Abstract
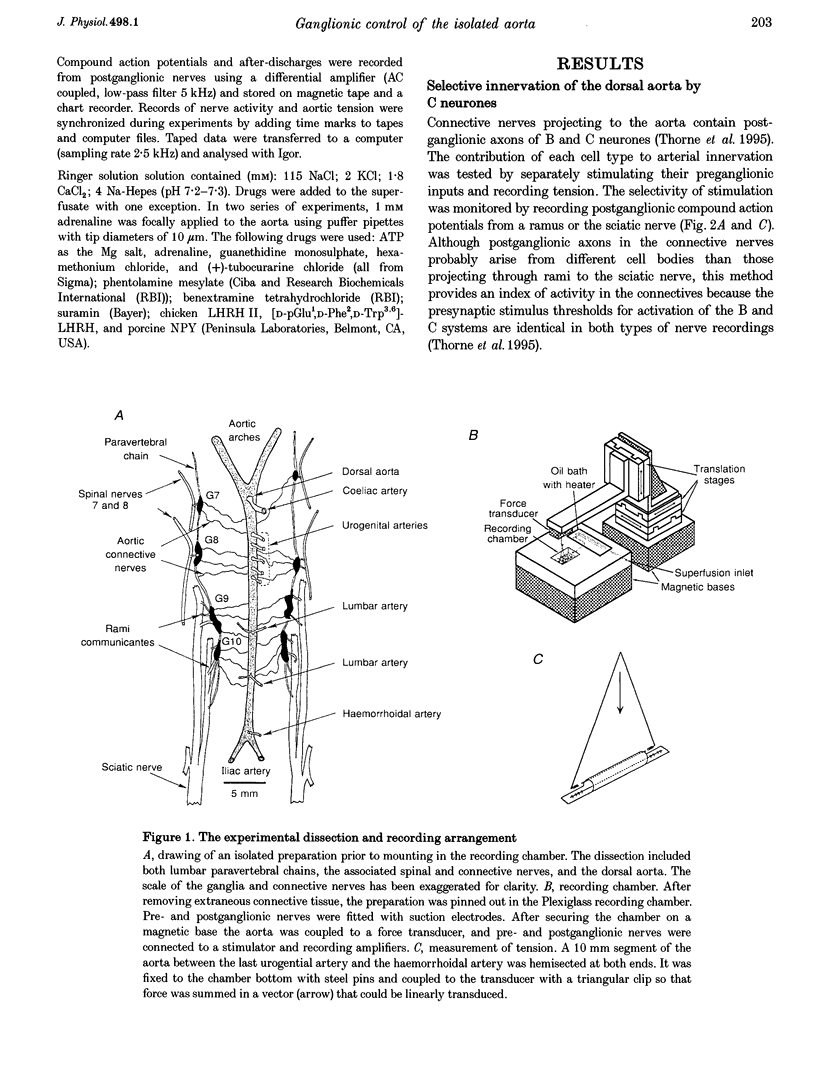
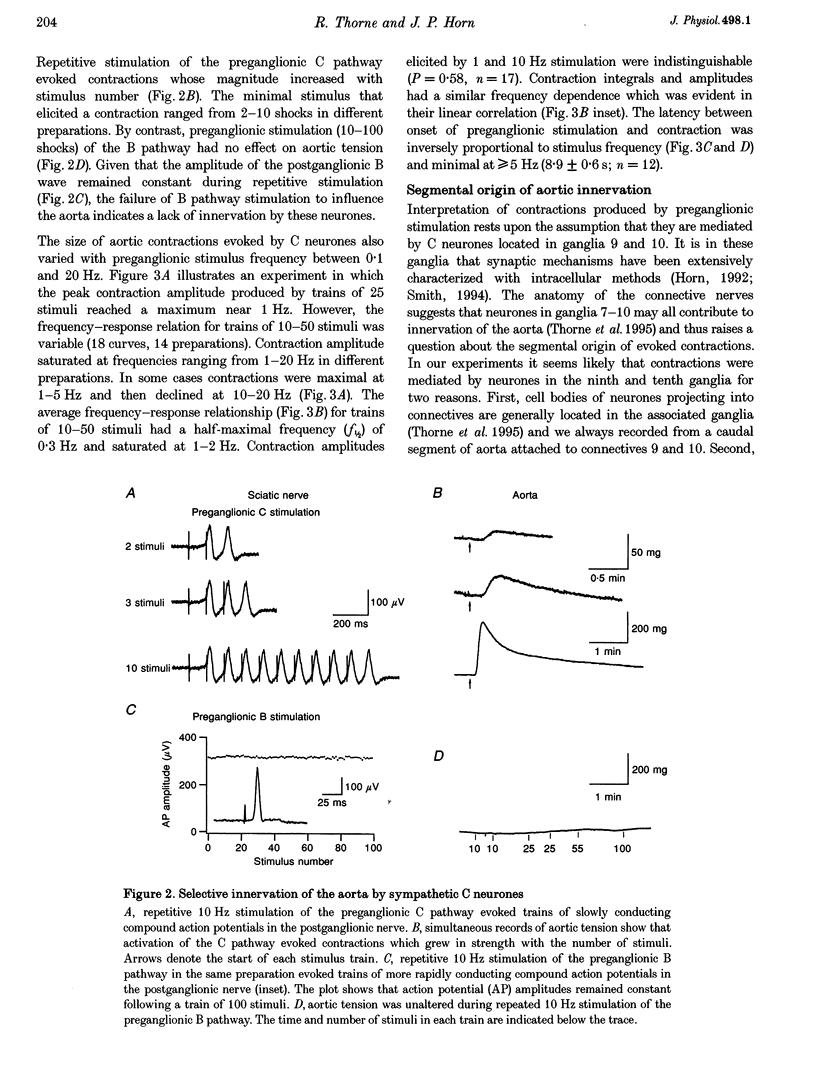
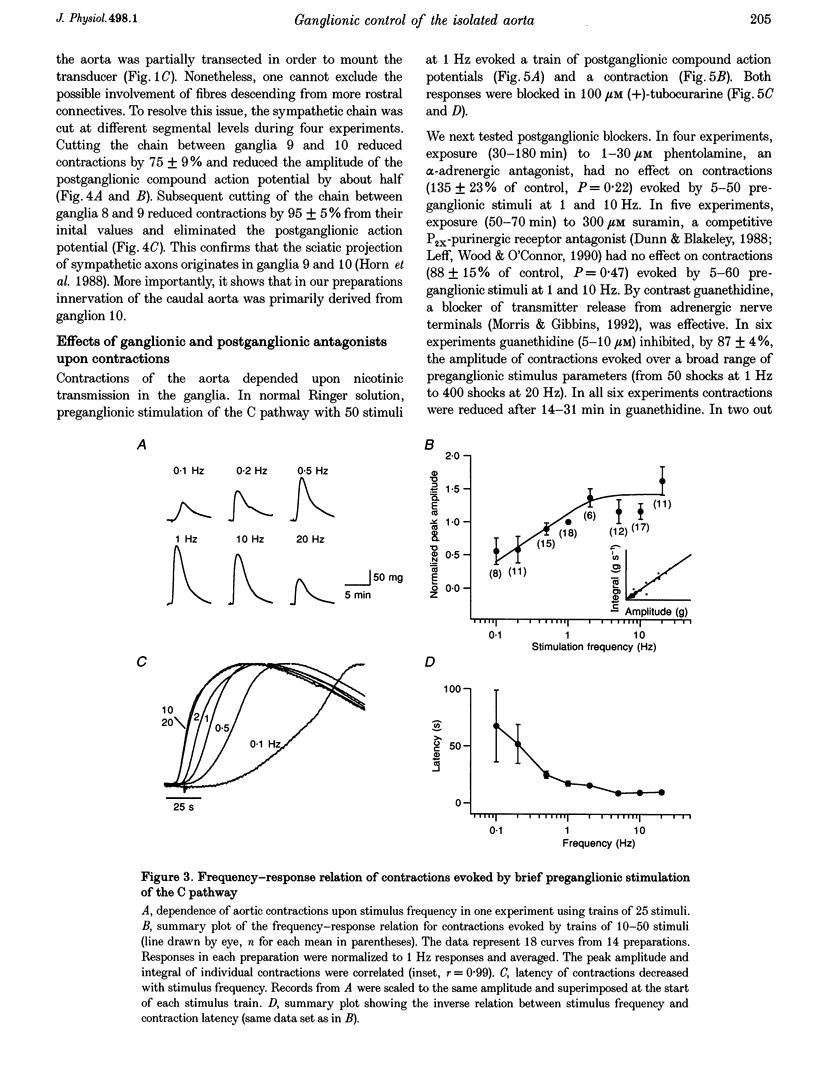
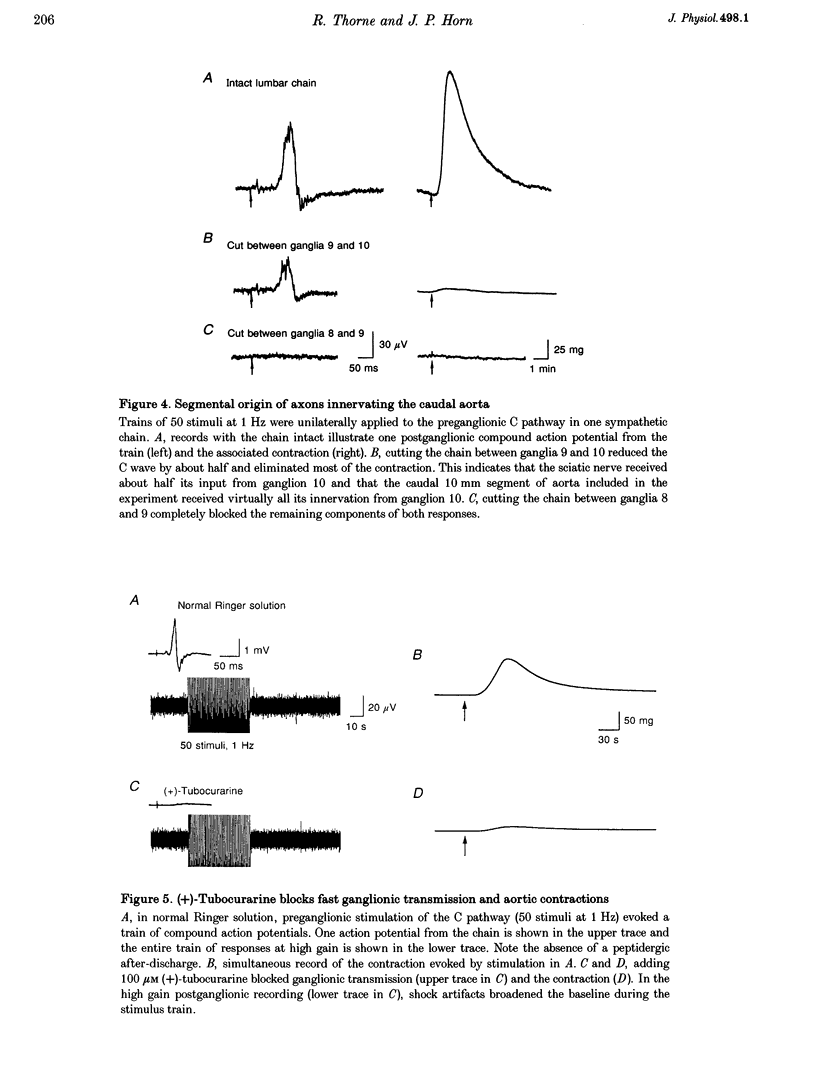
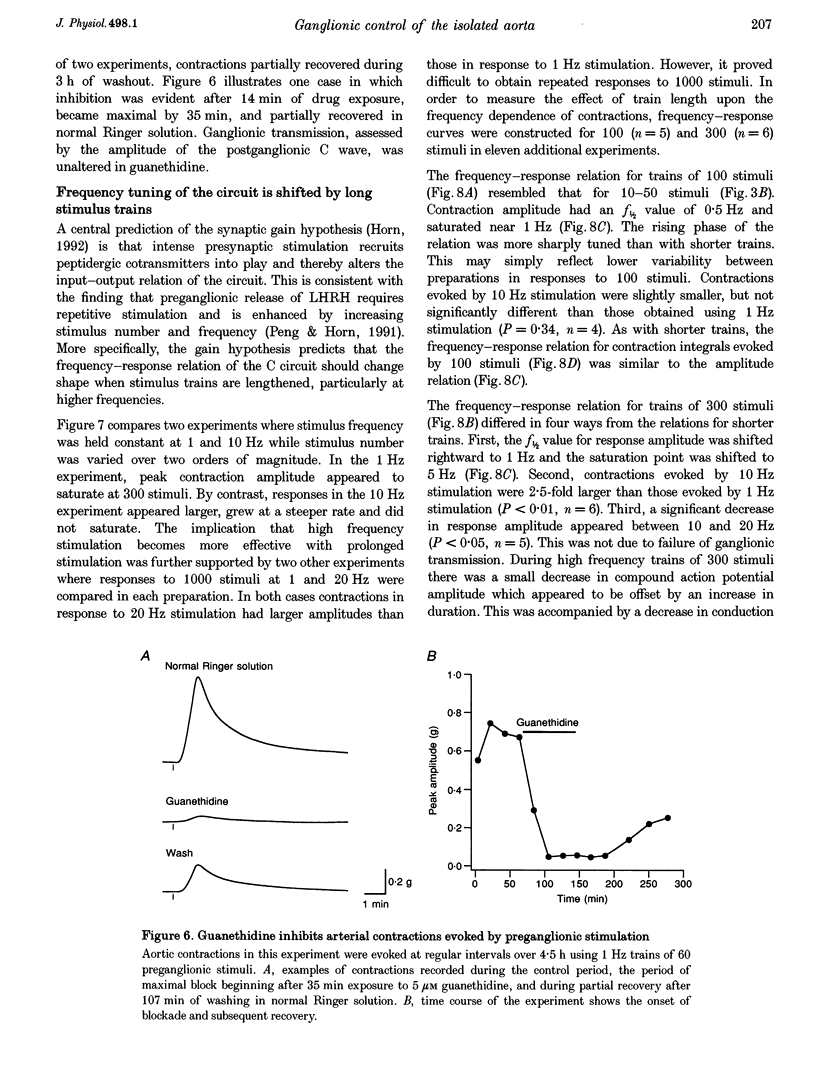
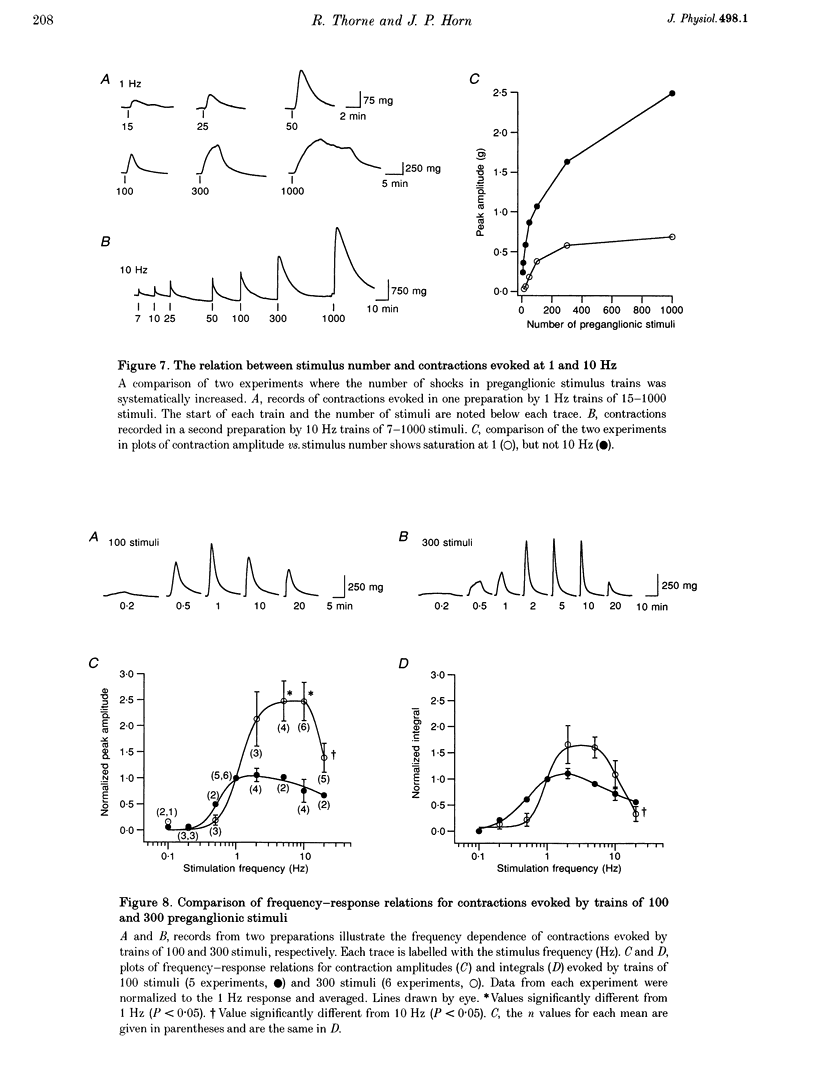
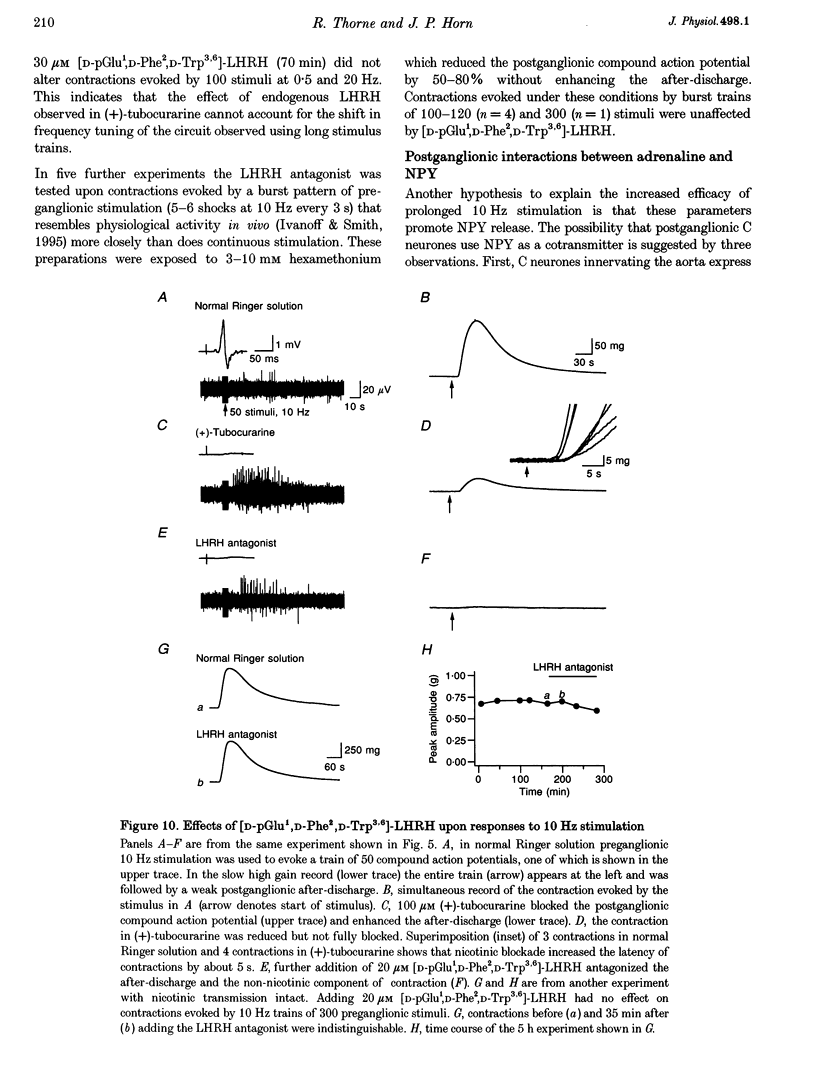
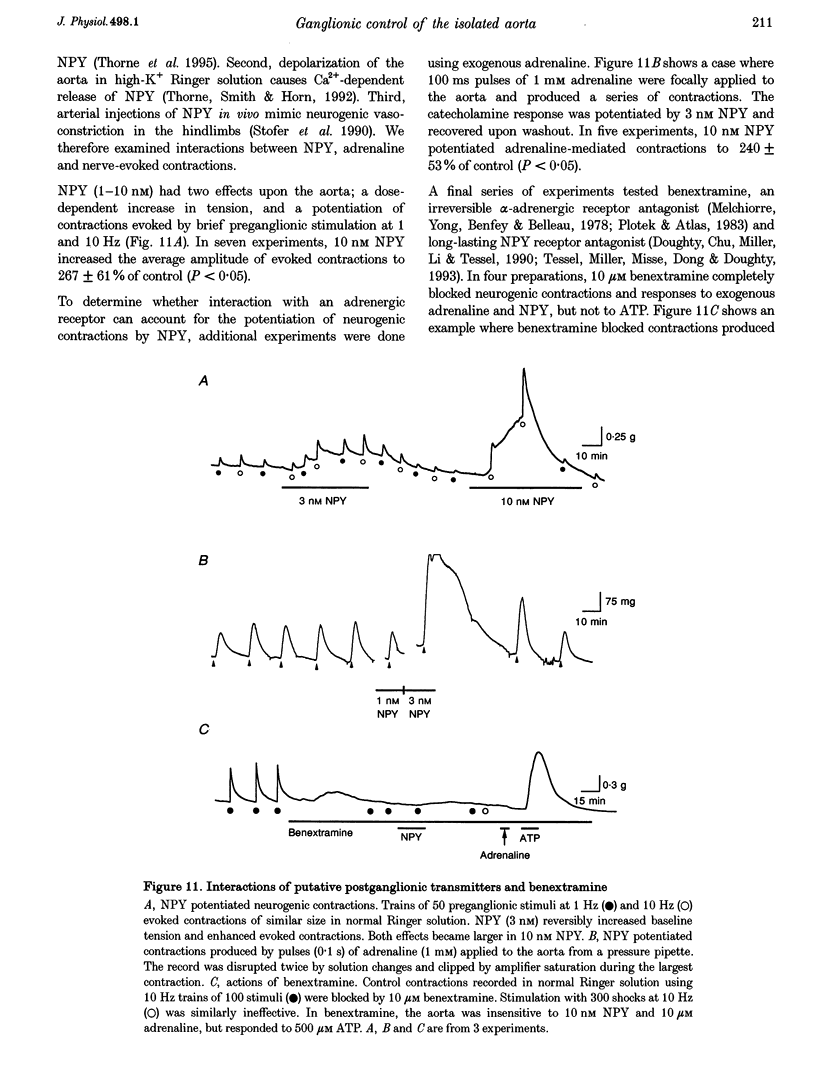
1. The relation between preganglionic activity and arterial tone was studied in preparations of bullfrog lumbar sympathetic ganglia 7-10 and the dorsal aorta. 2. Two or more stimuli evoked contractions when applied to the preganglionic C, but not the B pathway. Contractions were blocked when transmission in ganglia 9 and 10 was disrupted by cutting the sympathetic chain or adding (+)-tubocurarine. Contractions were antagonized by postganglionic action of guanethidine, but not by phentolamine or suramin. 3. Aortic responses to short trains (10-100 stimuli) were half-maximal at 0.3-0.5 Hz, saturated near 1 Hz and had a minimum latency of 8.9 s. By contrast, responses