Abstract
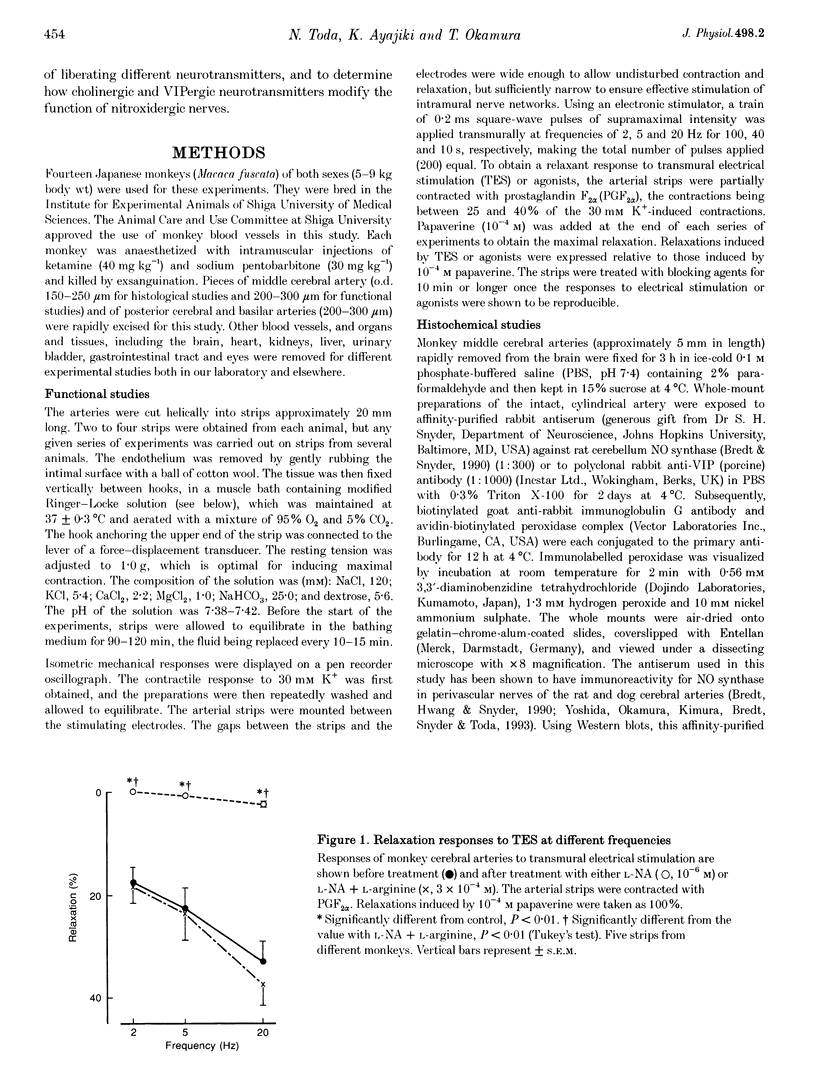
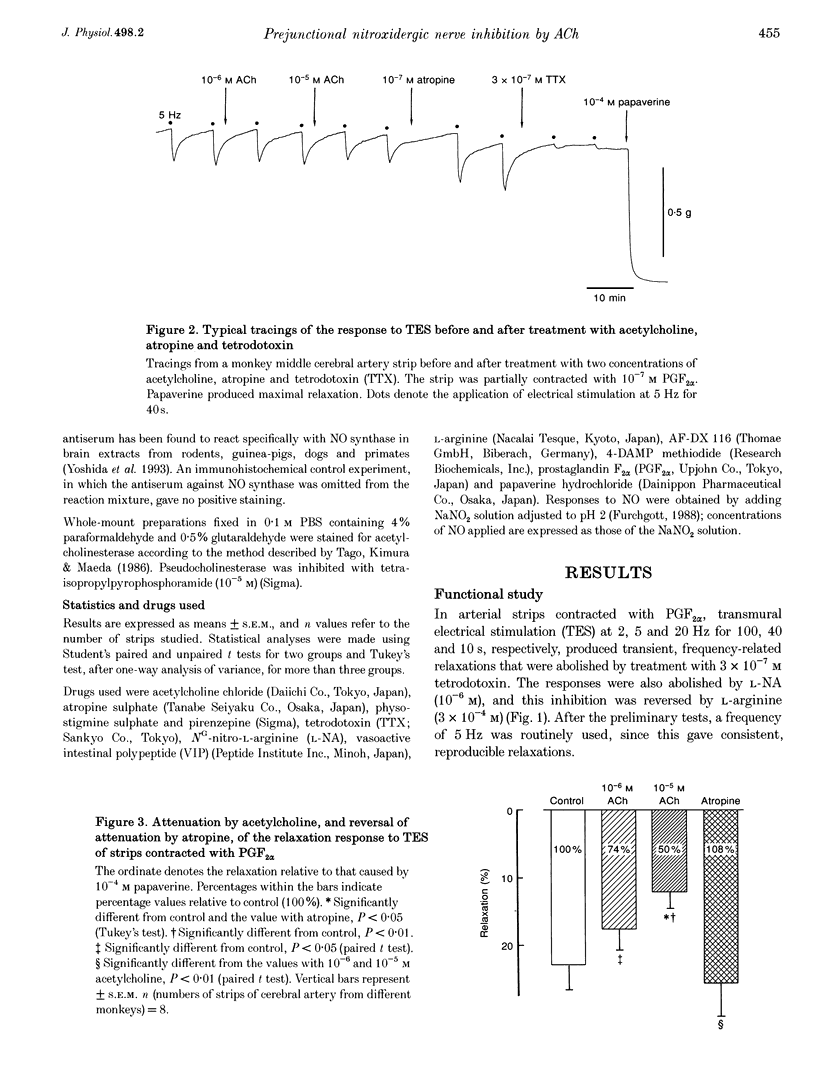
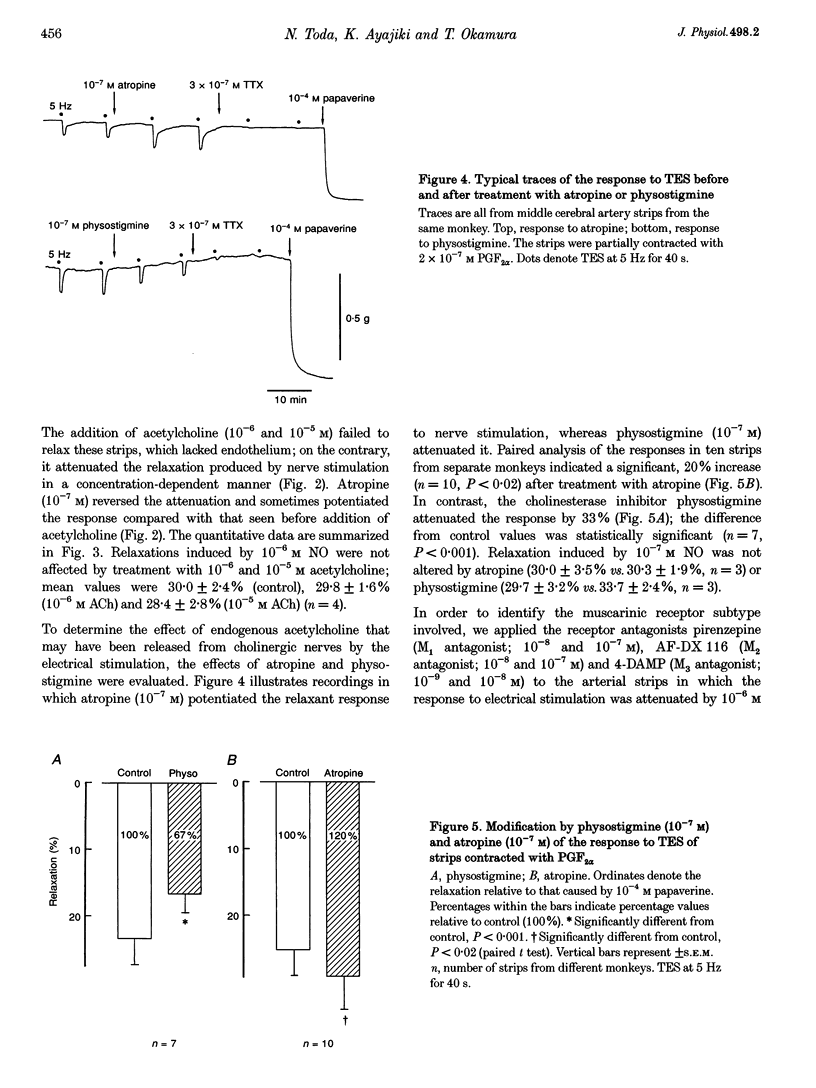
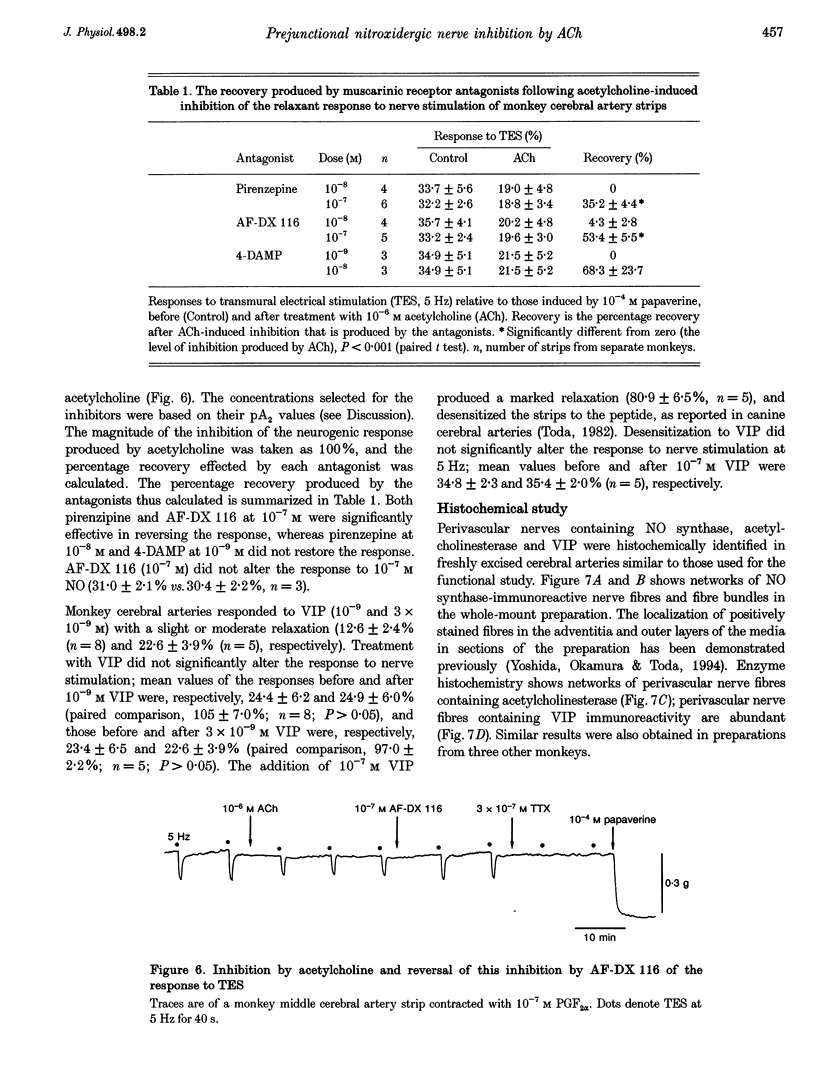
1. Modification by endogenous or exogenous acetylcholine and vasoactive intestinal polypeptide (VIP) of vasodilatation mediated by nitric oxide (NO) released from nitroxidergic nerves was studied in isolated monkey cerebral arteries. In arterial strips denuded of endothelium, transmural electrical stimulation (2-20 Hz) produced relaxations that were abolished by tetrodotoxin. 2. The relaxation response was attenuated by acetylcholine, and the attenuation was reversed by atropine. Attenuation was also observed with AF-DX 116, an antagonist of the muscarinic acetylcholine receptor subtype, M2. NO-induced relaxation was not affected by acetylcholine. Neurogenic relaxation was also inhibited by physostigmine and potentiated by atropine. 3. VIP in concentrations that elicited slight relaxation did not alter the response to nerve stimulation. In the strips showing tachyphylaxis to VIP, the neurogenic response was not inhibited. 4. Histochemical studies of whole-mount preparations revealed nerve fibres with NO synthase and VIP immunoreactivity, and also acetylcholinesterase, suggesting the presence of perivascular nitroxidergic, VIPergic and cholinergic innervation. 5. It is concluded that the actions of nitroxidergic nerve fibres on the monkey cerebral artery are inhibited by nerve-released acetylcholine acting on prejunctional muscarinic receptors, possibly of the M2 subtype. Despite the presence of VIP immunoreactive nerve fibres and the ability of exogenous VIP to relax the artery, there is no evidence supporting either a prejunctional modulation of nitroxidergic nerve function by VIP or a role for VIP as a vasodilatory neurotransmitter.
