Abstract
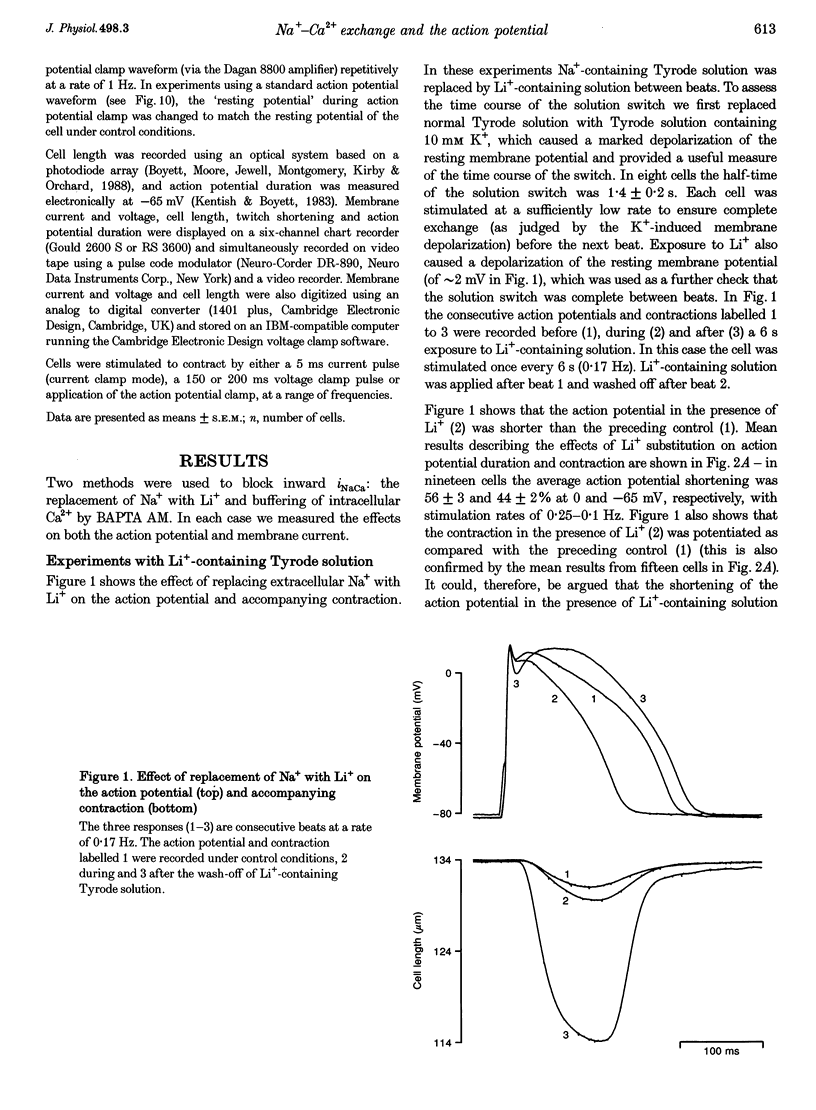
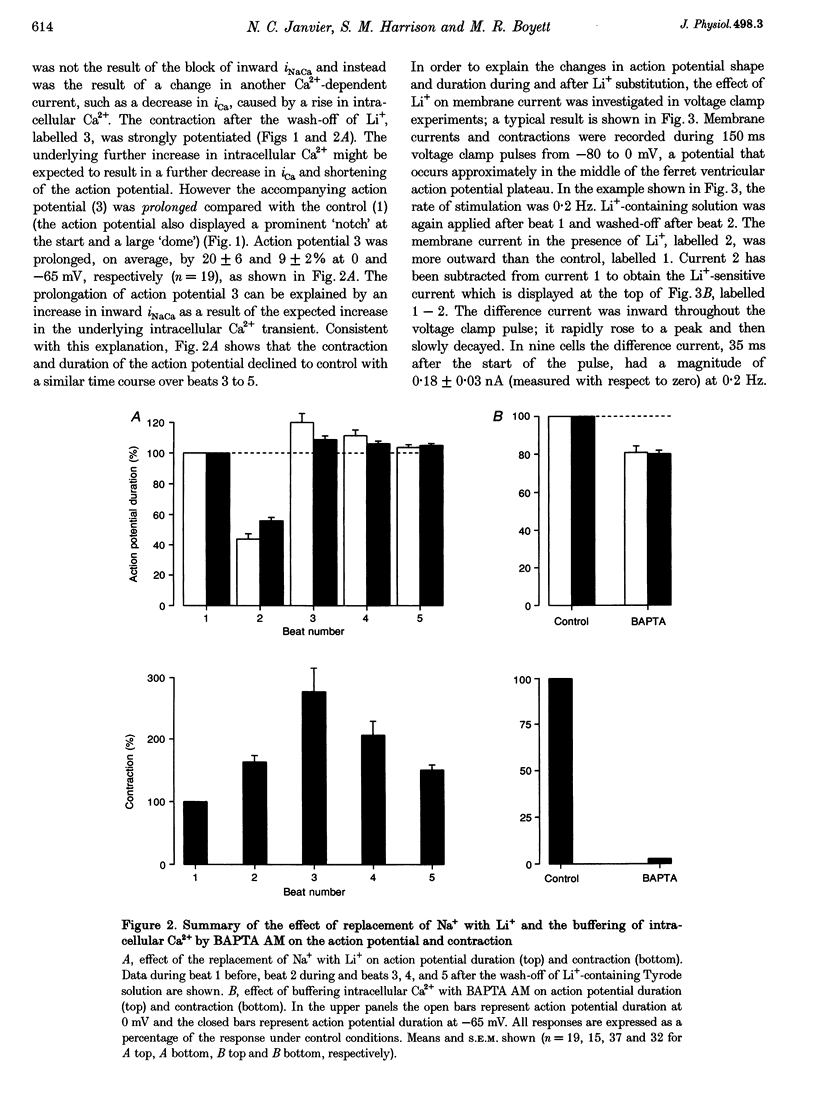
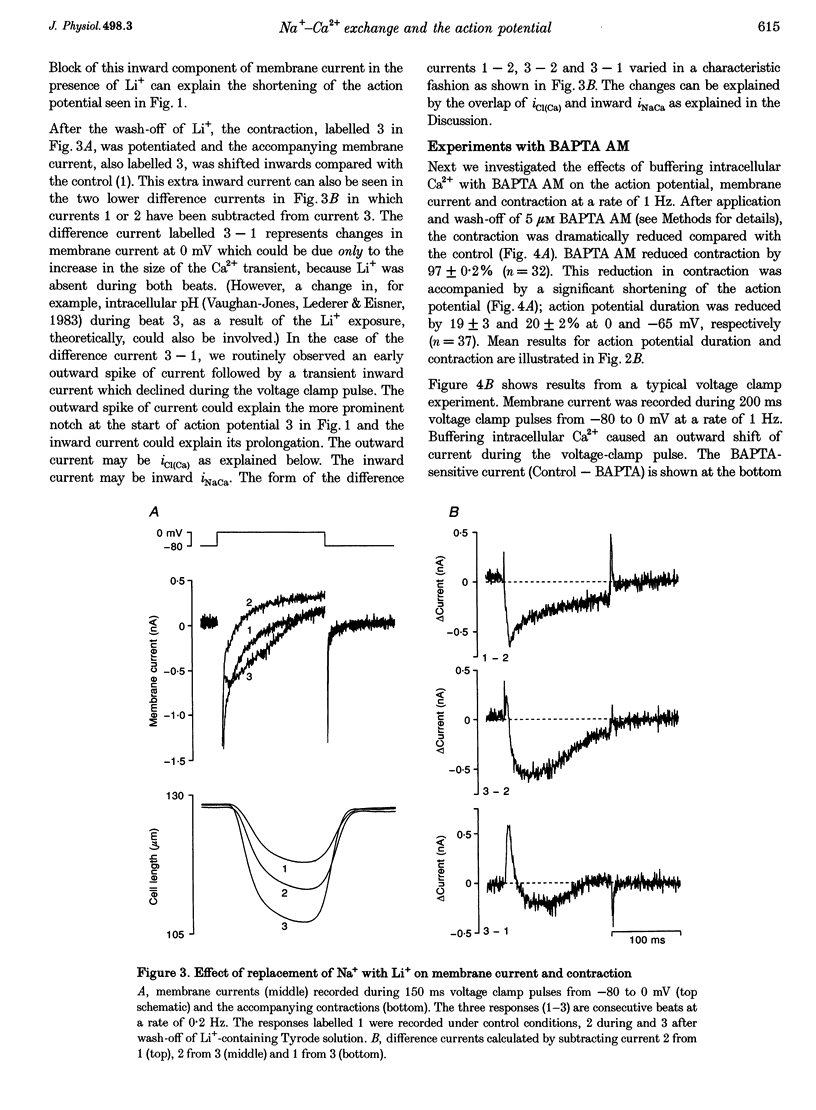
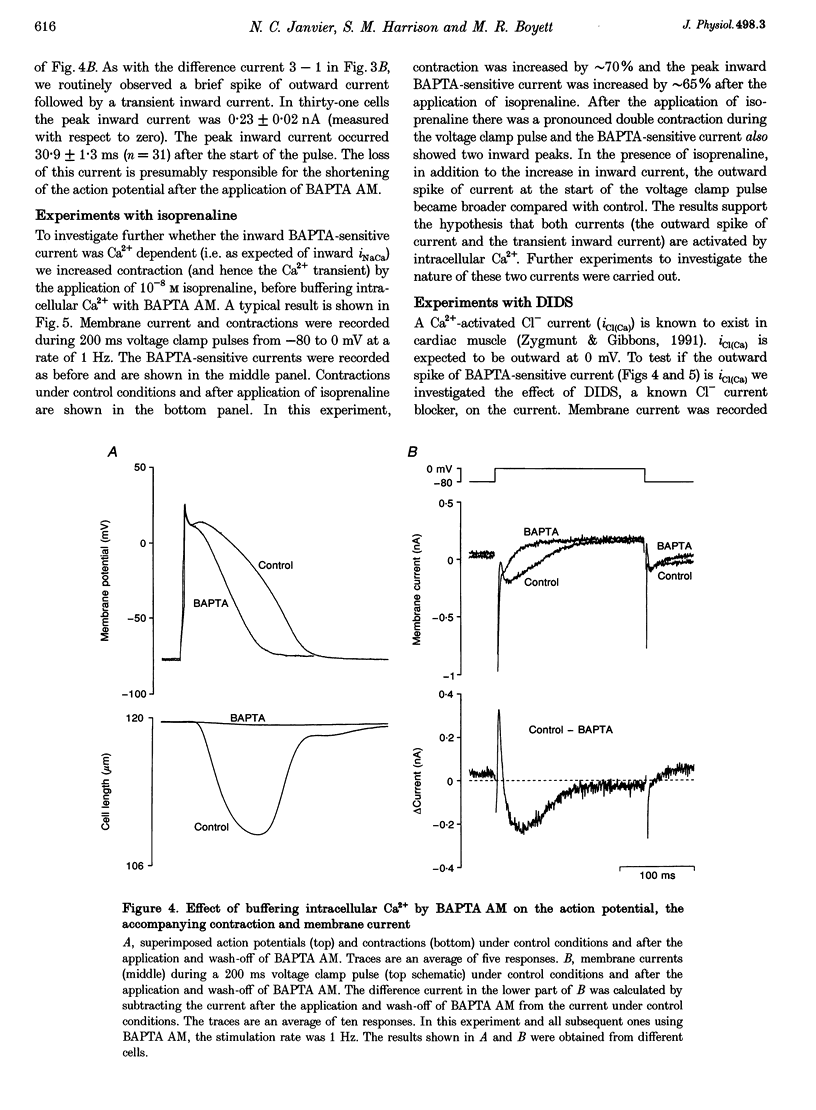
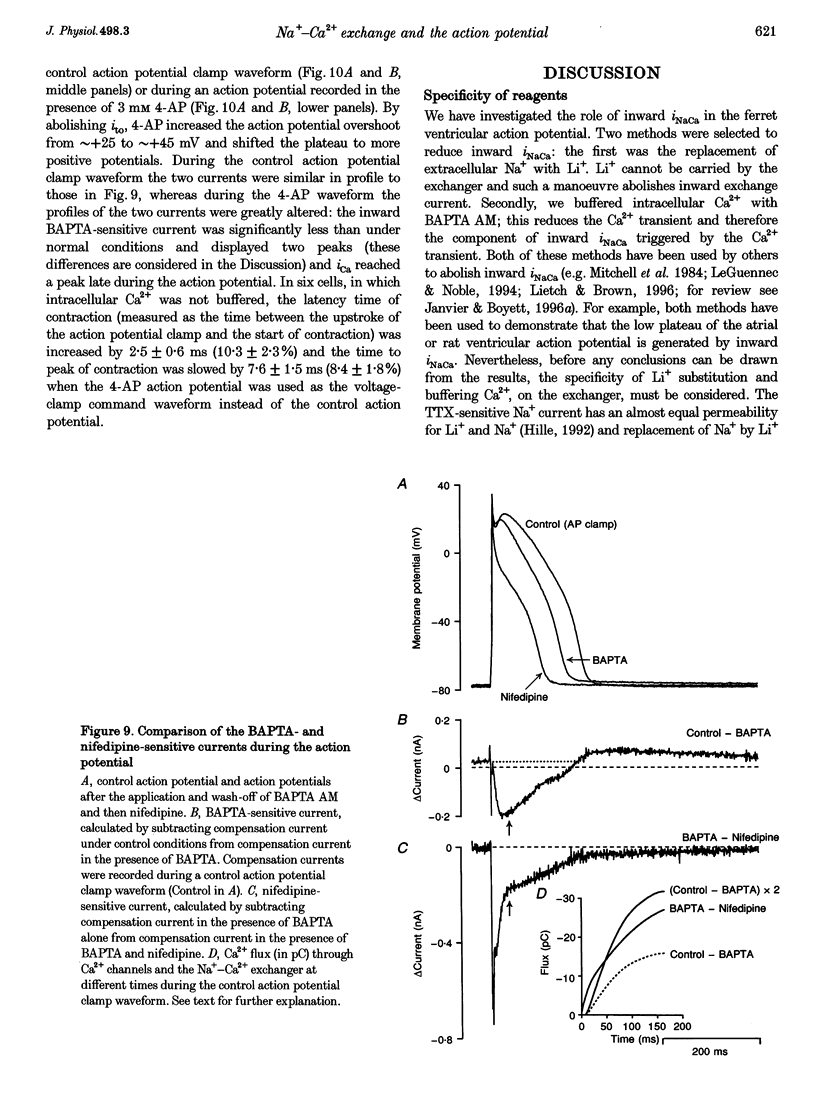
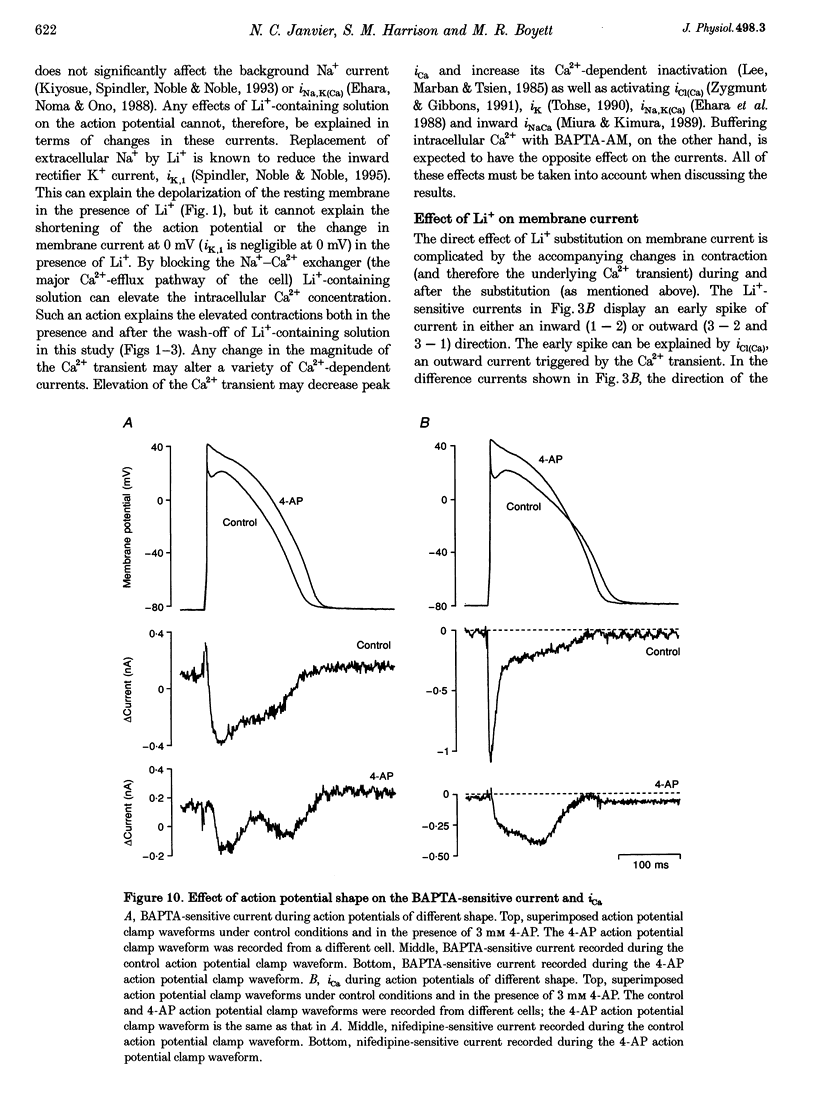
1. Inward Na(+)-Ca2+ exchange current (iNaCa) was either blocked in ferret ventricular cells by replacing extracellular Na+ with Li+ or substantially reduced by the almost complete elimination of the Ca2+ transient by buffering intracellular Ca2+ with the acetoxymethyl ester form of BAPTA (BAPTA AM). 2. During square wave voltage clamp pulses to 0 mV, replacing extracellular Na+ with Li+ or buffering intracellular Ca2+ with BAPTA AM resulted in the loss of a transient inward current. This current was increased by the application of isoprenaline (expected to increase the underlying Ca2+ transient) and displayed the voltage-dependent characteristics of inward iNaCa. 3. Replacing extracellular Na+ with Li+ or buffering intracellular Ca2+ caused a significant shortening of the action potential (at -65 mV, 44 +/- 2% with Li+ and 20 +/- 2% with BAPTA AM). The shortening can be explained by changes in iNaCa. 