power C, Gallo R C, Major E O. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duh E J, Maury W J, Folks T M, Fauci A S, Rabson A B. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci USA. 1989;86:5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein L G, Gendelman H E. Human immunodeficiency virus type 1 infection of the nervous system: pathogenetic mechanisms. Ann Neurol. 1993;33:429–436. doi: 10.1002/ana.410330502. [DOI] [PubMed] [Google Scholar]
- 10.Gelbard H A, Dzenko K A, DiLoreto D, del Cerro C, del Cerro M, Epstein L G. Neurotoxic effects of tumor necrosis factor alpha in primary human neuronal cultures are mediated by activation of the glutamate AMPA receptor subtype: implications for AIDS neuropathogenesis. Dev Neurosci. 1993;15:417–422. doi: 10.1159/000111367. [DOI] [PubMed] [Google Scholar]
- 11.Hallensleben W, Biro L, Sauder C, Hausmann J, Asensio V C, Campbell I L, Staeheli P. A polymorphism in the mouse crg-2/IP-10 gene complicates chemokine gene expression analysis using a commercial ribonuclease protection assay. J Immunol Methods. 2000;234:149–151. doi: 10.1016/s0022-1759(99)00197-0. [DOI] [PubMed] [Google Scholar]
- 12.Hasenkrug K J, Robertson S J, Porti J, McAtee F, Nishio J, Chesebro B. Two separate envelope regions influence induction of brain disease by a polytropic murine retrovirus (FMCF98) J Virol. 1996;70:4825–4828. doi: 10.1128/jvi.70.7.4825-4828.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrmann M, Hagenhofer M, Kalden J R. Retroviruses and systemic lupus erythematosus. Immunol Rev. 1996;152:145–156. doi: 10.1111/j.1600-065x.1996.tb00914.x. [DOI] [PubMed] [Google Scholar]
- 14.Izumo S, Umehara F, Kashio N, Kubota R, Sato E, Osame M. Neuropathology of HTLV-1-associated myelopathy (HAM/TSP) Leukemia. 1997;11(Suppl. 3):82–84. [PubMed] [Google Scholar]
- 15.Joe A K, Foo H H, Kleeman L, Levine B. The transmembrane domains of Sindbis virus envelope glycoproteins induce cell death. J Virol. 1998;72:3935–3943. doi: 10.1128/jvi.72.5.3935-3943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnstone M, Gearing A J, Miller K M. A central role for astrocytes in the inflammatory response to beta-amyloid; chemokines, cytokines and reactive oxygen species are produced. J Neuroimmunol. 1999;93:182–193. doi: 10.1016/s0165-5728(98)00226-4. [DOI] [PubMed] [Google Scholar]
- 17.Kolson D L, Lavi E, Gonzalez-Scarano F. The effects of human immunodeficiency virus in the central nervous system. Adv Virus Res. 1998;50:1–47. doi: 10.1016/s0065-3527(08)60804-0. [DOI] [PubMed] [Google Scholar]
- 18.Lannuzel A, Barnier J V, Hery C, Huynh V T, Guibert B, Gray F, Vincent J D, Tardieu M. Human immunodeficiency virus type 1 and its coat protein gp120 induce apoptosis and activate JNK and ERK mitogen-activated protein kinases in human neurons. Ann Neurol. 1997;42:847–856. doi: 10.1002/ana.410420605. [DOI] [PubMed] [Google Scholar]
- 19.Licinio J. Central nervous system cytokines and their relevance for neurotoxicity and apoptosis. J Neural Transm Suppl. 1997;49:169–175. doi: 10.1007/978-3-7091-6844-8_18. [DOI] [PubMed] [Google Scholar]
- 20.Lynch W P, Czub S, McAtee F J, Hayes S F, Portis J L. Murine retrovirus-induced spongiform encephalopathy: productive infection of microglia and cerebellar neurons in accelerated CNS disease. Neuron. 1991;7:365–379. doi: 10.1016/0896-6273(91)90289-c. [DOI] [PubMed] [Google Scholar]