Full text
PDF
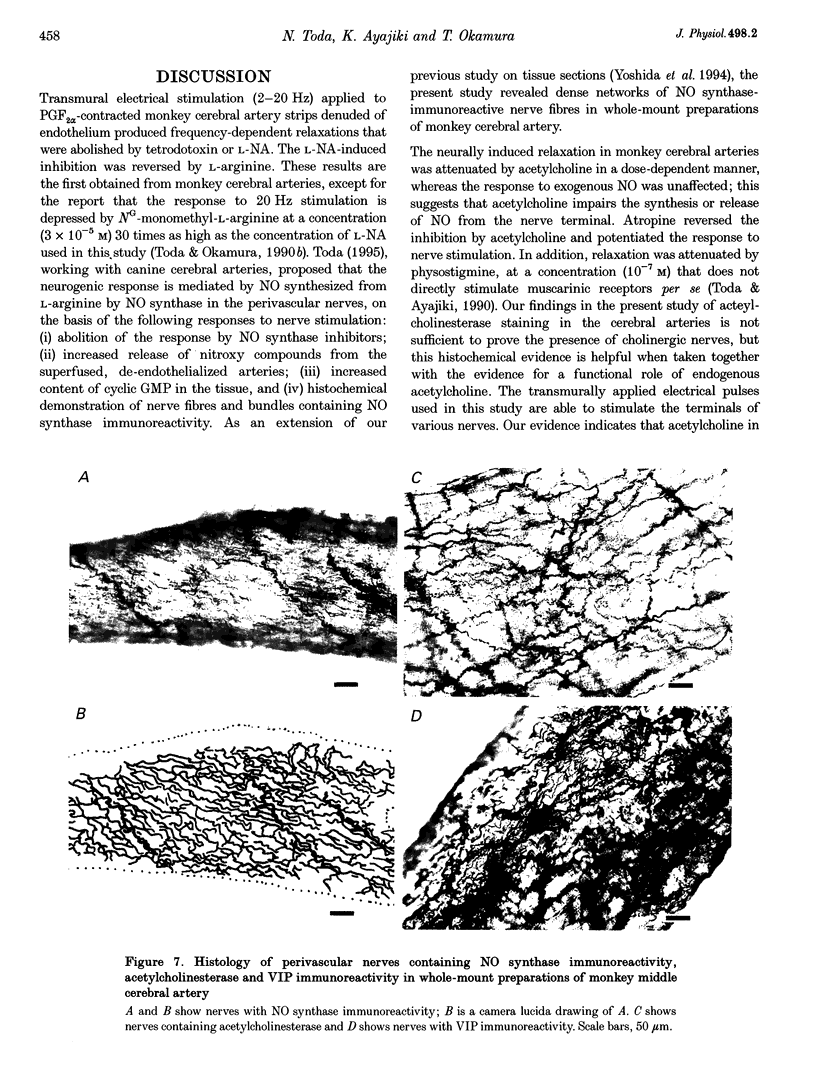
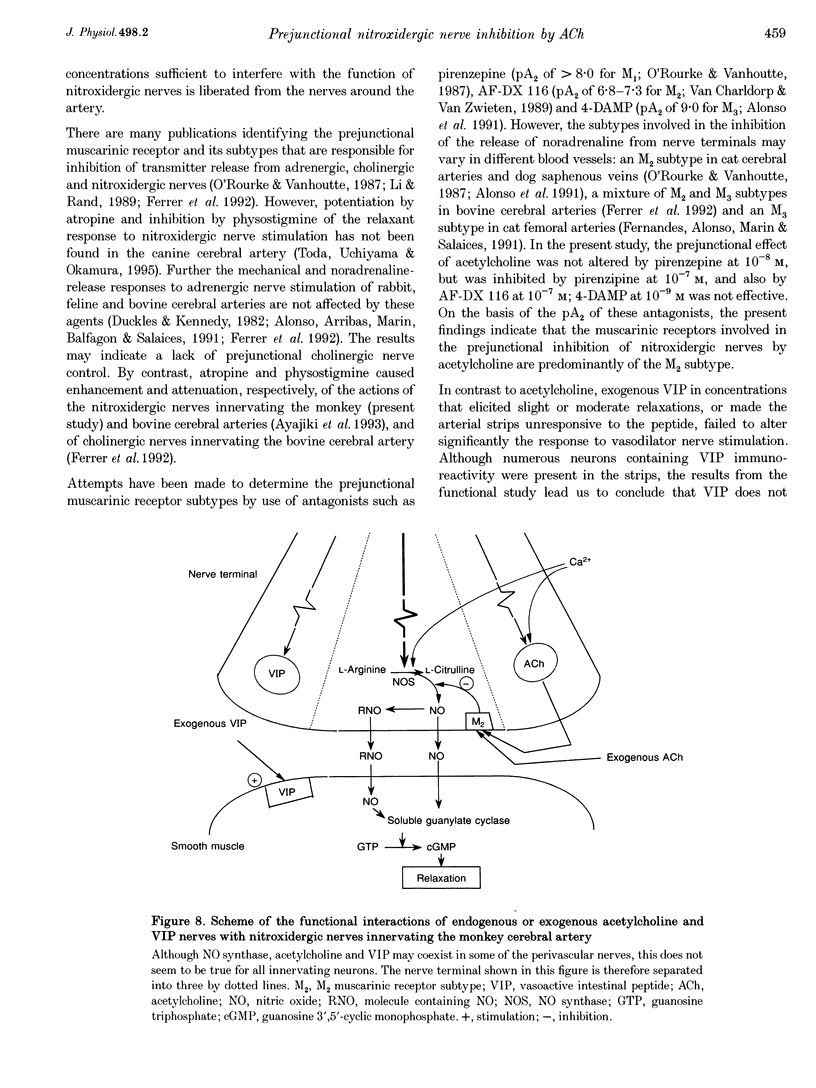


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso M. J., Arribas S., Marín J., Balfagón G., Salaices M. Presynaptic M2-muscarinic receptors on noradrenergic nerve endings and endothelium-derived M3 receptors in cat cerebral arteries. Brain Res. 1991 Dec 13;567(1):76–82. doi: 10.1016/0006-8993(91)91438-7. [DOI] [PubMed] [Google Scholar]
- Ayajiki K., Okamura T., Toda N. Neurogenic relaxations caused by nicotine in isolated cat middle cerebral arteries. J Pharmacol Exp Ther. 1994 Aug;270(2):795–801. [PubMed] [Google Scholar]
- Ayajiki K., Okamura T., Toda N. Nitric oxide mediates, and acetylcholine modulates, neurally induced relaxation of bovine cerebral arteries. Neuroscience. 1993 Jun;54(3):819–825. doi: 10.1016/0306-4522(93)90251-a. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckles S. P., Kennedy C. D. Cerebral blood vessels: effects of exogenous acetylcholine and field stimulation on norepinephrine release. J Pharmacol Exp Ther. 1982 Sep;222(3):562–565. [PubMed] [Google Scholar]
- Fernándes F. A., Alonso M. J., Marín J., Salaices M. M3-muscarinic receptor mediates prejunctional inhibition of noradrenaline release and the relaxation in cat femoral artery. J Pharm Pharmacol. 1991 Sep;43(9):644–649. doi: 10.1111/j.2042-7158.1991.tb03555.x. [DOI] [PubMed] [Google Scholar]
- Ferrer M., Galván R., Marín J., Balfagón G. Presynaptic muscarinic receptor subtypes involved in the inhibition of acetylcholine and noradrenaline release in bovine cerebral arteries. Naunyn Schmiedebergs Arch Pharmacol. 1992 Jun;345(6):619–626. doi: 10.1007/BF00164574. [DOI] [PubMed] [Google Scholar]
- Hara H., Hamill G. S., Jacobowitz D. M. Origin of cholinergic nerves to the rat major cerebral arteries: coexistence with vasoactive intestinal polypeptide. Brain Res Bull. 1985 Feb;14(2):179–188. doi: 10.1016/0361-9230(85)90077-2. [DOI] [PubMed] [Google Scholar]