4. The action potential clamp technique was used to measure the BAPTA-sensitive current (putative iNaCa) and the Ca2+ current (ica; measured using nifedipine) during the action potential. Under control conditions, the inward BAPTA-sensitive current makes approximately the same contribution as iCa during much of the action potential plateau. These results suggest an important role for inward iNaCa in the ferret ventricular action potential.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouchard R. A., Clark R. B., Giles W. R. Regulation of unloaded cell shortening by sarcolemmal sodium-calcium exchange in isolated rat ventricular myocytes. J Physiol. 1993 Sep;469:583–599. doi: 10.1113/jphysiol.1993.sp019831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R., Kirby M. S., Orchard C. H. Rapid regulation of the 'second inward current' by intracellular calcium in isolated rat and ferret ventricular myocytes. J Physiol. 1988 Dec;407:77–102. doi: 10.1113/jphysiol.1988.sp017404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R., Moore M., Jewell B. R., Montgomery R. A., Kirby M. S., Orchard C. H. An improved apparatus for the optical recording of contraction of single heart cells. Pflugers Arch. 1988 Dec;413(2):197–205. doi: 10.1007/BF00582531. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Lederer W. J. A novel experimental chamber for single-cell voltage-clamp and patch-clamp applications with low electrical noise and excellent temperature and flow control. Pflugers Arch. 1986 May;406(5):536–539. doi: 10.1007/BF00583378. [DOI] [PubMed] [Google Scholar]
- Cheng H., Cannell M. B., Lederer W. J. Propagation of excitation-contraction coupling into ventricular myocytes. Pflugers Arch. 1994 Oct;428(3-4):415–417. doi: 10.1007/BF00724526. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Noble D., Noble S. J., Powell T., Spindler A. J., Twist V. W. Sodium-calcium exchange during the action potential in guinea-pig ventricular cells. J Physiol. 1989 Apr;411:639–661. doi: 10.1113/jphysiol.1989.sp017596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehara T., Noma A., Ono K. Calcium-activated non-selective cation channel in ventricular cells isolated from adult guinea-pig hearts. J Physiol. 