Abstract
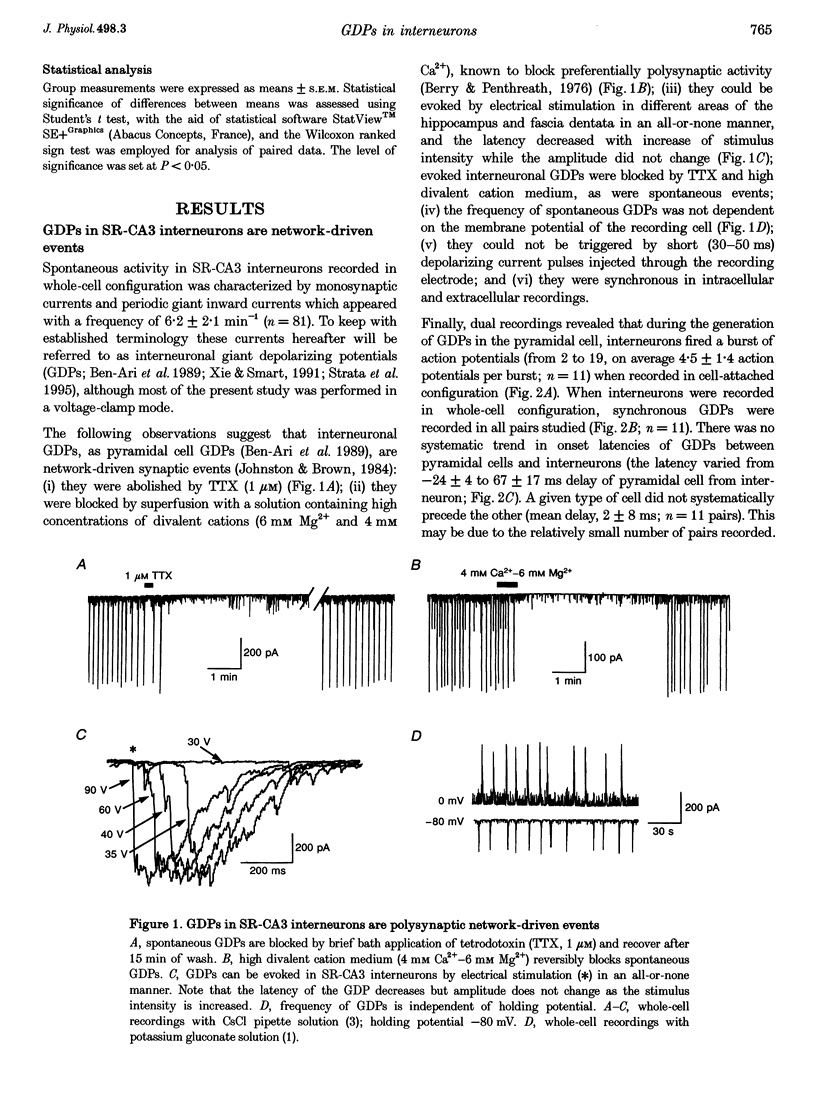
1. Cell-attached and whole-cell recordings from interneurons localized in the stratum radiatum of the CA3 subfield (SR-CA3) of neonatal (postnatal days 2-5) rat hippocampal slices were performed to study their activity during the generation of GABAergic giant depolarizing potentials (GDPs) in CA3 pyramidal cells. 2. Dual recordings revealed that during the generation of GDPs in CA3 pyramidal cells, the interneurons fire bursts of spikes, on average 4.5 +/- 1.4 spikes per burst (cell-attached mode). There bursts were induced by periodical large inward currents (interneuronal GDPs) recorded in whole-cell mode. 3. Interneuronal GDPs revealed typical features of polysynaptic neuronal network-driven events: they were blocked by TTX and by high divalent cation medium and they could be evoked in an all-or-none manner by electrical stimulation in different regions of the hippocampus. The network elements required for the generation of GDPs are present in local CA3 circuits since spontaneous GDPs were present in the isolated CA3 subfield of the hippocampal slice. 4. Interneuronal GDPs were mediated by GABAA and glutamate receptors, since: (i) their reversal potential strongly depended on [Cl-]i; (ii) at the reversal potential of GABAA postsynaptic currents an inward component of GDPs was composed of events with the same kinetics as alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) receptor-mediated EPSCs; and (iii) once GABAA receptors were blocked intracellularly by dialysis with F(-)-MgATP-free solution, the remaining component of interneuronal GDPs reversed near 0 mV and rectified at membrane potentials more negative than -20 mV, suggesting an important contribution of NMDA receptors in addition to AMPA receptors. 5. In cell-attached recordings from interneurons, electrical stimulation in the stratum radiatum evoked a burst of spikes that corresponded to evoked GDPs. Pharmacological study of this response revealed that excitation of SR-CA3 interneurons during GDPs is determined by the co-operative depolarizing actions mediated by GABAA and glutamate (AMPA and NMDA) receptors. Interestingly, after blockade of AMPA receptors, GABAA receptor-mediated depolarization enabled the activation of NMDA receptors presumably via attenuation of their voltage-dependent magnesium block. 6. It is concluded that synchronous activation of SR-CA3 interneurons during generation of GDPs is mediated synaptically and is determined by the co-operation of (i) excitatory GABAergic connections between interneurons and (ii) glutamatergic connections to interneurons originating presumably from the pyramidal cells.
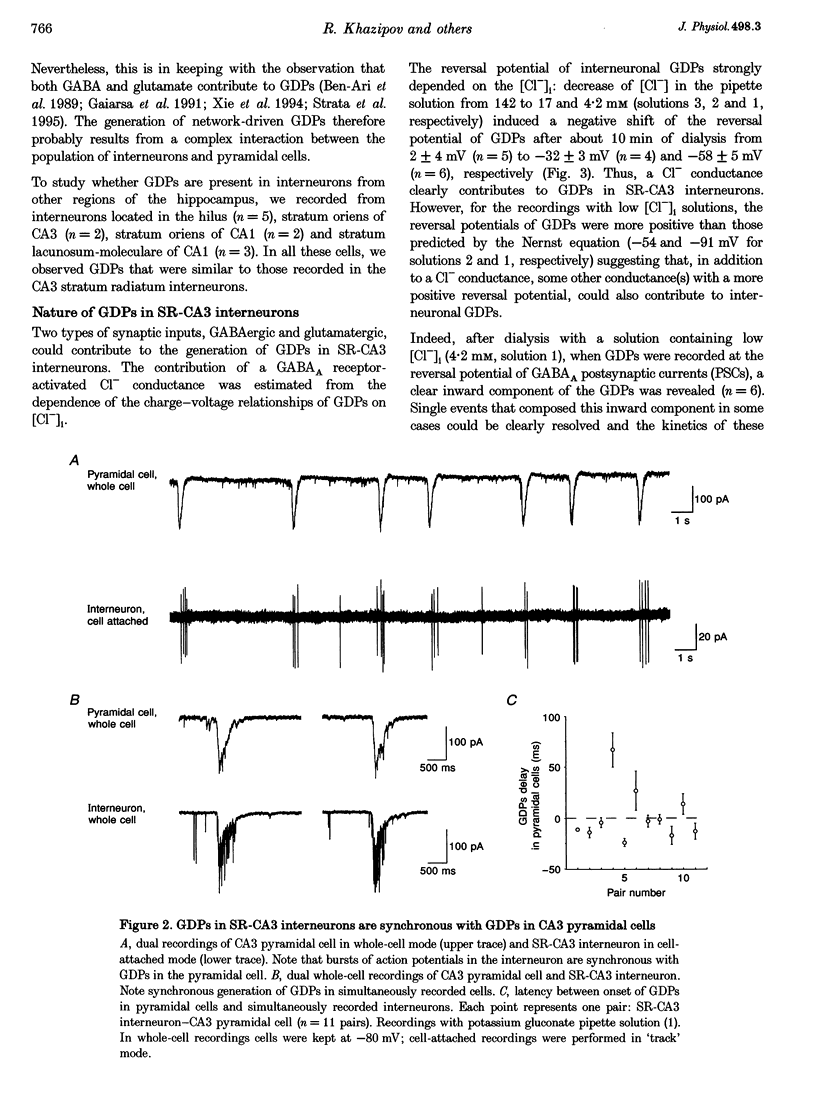
Full text
PDF

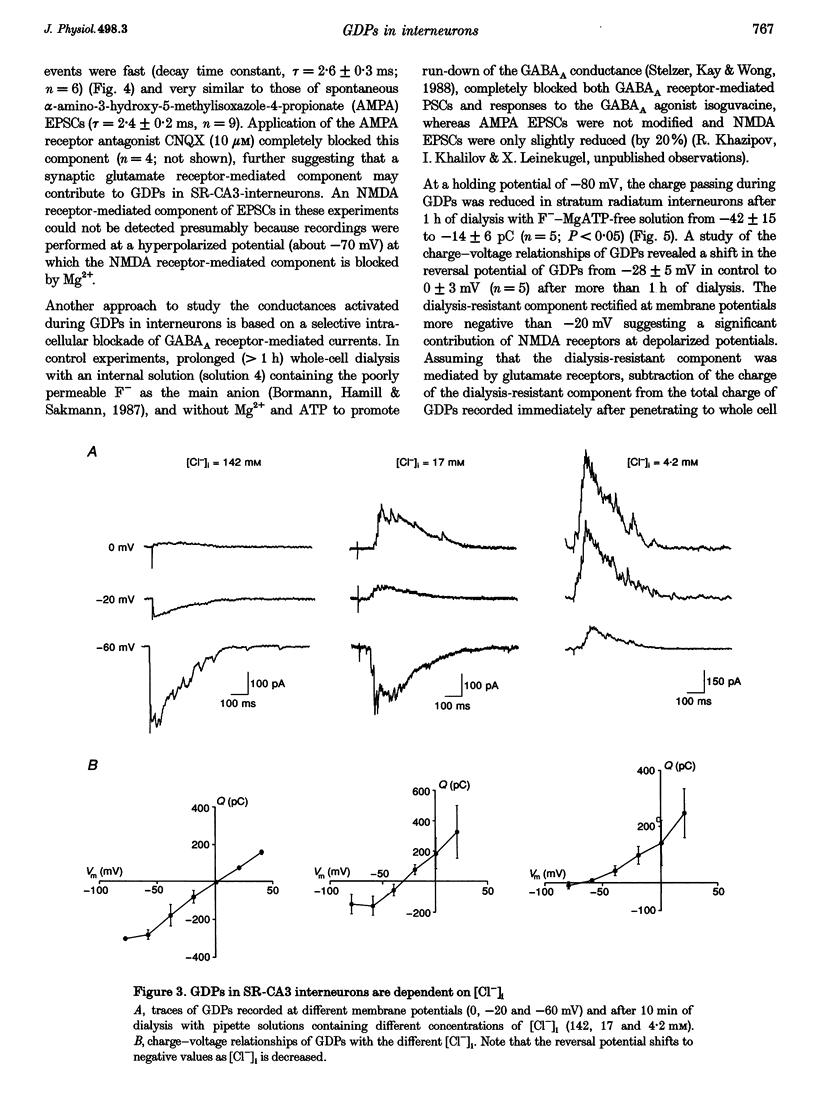
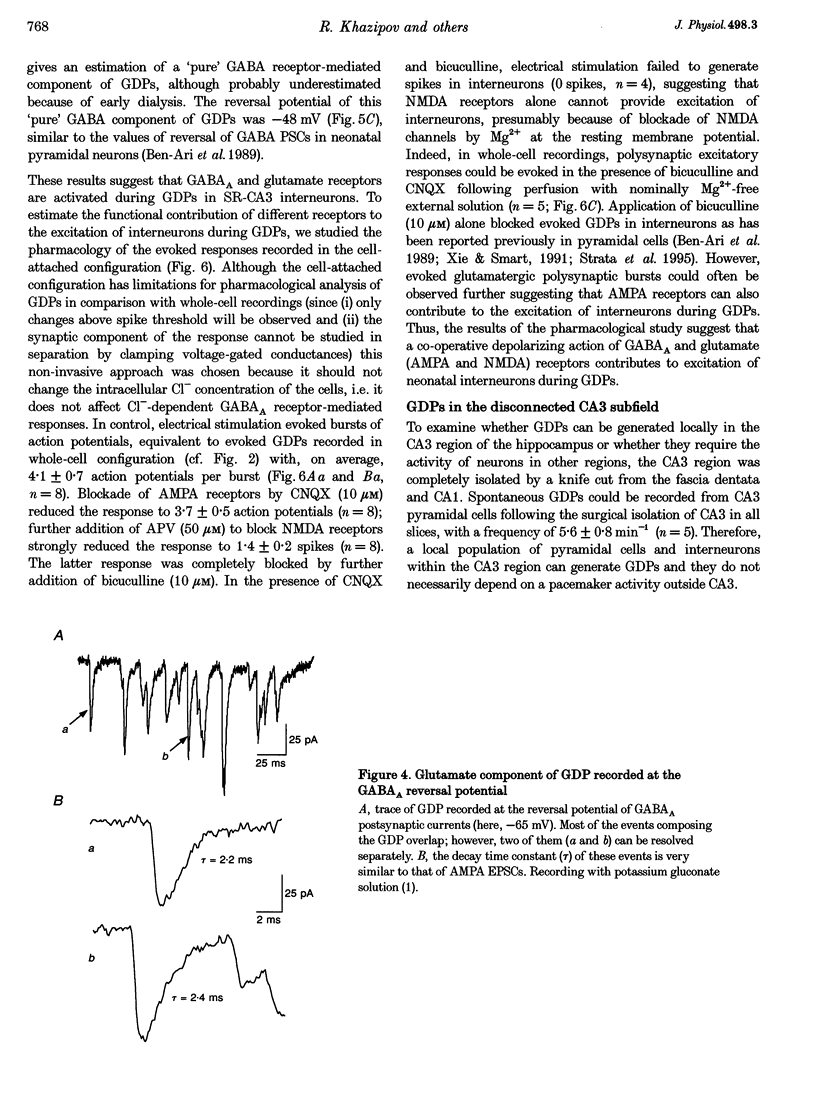
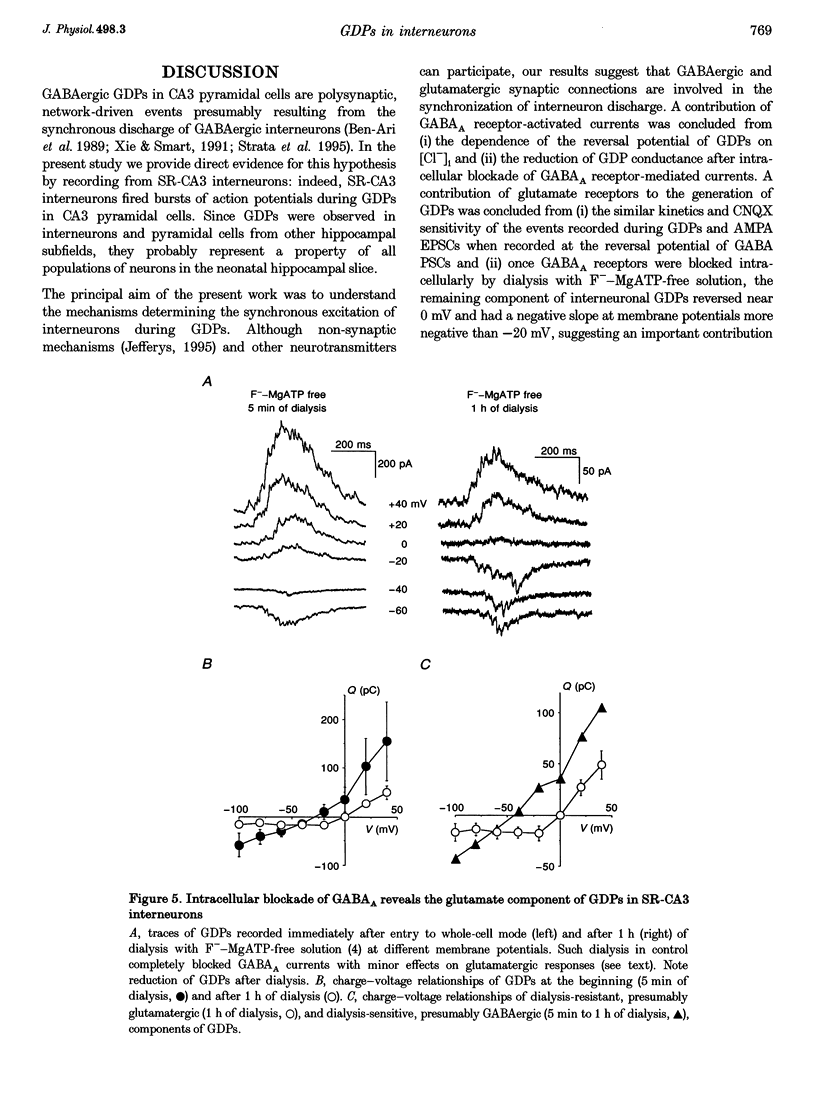
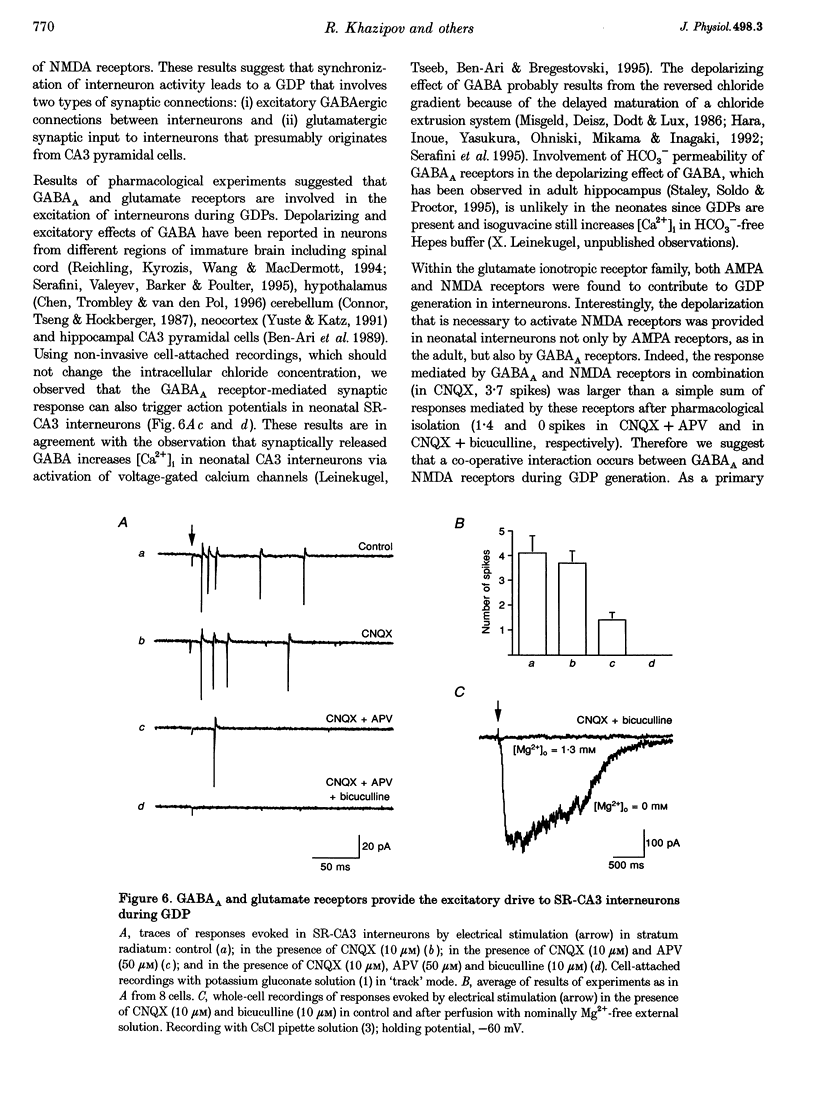