- 21.Masuda M, Masuda M, Ruscetti S K, Hoffman P M. Molecular mechanism for retroviral neuropathogenesis: possible involvement of capillary endothelial cells. Leukemia. 1997;11(Suppl. 3):233–235. [PubMed] [Google Scholar]
- 22.Mennicken F, Maki R, de Souza E B, Quirion R. Chemokines and chemokine receptors in the CNS: a possible role in neuroinflammation and patterning. Trends Pharmacol Sci. 1999;20:73–78. doi: 10.1016/s0165-6147(99)01308-5. [DOI] [PubMed] [Google Scholar]
- 23.Miller R J, Meucci O. AIDS and the brain: is there a chemokine connection? Trends Neurosci. 1999;22:471–479. doi: 10.1016/s0166-2236(99)01408-3. [DOI] [PubMed] [Google Scholar]
- 24.Oh J W, Schwiebert L M, Benveniste E N. Cytokine regulation of CC and CXC chemokine expression by human astrocytes. J Neurovirol. 1999;5:82–94. doi: 10.3109/13550289909029749. [DOI] [PubMed] [Google Scholar]
- 25.Park B H, Lavi E, Blank K J, Gaulton G N. Intracerebral hemorrhages and syncytium formation induced by endothelial cell infection with a murine leukemia virus. J Virol. 1993;67:6015–6024. doi: 10.1128/jvi.67.10.6015-6024.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson P K, Hu S, Salak-Johnson J, Molitor T W, Chao C C. Differential production of and migratory response to beta chemokines by human microglia and astrocytes. J Infect Dis. 1997;175:478–481. doi: 10.1093/infdis/175.2.478. [DOI] [PubMed] [Google Scholar]
- 27.Poli A, Abramo F, Di Iorio C, Cantile C, Carli M A, Pollera C, Vago L, Tosoni A, Costanzi G. Neuropathology in cats experimentally infected with feline immunodeficiency virus: a morphological, immunocytochemical and morphometric study. J Neurovirol. 1997;3:361–368. doi: 10.3109/13550289709030750. [DOI] [PubMed] [Google Scholar]
- 28.Portis J L, Czub S, Robertson S, McAtee F, Chesebro B. Characterization of a neurologic disease induced by a polytropic murine retrovirus: evidence for differential targeting of ecotropic and polytropic viruses in the brain. J Virol. 1995;69:8070–8075. doi: 10.1128/jvi.69.12.8070-8075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poulsen D J, Favara C, Snyder E Y, Portis J, Chesebro B. Increased neurovirulence of polytropic mouse retroviruses delivered by inoculation of brain with infected neural stem cells. Virology. 1999;263:23–29. doi: 10.1006/viro.1999.9917. [DOI] [PubMed] [Google Scholar]
- 30.Poulsen D J, Robertson S J, Favara C A, Portis J L, Chesebro B W. Mapping of a neurovirulence determinant within the envelope protein of a polytropic murine retrovirus: induction of central nervous system disease by low levels of virus. Virology. 1998;248:199–207. doi: 10.1006/viro.1998.9258. [DOI] [PubMed] [Google Scholar]
- 31.Power C, McArthur J C, Johnson R T, Griffin D E, Glass J D, Perryman S, Chesebro B. Demented and nondemented patients with AIDS differ in brain-derived human immunodeficiency virus type 1 envelope sequences. J Virol. 1994;68:4643–4649. doi: 10.1128/jvi.68.7.4643-4649.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ransohoff R M, Tani M, Glabinski A R, Chernosky A, Krivacic K, Peterson J W, Chien H F, Trapp B D. Chemokines and chemokine receptors in model neurological pathologies: molecular and immunocytochemical approaches. Methods Enzymol. 1997;287:319–348. doi: 10.1016/s0076-6879(97)87023-1. [DOI] [PubMed] [Google Scholar]