1988 Sep;403:117–133. doi: 10.1113/jphysiol.1988.sp017242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horackova M., Beresewicz A., Isenberg G. Effect of reduced Na gradient on electrical activity in isolated bovine and feline ventricular myocytes. Can J Physiol Pharmacol. 1988 Mar;66(3):222–232. doi: 10.1139/y88-038. [DOI] [PubMed] [Google Scholar]
- Janvier N. C., Boyett M. R. The role of Na-Ca exchange current in the cardiac action potential. Cardiovasc Res. 1996 Jul;32(1):69–84. [PubMed] [Google Scholar]
- Kentish J. C., Boyett M. R. A simple electronic circuit for monitoring changes in the duration of the action potential. Pflugers Arch. 1983 Aug;398(3):233–235. doi: 10.1007/BF00657157. [DOI] [PubMed] [Google Scholar]
- Kirschenlohr H. L., Grace A. A., Clarke S. D., Shachar-Hill Y., Metcalfe J. C., Morris P. G., Smith G. A. Calcium measurements with a new high-affinity n.m.r. indicator in the isolated perfused heart. Biochem J. 1993 Jul 15;293(Pt 2):407–411. doi: 10.1042/bj2930407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyosue T., Spindler A. J., Noble S. J., Noble D. Background inward current in ventricular and atrial cells of the guinea-pig. Proc Biol Sci. 1993 Apr 22;252(1333):65–74. doi: 10.1098/rspb.1993.0047. [DOI] [PubMed] [Google Scholar]