to 300 stimuli were half-maximal at 1 Hz and became 2.5-fold larger at 10 Hz. 4. Exogenous luteinizing hormone releasing hormone (LHRH) potentiated preganglionically evoked contractions. Endogenous LHRH mediated contractions evoked by 10 Hz stimulation in (+)-tubocurarine. These responses had a longer latency than in normal Ringer solution and were blocked by [D-pGlu1, D-Phe2, D-Trp3.6]-LHRH. The LHRH antagonist did not alter contractions evoked by continuous stimulation in normal Ringer solution or by bursts of stimuli in hexamethonium. 5. Exogenous neuropeptide Y (NPY) potentiated neurogenic contractions and responses to adrenaline. Benextramine blocked contractions produced by nerve stimulation, adrenaline and NPY, but not ATP. 6. The results show that contractions of the isolated aorta are tuned to physiological frequencies of activity in sympathetic C neurones. Peptidergic cotransmission in the ganglia can increase arterial tension, but not during synchronous activation of primary nicotinic synapses. It is suggested that the physiological role of LHRH arises from interactions with subthreshold nicotinic EPSPs and that postganglionic release of NPY shifts frequency tuning of the circuit during prolonged activity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowers C. W. Superfluous neurotransmitters? Trends Neurosci. 1994 Aug;17(8):315–320. doi: 10.1016/0166-2236(94)90168-6. [DOI] [PubMed] [Google Scholar]
- Dodd J., Horn J. P. A reclassification of B and C neurones in the ninth and tenth paravertebral sympathetic ganglia of the bullfrog. J Physiol. 1983 Jan;334:255–269. doi: 10.1113/jphysiol.1983.sp014493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J., Horn J. P. Muscarinic inhibition of sympathetic C neurones in the bullfrog. J Physiol. 1983 Jan;334:271–291. doi: 10.1113/jphysiol.1983.sp014494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty M. B., Chu S. S., Miller D. W., Li K., Tessel R. E. Benextramine: a long-lasting neuropeptide Y receptor antagonist. Eur J Pharmacol. 1990 Aug 21;185(1):113–114. doi: 10.1016/0014-2999(90)90218-u. [DOI] [PubMed] [Google Scholar]