- 33.Robertson S J, Hasenkrug K J, Chesebro B, Portis J L. Neurologic disease induced by polytropic murine retroviruses: neurovirulence determined by efficiency of spread to microglial cells. J Virol. 1997;71:5287–5294. doi: 10.1128/jvi.71.7.5287-5294.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarzotti M. Immunologic tolerance. Curr Opin Hematol. 1997;4:48–52. doi: 10.1097/00062752-199704010-00008. [DOI] [PubMed] [Google Scholar]
- 35.Sauder C, de la Torre J C. Cytokine expression in the rat central nervous system following perinatal Borna disease virus infection. J Neuroimmunol. 1999;96:29–45. doi: 10.1016/s0165-5728(98)00272-0. [DOI] [PubMed] [Google Scholar]
- 36.Schmidtmayerova H, Nottet H S, Nuovo G, Raabe T, Flanagan C R, Dubrovsky L, Gendelman H E, Cerami A, Bukrinsky M, Sherry B. Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci USA. 1996;93:700–704. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sopper S, Demuth M, Stahl-Hennig C, Hunsmann G, Plesker R, Coulibaly C, Czub S, Ceska M, Koutsilieri E, Riederer P, Brinkmann R, Katz M, ter Meulin V. The effect of simian immunodeficiency virus infection in vitro and in vivo on the cytokine production of isolated microglia and peripheral macrophages from rhesus monkey. Virology. 1996;220:320–329. doi: 10.1006/viro.1996.0320. [DOI] [PubMed] [Google Scholar]
- 38.Stalder A K, Carson M J, Pagenstecher A, Asensio V C, Kincaid C, Benedict M, Powell H C, Masliah E, Campbell I L. Late-onset chronic inflammatory encephalopathy in immune-competent and severe combined immune-deficient (SCID) mice with astrocyte-targeted expression of tumor necrosis factor. Am J Pathol. 1998;153:767–783. doi: 10.1016/S0002-9440(10)65620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzumura A, Sawada M, Makino M, Takayanagi T. Propentofylline inhibits production of TNFalpha and infection of LP-BM5 murine leukemia virus in glial cells. J Neurovirol. 1998;4:553–559. doi: 10.3109/13550289809113500. [DOI] [PubMed] [Google Scholar]
- 40.Trgovcich J, Ryman K, Extrom P, Eldridge J C, Aronson J F, Johnston R E. Sindbis virus infection of neonatal mice results in a severe stress response. Virology. 1997;227:234–238. doi: 10.1006/viro.1996.8289. [DOI] [PubMed] [Google Scholar]
- 41.Vitkovic L, Stover E, Koslow S H. Animal models recapitulate aspects of HIV/CNS disease. AIDS Res Hum Retrovir. 1995;11:753–759. doi: 10.1089/aid.1995.11.753. [DOI] [PubMed] [Google Scholar]
- 42.Wesselingh S L, Power C, Glass J D, Tyor W R, McArthur J C, Farber J M, Griffin J W, Griffin D E. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann Neurol. 1993;33:576–582. doi: 10.1002/ana.410330604. [DOI] [PubMed] [Google Scholar]
- 43.Westmoreland S V, Kolson D, Gonzalez-Scarano F. Toxicity of TNF alpha and platelet activating factor for human NT2N neurons: a tissue culture model for human immunodeficiency virus dementia. J Neurovirol. 1996;2:118–126. doi: 10.3109/13550289609146545. [DOI] [PubMed] [Google Scholar]
- 44.Xiao B G, Mousa A, Kivisakk P, Seiger A, Bakhiet M, Link H. Induction of beta-family chemokines mRNA in human embryonic astrocytes by inflammatory cytokines and measles virus protein. J Neurocytol. 1998;27:575–580. doi: 10.1023/a:1006918110952. [DOI] [PubMed] [Google Scholar]
- 45.Yeung M C, Pulliam L, Lau A S. The HIV envelope protein gp120 is toxic to human brain-cell cultures through the induction of interleukin-6 and tumor necrosis factor-alpha. AIDS. 1995;9:137–143. [PubMed] [Google Scholar]