Abstract
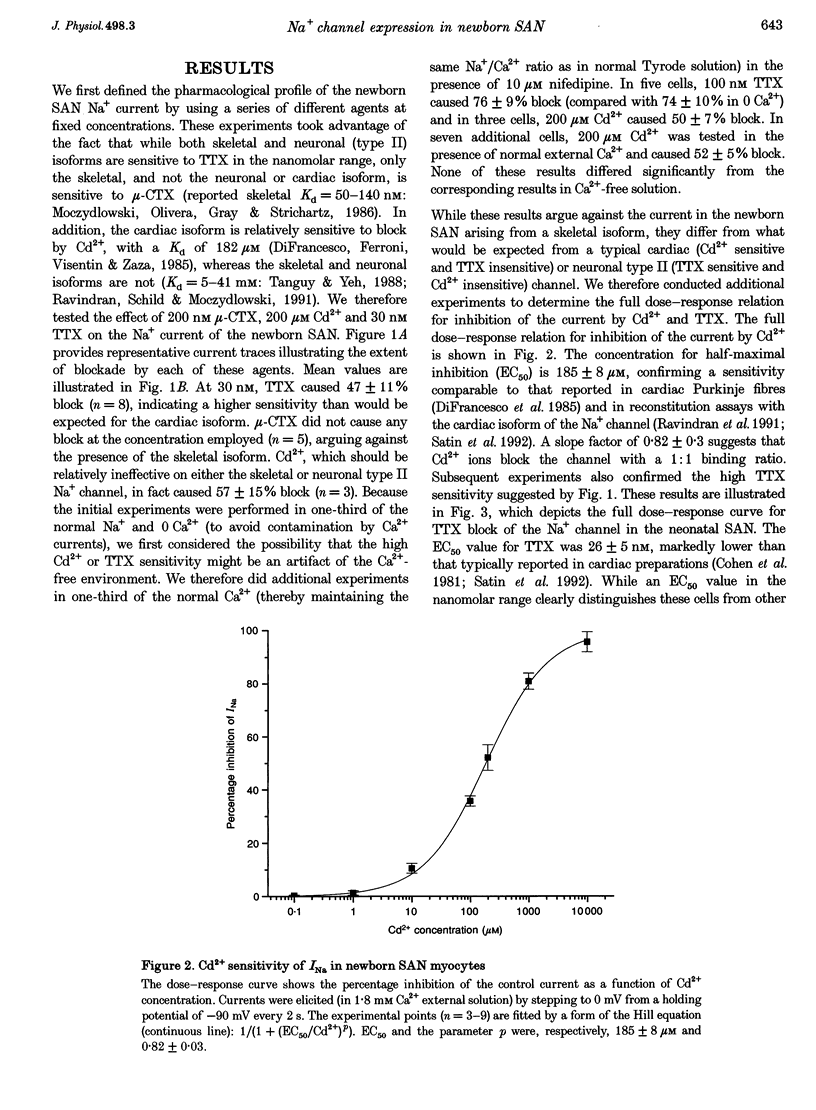
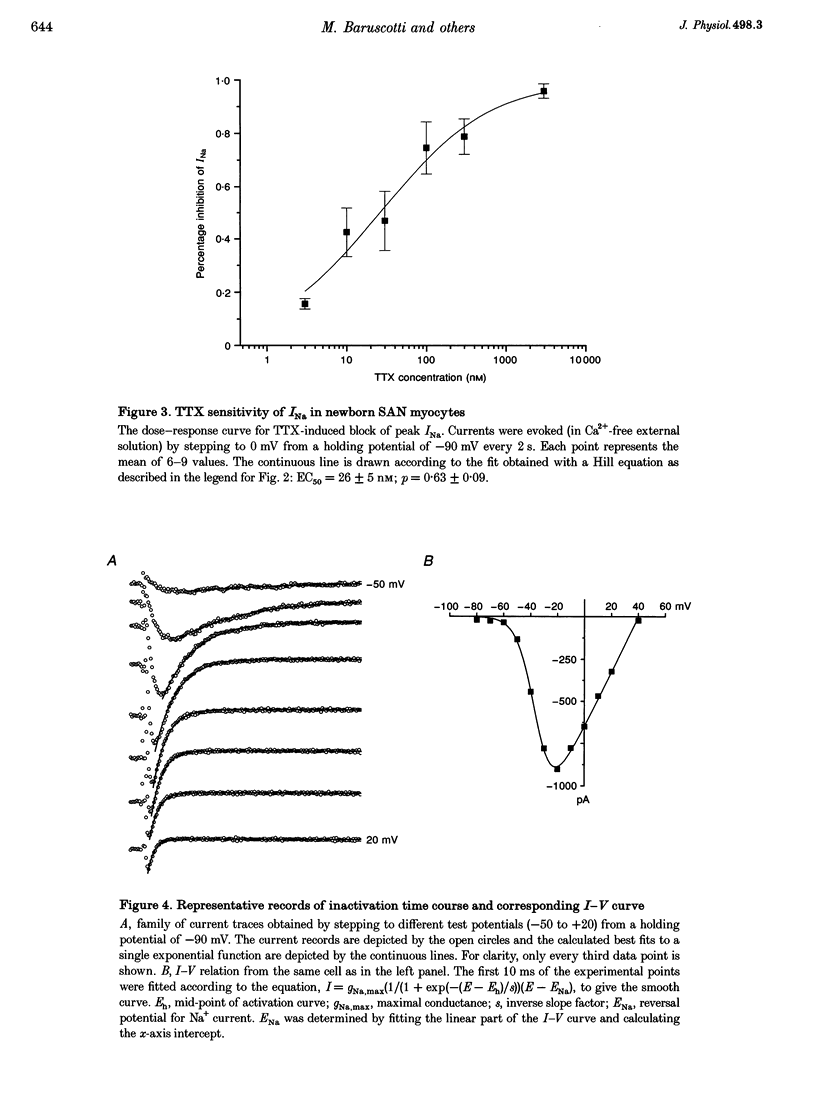
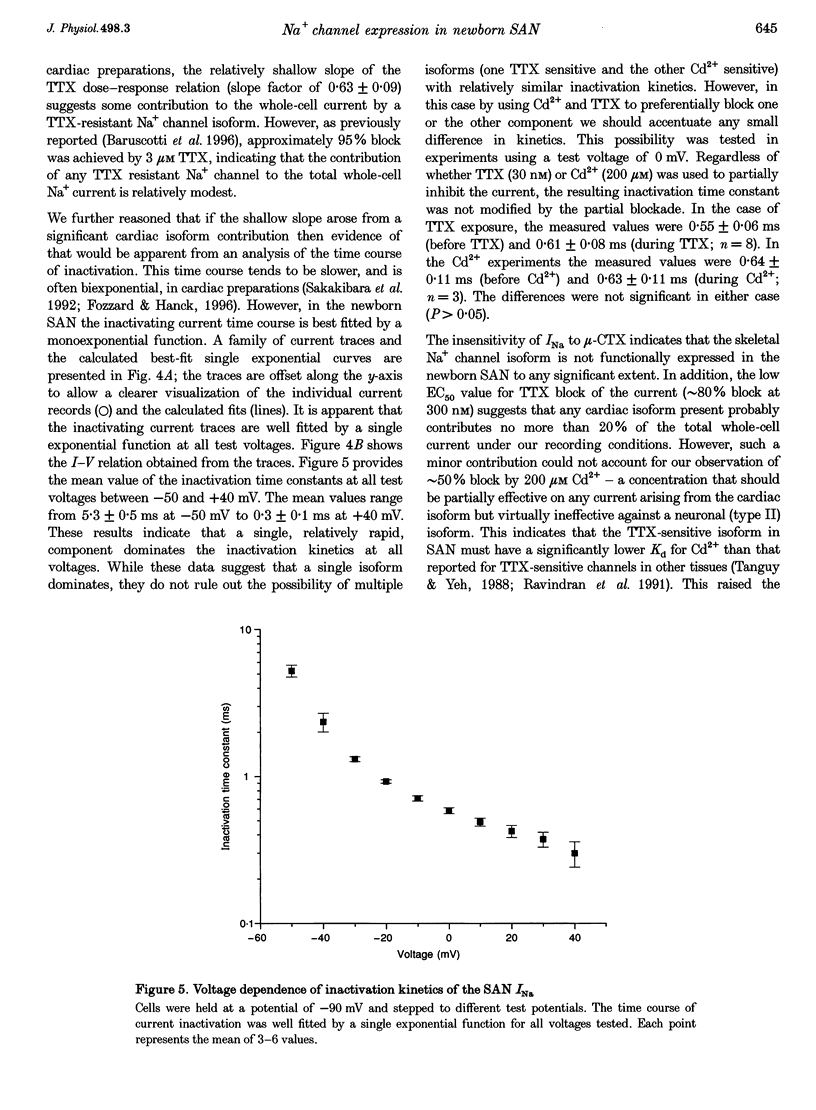
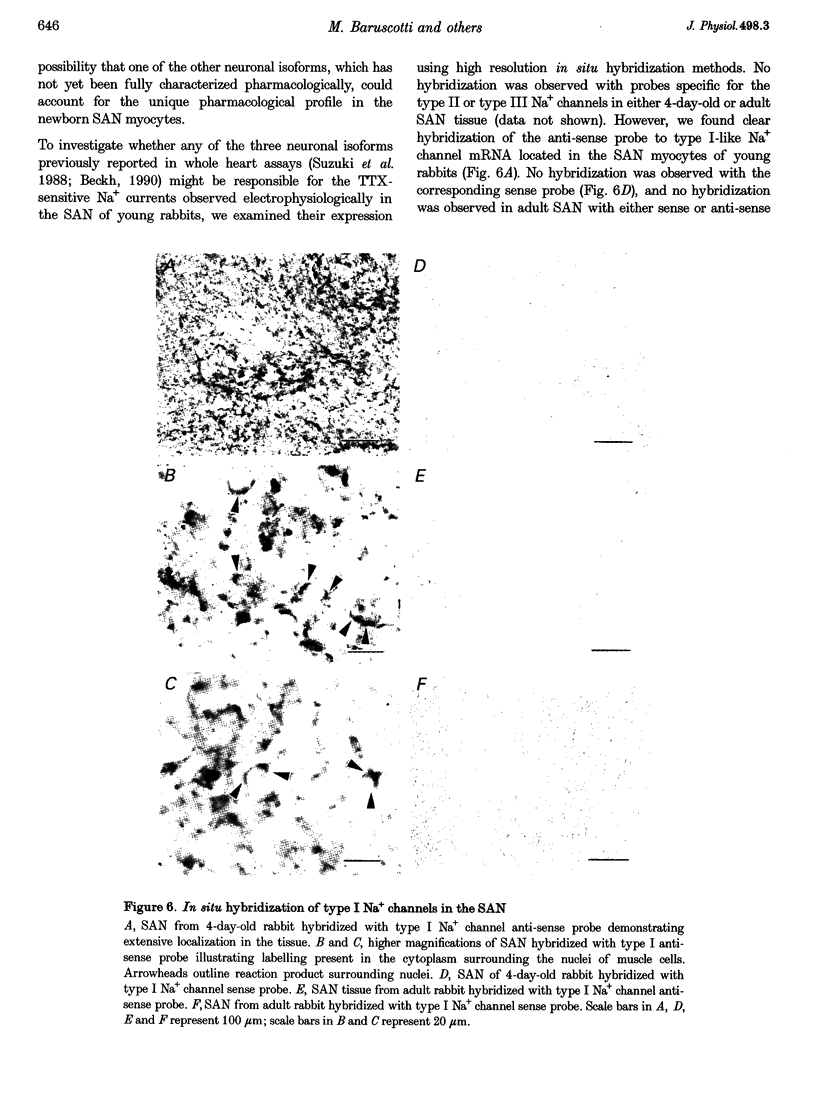
1. Newborn rabbit sino-atrial node (SAN) myocytes were recently found to express a tetrodotoxin (TTX)-sensitive Na+ current. We now report that the dose-response relation indicates that this SAN Na+ channel has unusually high TTX sensitivity, with half-maximal inhibition (26 +/- 5 nM) which is more typical of neuronal than cardiac tissue. 2. Additional characterization used mu-conotoxin GIIIA and Cd2+ as relatively selective blockers of the skeletal and cardiac isoforms, respectively. mu-Conotoxin GIIIA had no effect on the current recorded from SAN myocytes, but the Cd2+ sensitivity was unexpectedly high for a neuronal isoform (half-maximal inhibition = 185 +/- 8 microM). 3. Analysis of the time constant of inactivation did not reveal evidence of multiple inactivation processes, with the data well fitted by a single, relatively rapid exponential (inactivation time constant = 0.58 +/- 0.03 ms at 0 mV). 4. In situ hybridization with anti-sense cDNA probes was used to test for expression of neuronal type I, II and III Na+ channel isoforms. Myocardial cells in newborn SAN tissue exhibited clear hybridization to the type I, but not the type II or III probes. No hybridization was observed in adult SAN tissue with any of the three probes. 