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Ari Y., Cherubini E., Corradetti R., Gaiarsa J. L. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989 Sep;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y., Tseeb V., Raggozzino D., Khazipov R., Gaiarsa J. L. gamma-Aminobutyric acid (GABA): a fast excitatory transmitter which may regulate the development of hippocampal neurones in early postnatal life. Prog Brain Res. 1994;102:261–273. doi: 10.1016/S0079-6123(08)60545-2. [DOI] [PubMed] [Google Scholar]
- Berry M. S., Pentreath V. W. Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res. 1976 Mar 19;105(1):1–20. doi: 10.1016/0006-8993(76)90919-7. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash S., Zucker R. S., Poo M. M. Spread of synaptic depression mediated by presynaptic cytoplasmic signaling. Science. 1996 May 17;272(5264):998–1001. doi: 10.1126/science.272.5264.998. [DOI] [PubMed] [Google Scholar]
- Chen G., Trombley P. Q., van den Pol A. N. Excitatory actions of GABA in developing rat hypothalamic neurones. J Physiol. 1996 Jul 15;494(Pt 2):451–464. doi: 10.1113/jphysiol.1996.sp021505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E., Gaiarsa J. L., Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991 Dec;14(12):515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Cobb S. R., Buhl E. H., Halasy K., Paulsen O., Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995 Nov 2;378(6552):75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Tseng H. Y., Hockberger P. E. Depolarization- and transmitter-induced changes in intracellular Ca2+ of rat cerebellar granule cells in explant cultures. J Neurosci. 