- Kalsner S., Quillan M. Cholinergic contraction to field stimulation in coronary arteries of cattle. J Pharmacol Exp Ther. 1989 Jun;249(3):785–789. [PubMed] [Google Scholar]
- Lee T. J., Sarwinski S. J. Nitric oxidergic neurogenic vasodilation in the porcine basilar artery. Blood Vessels. 1991;28(5):407–412. doi: 10.1159/000158887. [DOI] [PubMed] [Google Scholar]
- Li C. G., Rand M. J. Prejunctional inhibition of non-adrenergic non-cholinergic transmission in the rat anococcygeus muscle. Eur J Pharmacol. 1989 Sep 1;168(1):107–110. doi: 10.1016/0014-2999(89)90640-7. [DOI] [PubMed] [Google Scholar]
- Minami Y., Kimura H., Aimi Y., Vincent S. R. Projections of nitric oxide synthase-containing fibers from the sphenopalatine ganglion to cerebral arteries in the rat. Neuroscience. 1994 Jun;60(3):745–759. doi: 10.1016/0306-4522(94)90502-9. [DOI] [PubMed] [Google Scholar]
- Morris J. L., Gibbins I. L., Kadowitz P. J., Herzog H., Kreulen D. L., Toda N., Claing A. Roles of peptides and other substances in cotransmission from vascular autonomic and sensory neurons. Can J Physiol Pharmacol. 1995 May;73(5):521–532. doi: 10.1139/y95-067. [DOI] [PubMed] [Google Scholar]
- O'Rourke S. T., Vanhoutte P. M. Subtypes of muscarinic receptors on adrenergic nerves and vascular smooth muscle of the canine saphenous vein. J Pharmacol Exp Ther. 1987 Apr;241(1):64–67. [PubMed] [Google Scholar]
- Tago H., Kimura H., Maeda T. Visualization of detailed acetylcholinesterase fiber and neuron staining in rat brain by a sensitive histochemical procedure. J Histochem Cytochem. 1986 Nov;34(11):1431–1438. doi: 10.1177/34.11.2430009. [DOI] [PubMed] [Google Scholar]
- Toda N., Ayajiki K. Cholinergic prejunctional inhibition of vasodilator nerve function in bovine basilar arteries. Am J Physiol. 1990 Apr;258(4 Pt 2):H983–H986. doi: 10.1152/ajpheart.1990.258.4.H983. [DOI] [PubMed] [Google Scholar]
- Toda N., Ayajiki K., Yoshida K., Kimura H., Okamura T. Impairment by damage of the pterygopalatine ganglion of nitroxidergic vasodilator nerve function in canine cerebral and retinal arteries. Circ Res. 1993 Jan;72(1):206–213. doi: 10.1161/01.res.72.1.206. [DOI] [PubMed] [Google Scholar]