5. It is concluded that the newborn SAN expresses a neuronal type I-like Na+ channel isoform, and that this probably accounts for the unusual characteristic of high sensitivity to both TTX and Cd2+.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baruscotti M., DiFrancesco D., Robinson R. B. A TTX-sensitive inward sodium current contributes to spontaneous activity in newborn rabbit sino-atrial node cells. J Physiol. 1996 Apr 1;492(Pt 1):21–30. doi: 10.1113/jphysiol.1996.sp021285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckh S. Differential expression of sodium channel mRNAs in rat peripheral nervous system and innervated tissues. FEBS Lett. 1990 Mar 26;262(2):317–322. doi: 10.1016/0014-5793(90)80218-8. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Lee K. S., Powell T. Sodium current in single rat heart muscle cells. J Physiol. 1981 Sep;318:479–500. doi: 10.1113/jphysiol.1981.sp013879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. J., Bean B. P., Colatsky T. J., Tsien R. W. Tetrodotoxin block of sodium channels in rabbit Purkinje fibers. Interactions between toxin binding and channel gating. J Gen Physiol. 1981 Oct;78(4):383–411. doi: 10.1085/jgp.78.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Visentin S., Zaza A. Cadmium-induced blockade of the cardiac fast Na channels in calf Purkinje fibres. Proc R Soc Lond B Biol Sci. 1985 Feb 22;223(1233):475–484. doi: 10.1098/rspb.1985.0013. [DOI] [PubMed] [Google Scholar]