- Dunn P. M., Blakeley A. G. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br J Pharmacol. 1988 Feb;93(2):243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh J. W., Pennefather P. S. Pharmacological and physiological properties of the after-hyperpolarization current of bullfrog ganglion neurones. J Physiol. 1987 Dec;394:315–330. doi: 10.1113/jphysiol.1987.sp016872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Edwards F. R. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989 Apr;69(2):546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- Honma S. Functional differentiation in sB and sC neurons of toad sympathetic ganglia. Jpn J Physiol. 1970 Jun 15;20(3):281–295. doi: 10.2170/jjphysiol.20.281. [DOI] [PubMed] [Google Scholar]
- Horn J. P., Fatherazi S., Stofer W. D. Differential projections of B and C sympathetic axons in peripheral nerves of the bullfrog. J Comp Neurol. 1988 Dec 22;278(4):570–580. doi: 10.1002/cne.902780408. [DOI] [PubMed] [Google Scholar]
- Hökfelt T. Neuropeptides in perspective: the last ten years. Neuron. 1991 Dec;7(6):867–879. doi: 10.1016/0896-6273(91)90333-u. [DOI] [PubMed] [Google Scholar]
- Jones S. W. Chicken II luteinizing hormone-releasing hormone inhibits the M-current of bullfrog sympathetic neurons. Neurosci Lett. 1987 Sep 23;80(2):180–184. doi: 10.1016/0304-3940(87)90650-1. [DOI] [PubMed] [Google Scholar]
- Leff P., Wood B. E., O'Connor S. E. Suramin is a slowly-equilibrating but competitive antagonist at P2x-receptors in the rabbit isolated ear artery. Br J Pharmacol. 1990 Nov;101(3):645–649. doi: 10.1111/j.1476-5381.1990.tb14134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libet B., Chichibu S., Tosaka T. Slow synaptic responses and excitability in sympathetic ganglia of the bullfrog. J Neurophysiol. 1968 May;31(3):383–395. doi: 10.1152/jn.1968.31.3.383. [DOI] [PubMed] [Google Scholar]
