Abstract
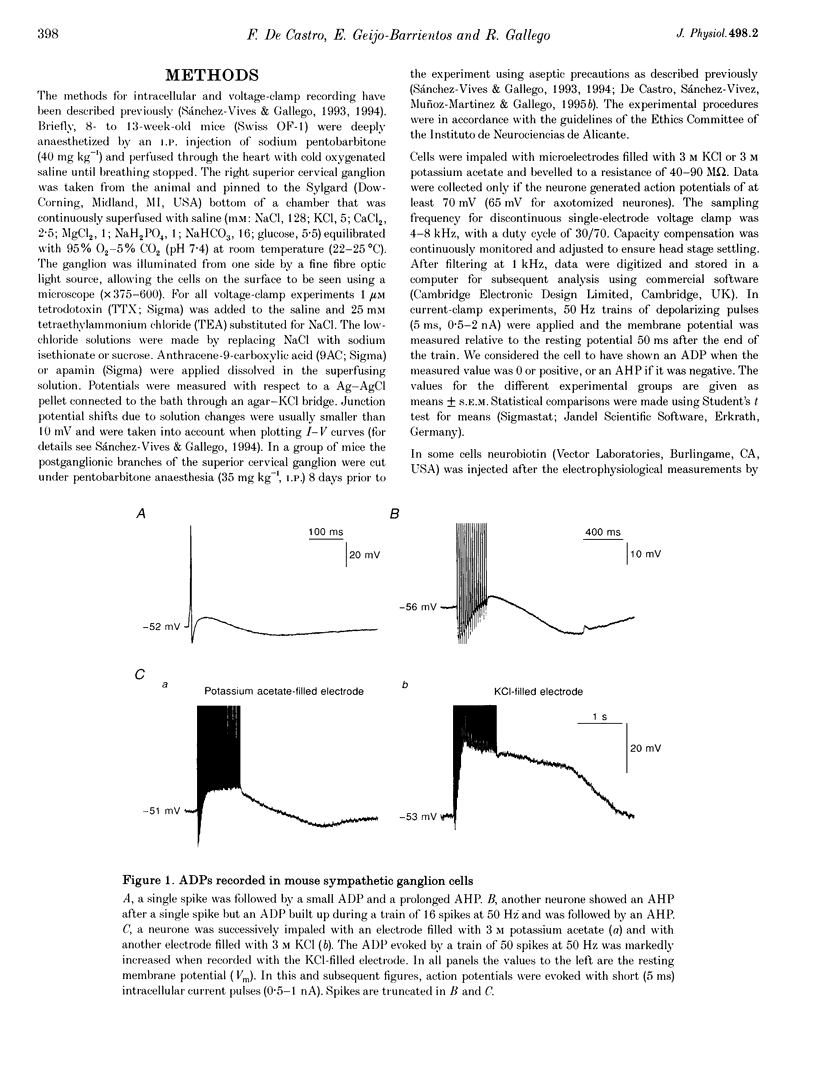
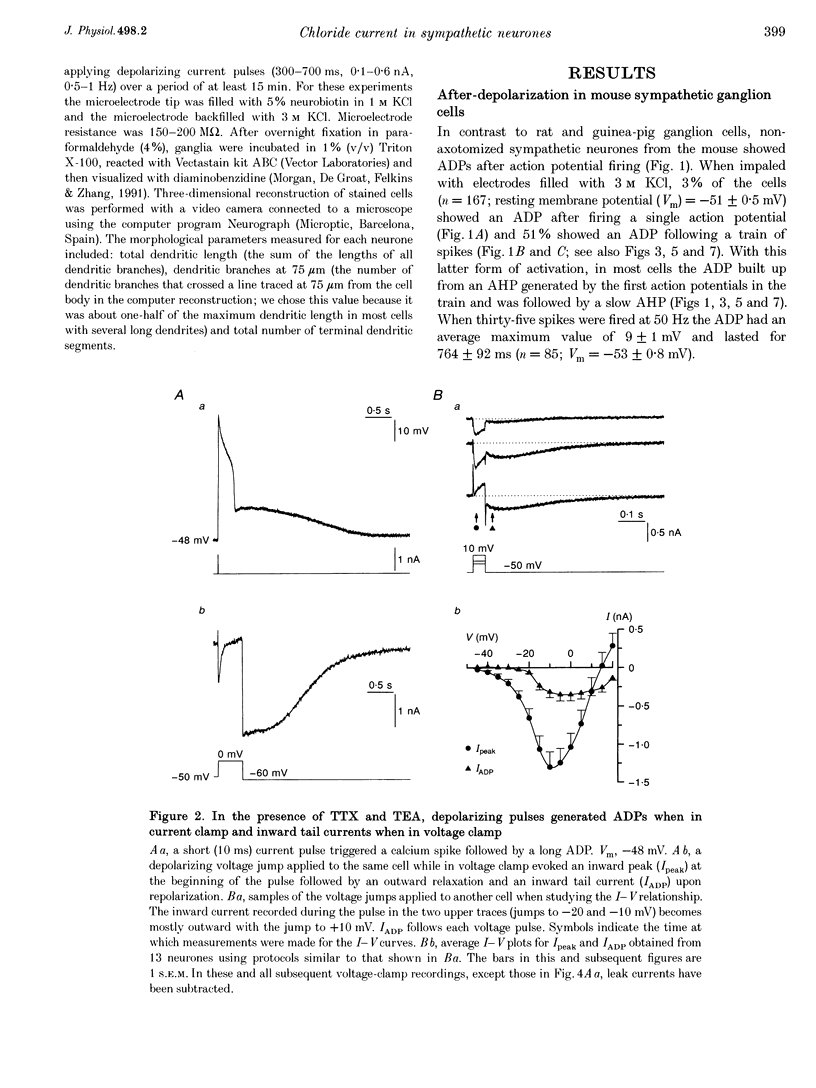
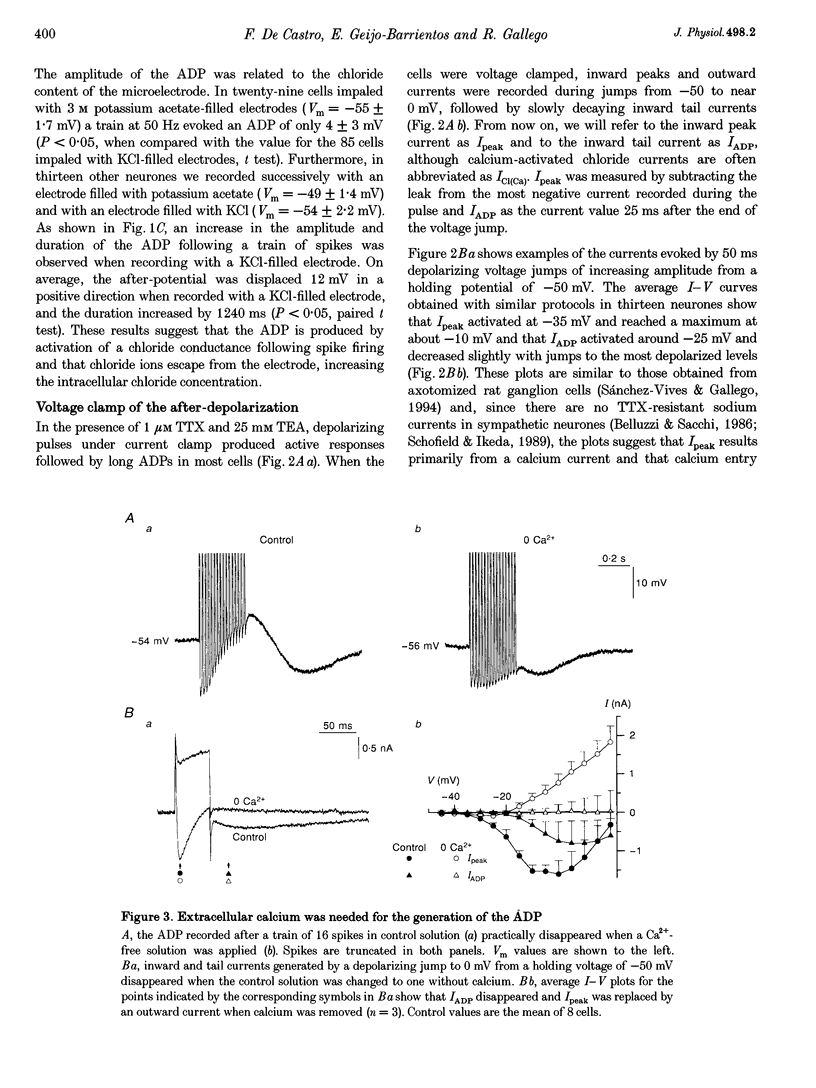
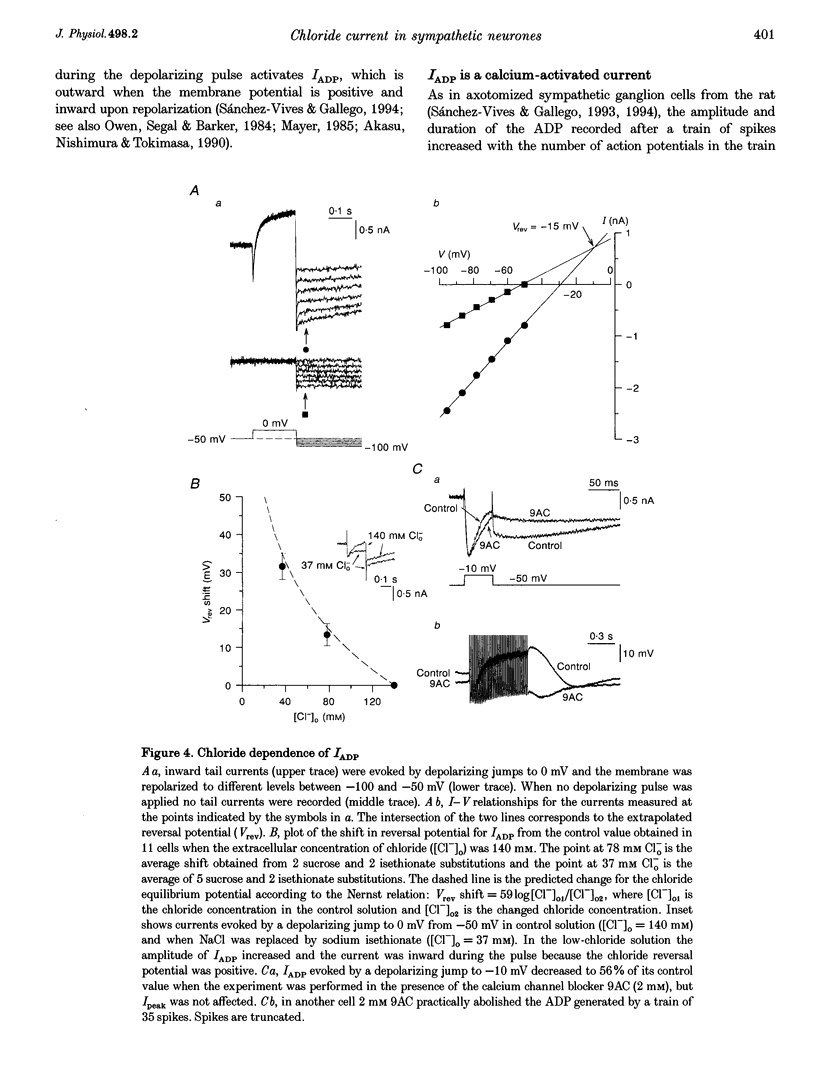
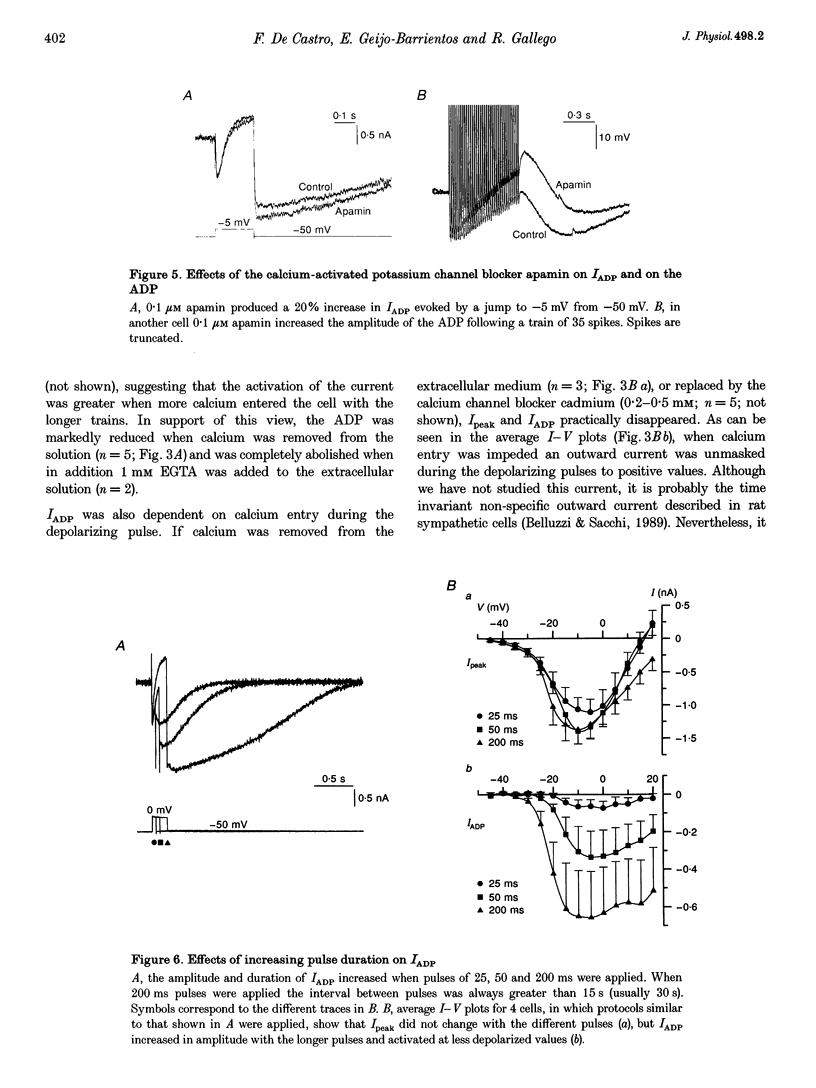
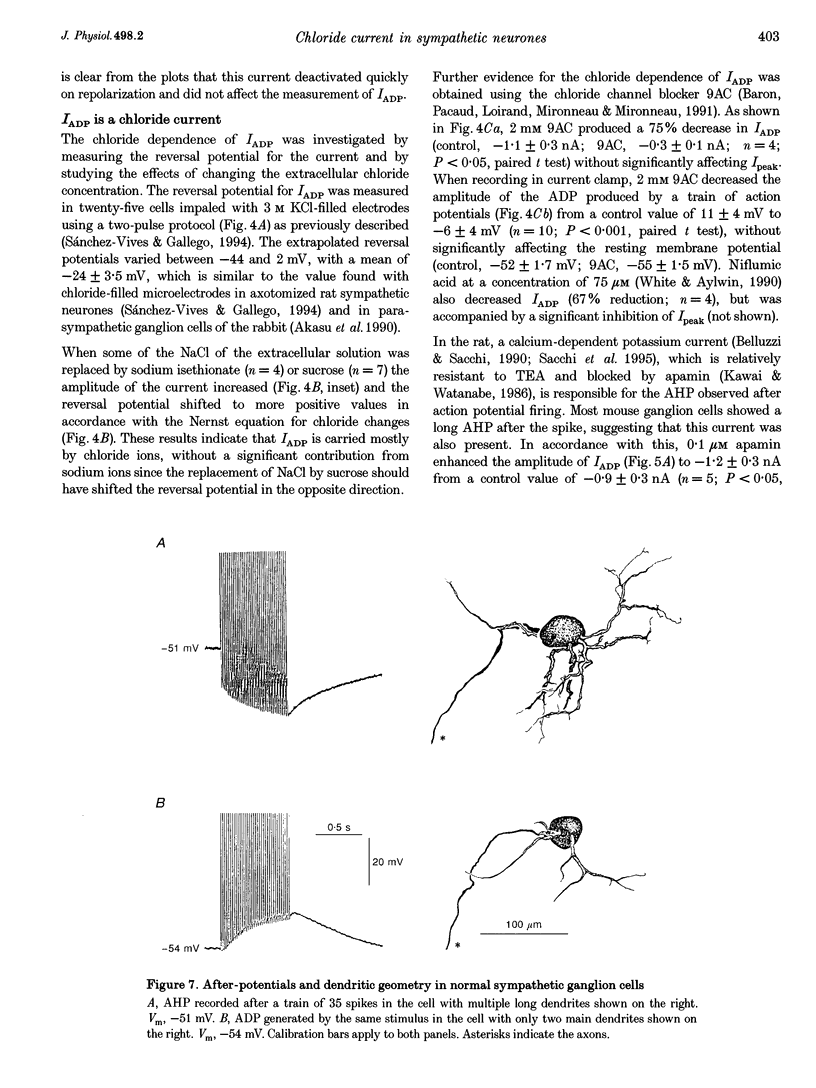
1. In rat sympathetic ganglion cells, axotomy induces the appearance of a depolarizing after-potential (ADP) produced by a calcium-activated chloride current. Here we report that this current is also present in normal sympathetic neurones from the mouse. 2. In an in vitro preparation of the superior cervical ganglion, an ADP was observed after spike firing in 50% of the cells studied with single-electrode current- and voltage-clamp techniques. 3. When the cells were voltage clamped at -50 mV in the presence of tetrodotoxin (TTX) and tetraethylammonium chloride (TEA), depolarizing jumps evoked inward calcium currents which were contaminated by outward chloride currents, followed by slowly decaying inward chloride tail currents. 4. The ADP and the inward tail currents disappeared when calcium was removed from the extracellular solution or when cadmium was added. 5. The reversal potential for the inward tail current was approximately -24 mV and was displaced in agreement with the Nernst equation for chloride when the extracellular NaCl was replaced by sucrose or sodium isethionate. The chloride channel blocker anthracene-9-carboxylic acid (9AC) inhibited both the ADP and the tail current. 6. Using intracellular injection of neurobiotin, we found that cells with shorter dendrites had larger ADPs. In axotomized ganglia practically all cells showed very pronounced ADPs. 7. We conclude that normal mouse sympathetic ganglion cells have a calcium-activated chloride current that generates an ADP. The channels responsible for this current are probably located in the dendrites.