- Felipe A., Knittle T. J., Doyle K. L., Tamkun M. M. Primary structure and differential expression during development and pregnancy of a novel voltage-gated sodium channel in the mouse. J Biol Chem. 1994 Dec 2;269(48):30125–30131. [PubMed] [Google Scholar]
- Fozzard H. A., Hanck D. A. Structure and function of voltage-dependent sodium channels: comparison of brain II and cardiac isoforms. Physiol Rev. 1996 Jul;76(3):887–926. doi: 10.1152/physrev.1996.76.3.887. [DOI] [PubMed] [Google Scholar]
- George A. L., Jr, Knittle T. J., Tamkun M. M. Molecular cloning of an atypical voltage-gated sodium channel expressed in human heart and uterus: evidence for a distinct gene family. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4893–4897. doi: 10.1073/pnas.89.11.4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorza L., Schiaffino S., Vitadello M. Heart conduction system: a neural crest derivative? Brain Res. 1988 Aug 9;457(2):360–366. doi: 10.1016/0006-8993(88)90707-x. [DOI] [PubMed] [Google Scholar]
- Gorza L., Vitadello M. Distribution of conduction system fibers in the developing and adult rabbit heart revealed by an antineurofilament antibody. Circ Res. 1989 Aug;65(2):360–369. doi: 10.1161/01.res.65.2.360. [DOI] [PubMed] [Google Scholar]