- Melchiorre C., Yong M. S., Benfey B. G., Belleau B. Molecular properties of the adrenergic alpha receptor. 2. Optimum covalent inhibition by two different prototypes of polyamine disulfides. J Med Chem. 1978 Nov;21(11):1126–1132. doi: 10.1021/jm00209a007. [DOI] [PubMed] [Google Scholar]
- Nilsson H., Goldstein M., Nilsson O. Adrenergic innervation and neurogenic response in large and small arteries and veins from the rat. Acta Physiol Scand. 1986 Jan;126(1):121–133. doi: 10.1111/j.1748-1716.1986.tb07795.x. [DOI] [PubMed] [Google Scholar]
- Nishi S., Soeda H., Koketsu K. Studies on sympathetic B and C neurons and patterns of pregnaglionic innervation. J Cell Physiol. 1965 Aug;66(1):19–32. doi: 10.1002/jcp.1030660103. [DOI] [PubMed] [Google Scholar]
- Plotek Y., Atlas D. Characterization of benextramine as an irreversible alpha-adrenergic blocker and as a blocker of potassium-activated calcium channels. Eur J Biochem. 1983 Jul 1;133(3):539–544. doi: 10.1111/j.1432-1033.1983.tb07497.x. [DOI] [PubMed] [Google Scholar]