1987 May;7(5):1384–1400. doi: 10.1523/JNEUROSCI.07-05-01384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine-Paton M., Cline H. T., Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- Corradetti R., Gaïarsa J. L., Ben-Ari Y. D-aminophosphonovaleric acid-sensitive spontaneous giant EPSPs in immature rat hippocampal neurones. Eur J Pharmacol. 1988 Sep 13;154(2):221–222. doi: 10.1016/0014-2999(88)90103-3. [DOI] [PubMed] [Google Scholar]
- Durand G. M., Kovalchuk Y., Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996 May 2;381(6577):71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- Feller M. B., Wellis D. P., Stellwagen D., Werblin F. S., Shatz C. J. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science. 1996 May 24;272(5265):1182–1187. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- Fukuda A., Mody I., Prince D. A. Differential ontogenesis of presynaptic and postsynaptic GABAB inhibition in rat somatosensory cortex. J Neurophysiol. 1993 Jul;70(1):448–452. doi: 10.1152/jn.1993.70.1.448. [DOI] [PubMed] [Google Scholar]
- Gaiarsa J. L., Tseeb V., Ben-Ari Y. Postnatal development of pre- and postsynaptic GABAB-mediated inhibitions in the CA3 hippocampal region of the rat. J Neurophysiol. 1995 Jan;73(1):246–255. doi: 10.1152/jn.1995.73.1.246. [DOI] [PubMed] [Google Scholar]