- Lalik P. H., Krafte D. S., Volberg W. A., Ciccarelli R. B. Characterization of endogenous sodium channel gene expressed in Chinese hamster ovary cells. Am J Physiol. 1993 Apr;264(4 Pt 1):C803–C809. doi: 10.1152/ajpcell.1993.264.4.C803. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Olivera B. M., Gray W. R., Strichartz G. R. Discrimination of muscle and neuronal Na-channel subtypes by binding competition between [3H]saxitoxin and mu-conotoxins. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5321–5325. doi: 10.1073/pnas.83.14.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu H., Zou A. R., Berkowitz G. A., Nathan R. D. Characterization of a TTX-sensitive Na+ current in pacemaker cells isolated from rabbit sinoatrial node. Am J Physiol. 1996 Jun;270(6 Pt 2):H2108–H2119. doi: 10.1152/ajpheart.1996.270.6.H2108. [DOI] [PubMed] [Google Scholar]
- Qu Y., Isom L. L., Westenbroek R. E., Rogers J. C., Tanada T. N., McCormick K. A., Scheuer T., Catterall W. A. Modulation of cardiac Na+ channel expression in Xenopus oocytes by beta 1 subunits. J Biol Chem. 1995 Oct 27;270(43):25696–25701. doi: 10.1074/jbc.270.43.25696. [DOI] [PubMed] [Google Scholar]
- Ravindran A., Schild L., Moczydlowski E. Divalent cation selectivity for external block of voltage-dependent Na+ channels prolonged by batrachotoxin. Zn2+ induces discrete substates in cardiac Na+ channels. J Gen Physiol. 1991 Jan;97(1):89–115. doi: 10.1085/jgp.97.