Full text
PDF
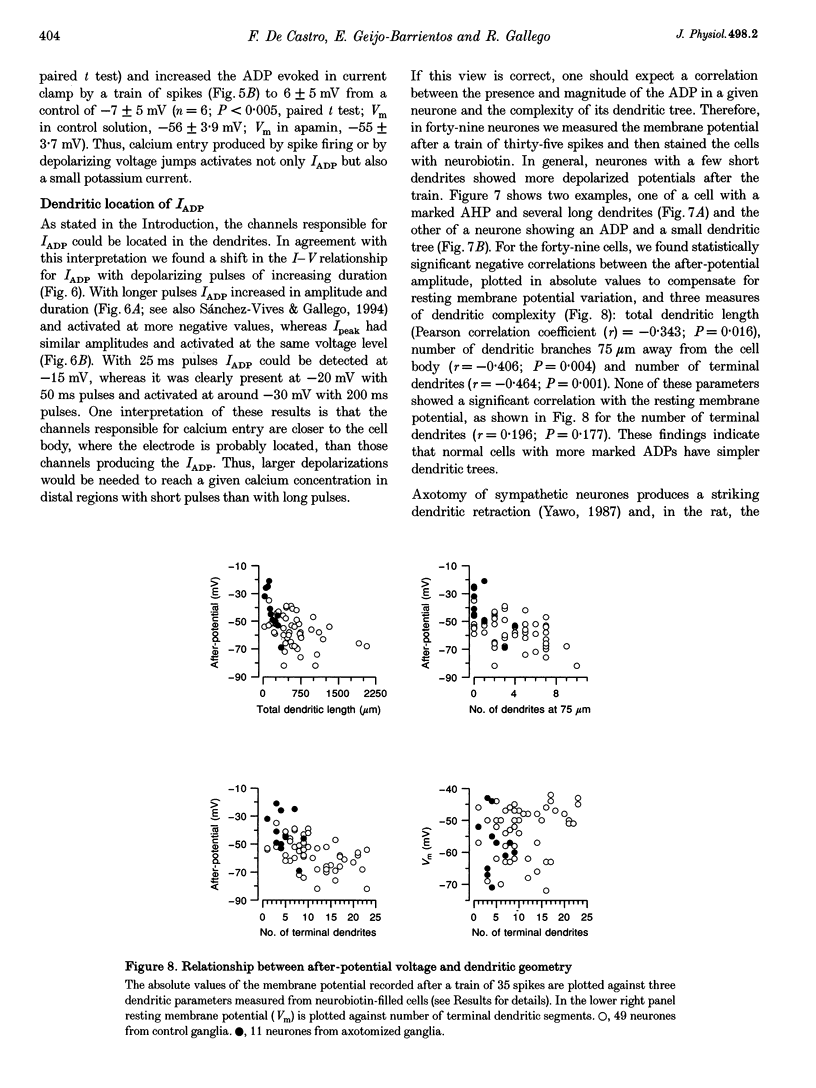
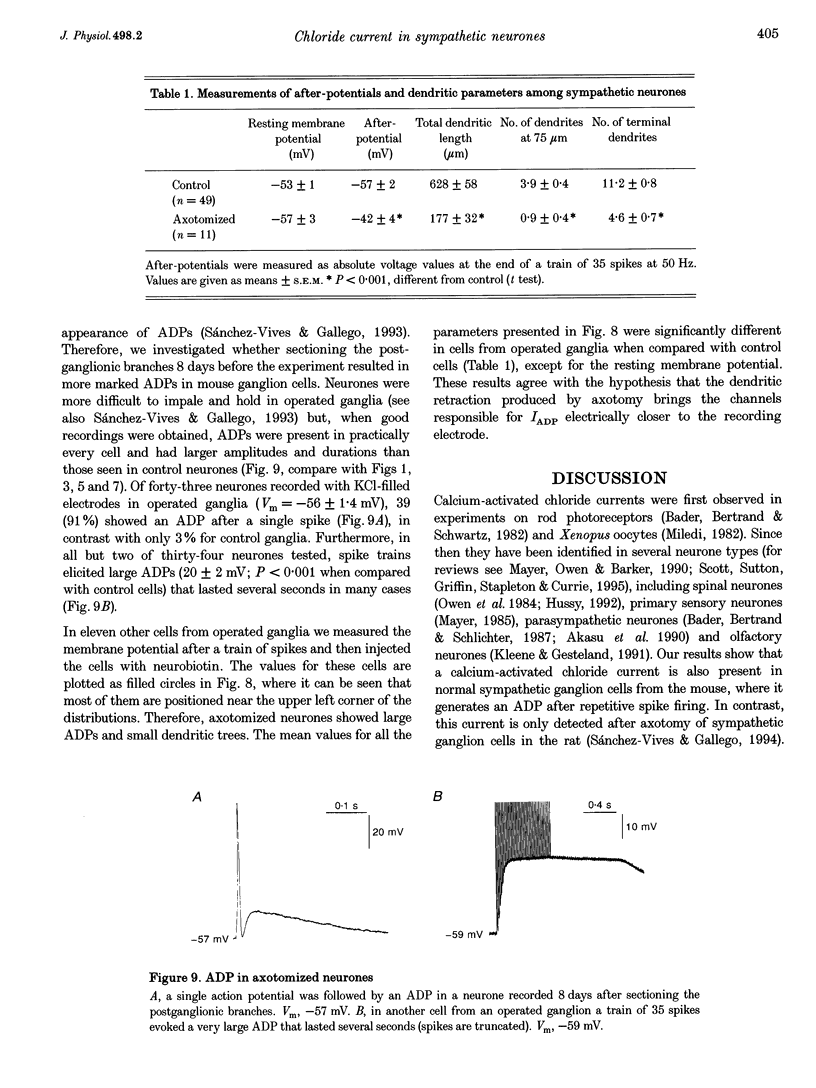



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akasu T., Nishimura T., Tokimasa T. Calcium-dependent chloride current in neurones of the rabbit pelvic parasympathetic ganglia. J Physiol. 1990 Mar;422:303–320. doi: 10.1113/jphysiol.1990.sp017985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Bertrand D., Schlichter R. Calcium-activated chloride current in cultured sensory and parasympathetic quail neurones. J Physiol. 1987 Dec;394:125–148. doi: 10.1113/jphysiol.1987.sp016863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Bertrand D., Schwartz E. A. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol. 1982 Oct;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K., Grafe P., Reddy M. M., ten Bruggencate G. Different types of potassium transport linked to carbachol and gamma-aminobutyric acid actions in rat sympathetic neurons. Neuroscience. 1984 Jul;12(3):917–927. doi: 10.1016/0306-4522(84)90179-9. [DOI] [PubMed] [Google Scholar]
- Baron A., Pacaud P., Loirand G., Mironneau C., Mironneau J. Pharmacological block of Ca(2+)-activated Cl- current in rat vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 1991 Dec;419(6):553–558. doi: 10.1007/BF00370294. [DOI] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O. A quantitative description of the sodium current in the rat sympathetic neurone. J Physiol. 1986 Nov;380:275–291. doi: 10.1113/jphysiol.1986.sp016285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O. Calcium currents in the normal adult rat sympathetic neurone. J Physiol. 1989 May;412:493–512. doi: 10.1113/jphysiol.1989.sp017628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O. The calcium-dependent potassium conductance in rat sympathetic neurones. J Physiol. 1990 Mar;422:561–583. doi: 10.1113/jphysiol.1990.sp018001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie K. P., Scott R. H. Calcium-activated currents in cultured neurones from rat dorsal root ganglia. Br J Pharmacol. 1992 Jul;106(3):593–602. doi: 10.1111/j.1476-5381.1992.tb14381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie K. P., Wootton J. F., Scott R. H. Activation of Ca(2+)-dependent Cl- currents in cultured rat sensory neurones by flash photolysis of DM-nitrophen. J Physiol. 