- Toda N. Mediation by nitric oxide of neurally-induced human cerebral artery relaxation. Experientia. 1993 Jan 15;49(1):51–53. doi: 10.1007/BF01928789. [DOI] [PubMed] [Google Scholar]
- Toda N., Okamura T. Possible role of nitric oxide in transmitting information from vasodilator nerve to cerebroarterial muscle. Biochem Biophys Res Commun. 1990 Jul 16;170(1):308–313. doi: 10.1016/0006-291x(90)91275-w. [DOI] [PubMed] [Google Scholar]
- Toda N. Relaxant responses to transmural stimulation and nicotine of dog and monkey cerebral arteries. Am J Physiol. 1982 Aug;243(2):H145–H153. doi: 10.1152/ajpheart.1982.243.2.H145. [DOI] [PubMed] [Google Scholar]
- Toda N., Uchiyama M., Okamura T. Prejunctional modulation of nitroxidergic nerve function in canine cerebral arteries. Brain Res. 1995 Nov 27;700(1-2):213–218. doi: 10.1016/0006-8993(95)00959-t. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Okamura T., Kimura H., Bredt D. S., Snyder S. H., Toda N. Nitric oxide synthase-immunoreactive nerve fibers in dog cerebral and peripheral arteries. Brain Res. 1993 Nov 26;629(1):67–72. doi: 10.1016/0006-8993(93)90482-3. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Okamura T., Toda N. Histological and functional studies on the nitroxidergic nerve innervating monkey cerebral, mesenteric and temporal arteries. Jpn J Pharmacol. 1994 Aug;65(4):351–359. doi: 10.1254/jjp.65.351. [DOI] [PubMed] [Google Scholar]
- Yoshioka K., Furuta T., Hayakawa A., Ishikawa N., Shigei T. Excitatory cholinergic innervation in canine portal and mesenteric veins. Am J Physiol. 1988 Aug;255(2 Pt 2):H288–H294. doi: 10.1152/ajpheart.1988.255.2.H288. [DOI] [PubMed] [Google Scholar]
- van Charldorp K. J., van Zwieten P. A. Comparison of the muscarinic receptors in the coronary artery, cerebral artery and atrium of the pig. Naunyn Schmiedebergs Arch Pharmacol. 1989 Apr;339(4):403–408. doi: 10.1007/BF00736054. [DOI] [PubMed] [Google Scholar]