- Gaiarsa Jean-Luc, Corradetti Renato, Cherubini Enrico, Ben-Ari Yehezkel. Modulation of GABA-mediated Synaptic Potentials by Glutamatergic Agonists in Neonatal CA3 Rat Hippocampal Neurons. Eur J Neurosci. 1991;3(4):301–309. doi: 10.1111/j.1460-9568.1991.tb00816.x. [DOI] [PubMed] [Google Scholar]
- Hara M., Inoue M., Yasukura T., Ohnishi S., Mikami Y., Inagaki C. Uneven distribution of intracellular Cl- in rat hippocampal neurons. Neurosci Lett. 1992 Aug 31;143(1-2):135–138. doi: 10.1016/0304-3940(92)90250-b. [DOI] [PubMed] [Google Scholar]
- Jefferys J. G. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiol Rev. 1995 Oct;75(4):689–723. doi: 10.1152/physrev.1995.75.4.689. [DOI] [PubMed] [Google Scholar]
- Leinekugel X., Tseeb V., Ben-Ari Y., Bregestovski P. Synaptic GABAA activation induces Ca2+ rise in pyramidal cells and interneurons from rat neonatal hippocampal slices. J Physiol. 1995 Sep 1;487(Pt 2):319–329. doi: 10.1113/jphysiol.1995.sp020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean H. A., Rovira C., Ben-Ari Y., Gaiarsa J. L. NMDA-dependent GABAA-mediated polysynaptic potentials in the neonatal rat hippocampal CA3 region. Eur J Neurosci. 1995 Jul 1;7(7):1442–1448. doi: 10.1111/j.1460-9568.1995.tb01139.x. [DOI] [PubMed] [Google Scholar]
- Misgeld U., Deisz R. A., Dodt H. U., Lux H. D. The role of chloride transport in postsynaptic inhibition of hippocampal neurons. Science. 1986 Jun 13;232(4756):1413–1415. doi: 10.1126/science.2424084. [DOI] [PubMed] [Google Scholar]