- Le Guennec J. V., Noble D. Effects of rapid changes of external Na+ concentration at different moments during the action potential in guinea-pig myocytes. J Physiol. 1994 Aug 1;478(Pt 3):493–504. doi: 10.1113/jphysiol.1994.sp020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch S. P., Brown H. F. Effect of raised extracellular calcium on characteristics of the guinea-pig ventricular action potential. J Mol Cell Cardiol. 1996 Mar;28(3):541–551. doi: 10.1006/jmcc.1996.0050. [DOI] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. The effects of ryanodine, EGTA and low-sodium on action potentials in rat and guinea-pig ventricular myocytes: evidence for two inward currents during the plateau. Br J Pharmacol. 1984 Mar;81(3):543–550. doi: 10.1111/j.1476-5381.1984.tb10107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y., Kimura J. Sodium-calcium exchange current. Dependence on internal Ca and Na and competitive binding of external Na and Ca. J Gen Physiol. 1989 Jun;93(6):1129–1145. doi: 10.1085/jgp.93.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Bett G. Reconstructing the heart: a challenge for integrative physiology. Cardiovasc Res. 1993 Oct;27(10):1701–1712. doi: 10.1093/cvr/27.10.1701. [DOI] [PubMed] [Google Scholar]
- Noble D., Noble S. J., Bett G. C., Earm Y. E., Ho W. K., So I. K. The role of sodium-calcium exchange during the cardiac action potential. Ann N Y Acad Sci. 1991;639:334–353. doi: 10.1111/j.1749-6632.1991.tb17323.x. [DOI] [PubMed] [Google Scholar]
- Terrar D. A., White E. Changes in cytosolic calcium monitored by inward currents during action potentials in guinea-pig ventricular cells. Proc R Soc Lond B Biol Sci. 1989 Nov 22;238(1291):171–188. doi: 10.1098/rspb.1989.0074. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Lederer W. J., Eisner D. A. Ca2+ ions can affect intracellular pH in mammalian cardiac muscle. Nature. 1983 Feb 10;301(5900):522–524. doi: 10.1038/301522a0. [DOI] [PubMed] [Google Scholar]
- Zygmunt A. C., Gibbons W. R. Calcium-activated chloride current in rabbit ventricular myocytes. Circ Res. 1991 Feb;68(2):424–437. doi: 10.1161/01.res.68.2.424. [DOI] [PubMed] [Google Scholar]