1995 Jan 15;482(Pt 2):291–307. doi: 10.1113/jphysiol.1995.sp020518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Castro F., Sánchez-Vives M. V., Muñoz-Martínez E. J., Gallego R. Effects of postganglionic nerve section on synaptic transmission in the superior cervical ganglion of the guinea-pig. Neuroscience. 1995 Aug;67(3):689–695. doi: 10.1016/0306-4522(95)00079-x. [DOI] [PubMed] [Google Scholar]
- Forehand C. J. Density of somatic innervation on mammalian autonomic ganglion cells is inversely related to dendritic complexity and preganglionic convergence. J Neurosci. 1985 Dec;5(12):3403–3408. doi: 10.1523/JNEUROSCI.05-12-03403.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan M., Dörge A., Beck F., Rick R. Intracellular electrolyte concentrations in rat sympathetic neurones measured with an electron microprobe. Pflugers Arch. 1984 Mar;400(3):274–279. doi: 10.1007/BF00581559. [DOI] [PubMed] [Google Scholar]
- Hussy N. Calcium-activated chloride channels in cultured embryonic Xenopus spinal neurons. J Neurophysiol. 1992 Dec;68(6):2042–2050. doi: 10.1152/jn.1992.68.6.2042. [DOI] [PubMed] [Google Scholar]
- Kawai T., Watanabe M. Blockade of Ca-activated K conductance by apamin in rat sympathetic neurones. Br J Pharmacol. 1986 Jan;87(1):225–232. doi: 10.1111/j.1476-5381.1986.tb10175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene S. J., Gesteland R. C. Calcium-activated chloride conductance in frog olfactory cilia. J Neurosci. 1991 Nov;11(11):3624–3629. doi: 10.1523/JNEUROSCI.11-11-03624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh S. J., Trouslard J., Leaney J. L., Brown D. A. Synergistic regulation of a neuronal chloride current by intracellular calcium and muscarinic receptor activation: a role for protein kinase C. Neuron. 1995 Sep;15(3):729–737. doi: 10.1016/0896-6273(95)90160-4. [DOI] [PubMed] [Google Scholar]
- Mayer M. L. A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture. J Physiol. 1985 Jul;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfee D. A., Yarowsky P. J. Calcium-dependent potentials in the mammalian sympathetic neurone. J Physiol. 1979 May;290(2):507–523. doi: 10.1113/jphysiol.1979.sp012787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C. W., de Groat W. C., Felkins L. A., Zhang S. J. Axon collaterals indicate broad intraspinal role for sacral preganglionic neurons. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6888–6892. doi: 10.1073/pnas.88.15.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D. G., Segal M., Barker J. L. A Ca-dependent Cl- conductance in cultured mouse spinal neurones. Nature. 1984 Oct 11;311(5986):567–570. doi: 10.1038/311567a0. [DOI] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol. 1975 Nov;252(2):429–463. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Lichtman J. W. Geometrical differences among homologous neurons in mammals. Science. 1985 Apr 19;228(4697):298–302. doi: 10.1126/science.3983631. [DOI] [PubMed] [Google Scholar]
- Sacchi O., Rossi M. L., Canella R. The slow Ca(2+)-activated K+ current, IAHP, in the rat sympathetic neurone. J Physiol. 1995 Feb 15;483(Pt 1):15–27. doi: 10.1113/jphysiol.1995.sp020564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield G. G., Ikeda S. R. Sodium and calcium currents of acutely isolated adult rat superior cervical ganglion neurons. Pflugers Arch. 1988 May;411(5):481–490. doi: 10.1007/BF00582368. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Sutton K. G., Griffin A., Stapleton S. R., Currie K. P. Aspects of calcium-activated chloride currents: a neuronal perspective. Pharmacol Ther. 1995 Jun;66(3):535–565. doi: 10.1016/0163-7258(95)00018-c. [DOI] [PubMed] [Google Scholar]
- Sánchez-Vives M. V., Gallego R. Calcium-dependent chloride current induced by axotomy in rat sympathetic neurons. J Physiol. 1994 Mar 15;475(3):391–400. doi: 10.1113/jphysiol.1994.sp020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Vives M. V., Gallego R. Effects of axotomy or target atrophy on membrane properties of rat sympathetic ganglion cells. J Physiol. 1993 Nov;471:801–815. doi: 10.1113/jphysiol.1993.sp019929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. M., Aylwin M. Niflumic and flufenamic acids are potent reversible blockers of Ca2(+)-activated Cl- channels in Xenopus oocytes. Mol Pharmacol. 1990 May;37(5):720–724. [PubMed] [Google Scholar]
- Yawo H. Changes in the dendritic geometry of mouse superior cervical ganglion cells following postganglionic axotomy. J Neurosci. 1987 Nov;7(11):3703–3711. doi: 10.1523/JNEUROSCI.07-11-03703.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]