- Reichling D. B., Kyrozis A., Wang J., MacDermott A. B. Mechanisms of GABA and glycine depolarization-induced calcium transients in rat dorsal horn neurons. J Physiol. 1994 May 1;476(3):411–421. doi: 10.1113/jphysiol.1994.sp020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini R., Valeyev A. Y., Barker J. L., Poulter M. O. Depolarizing GABA-activated Cl- channels in embryonic rat spinal and olfactory bulb cells. J Physiol. 1995 Oct 15;488(Pt 2):371–386. doi: 10.1113/jphysiol.1995.sp020973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer N. C. Spontaneous Ca2+ spikes and waves in embryonic neurons: signaling systems for differentiation. Trends Neurosci. 1994 Mar;17(3):115–118. doi: 10.1016/0166-2236(94)90120-1. [DOI] [PubMed] [Google Scholar]
- Staley K. J., Soldo B. L., Proctor W. R. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995 Aug 18;269(5226):977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- Stelzer A., Kay A. R., Wong R. K. GABAA-receptor function in hippocampal cells is maintained by phosphorylation factors. Science. 1988 Jul 15;241(4863):339–341. doi: 10.1126/science.2455347. [DOI] [PubMed] [Google Scholar]
- Strata F., Sciancalepore M., Cherubini E. Cyclic AMP-dependent modulation of giant depolarizing potentials by metabotropic glutamate receptors in the rat hippocampus. J Physiol. 1995 Nov 15;489(Pt 1):115–125. doi: 10.1113/jphysiol.1995.sp021035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington M. A., Traub R. D., Jefferys J. G. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995 Feb 16;373(6515):612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Xie X. M., Smart T. G. A physiological role for endogenous zinc in rat hippocampal synaptic neurotransmission. Nature. 1991 Feb 7;349(6309):521–524. doi: 10.1038/349521a0. [DOI] [PubMed] [Google Scholar]
- Xie X., Hider R. C., Smart T. G. Modulation of GABA-mediated synaptic transmission by endogenous zinc in the immature rat hippocampus in vitro. J Physiol. 1994 Jul 1;478(Pt 1):75–86. doi: 10.1113/jphysiol.1994.sp020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A., Bragin A., Nádasdy Z., Jandó G., Szabó I., Sik A., Buzsáki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995 Jan;15(1 Pt 1):30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A., Soltész I., Bragin A., Penttonen M., Sik A., Buzsáki G. Intracellular correlates of hippocampal theta rhythm in identified pyramidal cells, granule cells, and basket cells. Hippocampus. 1995;5(1):78–90. doi: 10.1002/hipo.450050110. [DOI] [PubMed] [Google Scholar]
- Yuste R., Katz L. C. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron. 1991 Mar;6(3):333–344. doi: 10.1016/0896-6273(91)90243-s. [DOI] [PubMed] [Google Scholar]
- Yuste R., Nelson D. A., Rubin W. W., Katz L. C. Neuronal domains in developing neocortex: mechanisms of coactivation. Neuron. 1995 Jan;14(1):7–17. doi: 10.1016/0896-6273(95)90236-8. [DOI] [PubMed] [Google Scholar]