- SKOK V. I. CONDUCTION IN TENTH GANGLION OF THE FROG SYMPATHETIC TRUNK. Fed Proc Transl Suppl. 1965 Mar-Apr;24:363–367. [PubMed] [Google Scholar]
- Shen W. X., Horn J. P. A presynaptic mechanism accounts for the differential block of nicotinic synapses on sympathetic B and C neurons by d-tubocurarine. J Neurosci. 1995 Jul;15(7 Pt 1):5025–5035. doi: 10.1523/JNEUROSCI.15-07-05025.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. A., Weight F. F. The pathway for the slow inhibitory postsynaptic potential in bullfrog sympathetic ganglia. J Neurophysiol. 1986 Sep;56(3):823–834. doi: 10.1152/jn.1986.56.3.823. [DOI] [PubMed] [Google Scholar]
- Stofer W. D., Fatherazi S., Horn J. P. Neuropeptide Y mimics a non-adrenergic component of sympathetic vasoconstriction in the bullfrog. J Auton Nerv Syst. 1990 Nov;31(2):141–151. doi: 10.1016/0165-1838(90)90071-p. [DOI] [PubMed] [Google Scholar]
- Tessel R. E., Miller D. W., Misse G. A., Dong X., Doughty M. B. Characterization of vascular postsynaptic neuropeptide Y receptor function and regulation. 1. NPY-induced constriction in isolated rat femoral artery rings is mediated by both Y1 and Y2 receptors: evidence from benextramine protection studies. J Pharmacol Exp Ther. 1993 Apr;265(1):172–177. [PubMed] [Google Scholar]
- Thorne R., Smith M. S., Horn J. P. Ganglionic and arterial release of neuropeptide Y by bullfrog sympathetic neurons. J Auton Nerv Syst. 1992 May 15;38(3):231–236. doi: 10.1016/0165-1838(92)90034-e. [DOI] [PubMed] [Google Scholar]
- Thorne R., Stofer W. D., Horn J. P. An aortic projection of lumbar paravertebral sympathetic neurons in the bullfrog. J Auton Nerv Syst. 1995 Dec 5;56(1-2):38–44. doi: 10.1016/0165-1838(95)00059-2. [DOI] [PubMed] [Google Scholar]
- Wilson J. X., Butchey J. K., Deshpande A. A. Chicken gonadotropin-releasing hormone II increases plasma catecholamines in the bullfrog. Neurosci Lett. 1988 Dec 19;95(1-3):354–358. doi: 10.1016/0304-3940(88)90684-2. [DOI] [PubMed] [Google Scholar]