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogart R. B., Cribbs L. L., Muglia L. K., Kephart D. D., Kaiser M. W. Molecular cloning of a putative tetrodotoxin-resistant rat heart Na+ channel isoform. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8170–8174. doi: 10.1073/pnas.86.20.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y., Wasserstrom J. A., Furukawa T., Jia H., Arentzen C. E., Hartz R. S., Singer D. H. Characterization of the sodium current in single human atrial myocytes. Circ Res. 1992 Sep;71(3):535–546. doi: 10.1161/01.res.71.3.535. [DOI] [PubMed] [Google Scholar]
- Satin J., Kyle J. W., Chen M., Bell P., Cribbs L. L., Fozzard H. A., Rogart R. B. A mutant of TTX-resistant cardiac sodium channels with TTX-sensitive properties. Science. 1992 May 22;256(5060):1202–1205. doi: 10.1126/science.256.5060.1202. [DOI] [PubMed] [Google Scholar]
- Schaller K. L., Krzemien D. M., McKenna N. M., Caldwell J. H. Alternatively spliced sodium channel transcripts in brain and muscle. J Neurosci. 1992 Apr;12(4):1370–1381. doi: 10.1523/JNEUROSCI.12-04-01370.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Beckh S., Kubo H., Yahagi N., Ishida H., Kayano T., Noda M., Numa S. Functional expression of cloned cDNA encoding sodium channel III. FEBS Lett. 1988 Feb 8;228(1):195–200. doi: 10.1016/0014-5793(88)80615-x. [DOI] [PubMed] [Google Scholar]



