Abstract
1. The time constants of motor and sensory nerve fibres were studied in normal human ulnar nerves by the method of latent addition, using threshold tracking to follow the recovery of excitability after brief conditioning current pulses. The 60 microseconds test and conditioning stimuli were applied at the wrist, and the conditioning stimuli were set to 90, 60, 30, -30, -60 and -90% of the control threshold current. Compound muscle action potentials were recorded from abductor digiti minimi, and sensory nerve action potentials from the little finger. 2. Recovery from depolarizing conditioning pulses was slower than recovery from hyperpolarizing pulses and strongly dependent on conditioning pulse amplitude. The voltage dependence of latent addition was attributed to subthreshold activation of sodium channels (local response). 3. Motor and sensory nerve excitability generally recovered from -90% hyperpolarizing pulses as the sum of two exponential components, although the slow component was negligible in some motor nerves. The fast component (time constant 43.3 +/- 2.0 microseconds, mean +/- S.E.M., n = 9) was similar between motor and sensory fibres in the same subject. It showed no consistent voltage dependence, and was attributed to a passive input time constant of the fibres. The slow component of recovery from hyperpolarizing pulses was greater in sensory than in motor fibres and was voltage dependent: it could be greatly increased in motor and sensory fibres by steady depolarization. It was attributed to a regenerative membrane current, active at the resting potential in sensory and at least some motor nerves. 4. The latent addition responses were compared with the computed responses of four theoretical models. Both motor and sensory responses were well fitted by a model in which a fraction of the sodium channels (less in motor than in sensory fibres) were activated at potentials 20 mV more negative than normal and at half the normal rate, and did not inactivate. 5. It is concluded that the differences in latent addition between motor and sensory fibres are primarily due to differences in non-classical, voltage-dependent ion channels, active close to the resting potential. These "threshold channels' may help to account for the longer strength-duration time constant of sensory fibres, for their lower rheobase, and for their greater tendency to fire repetitively.
Full text
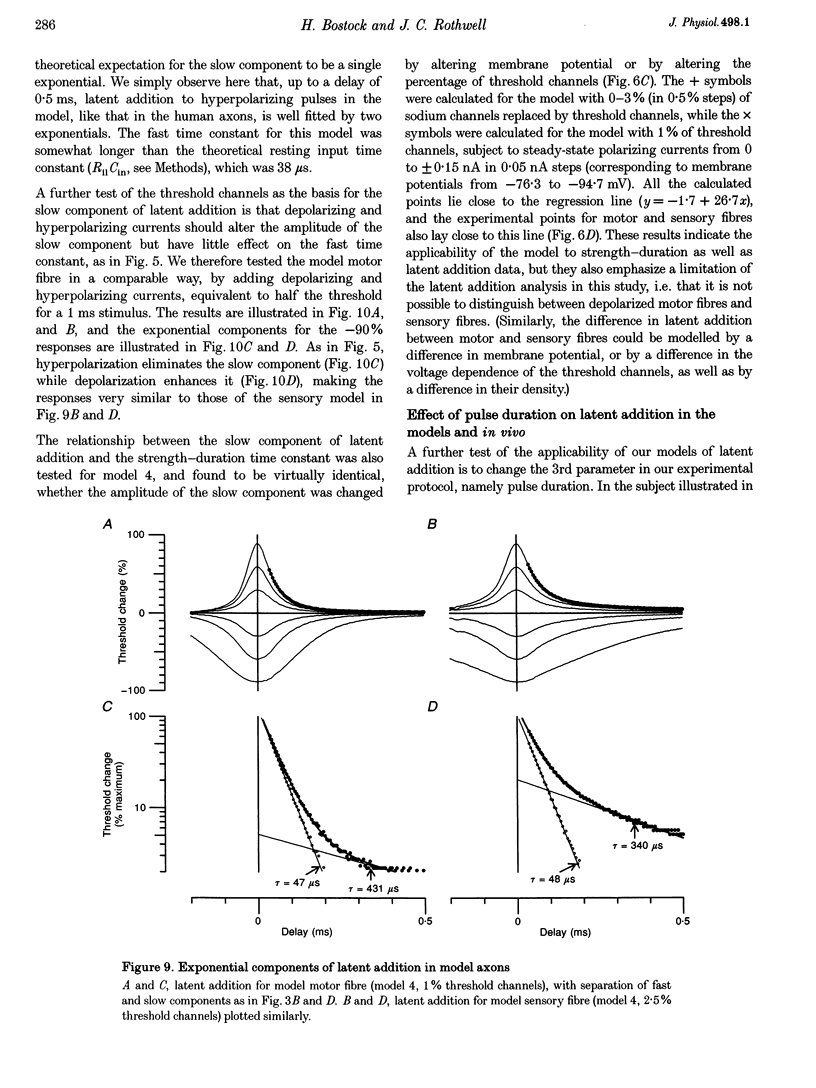
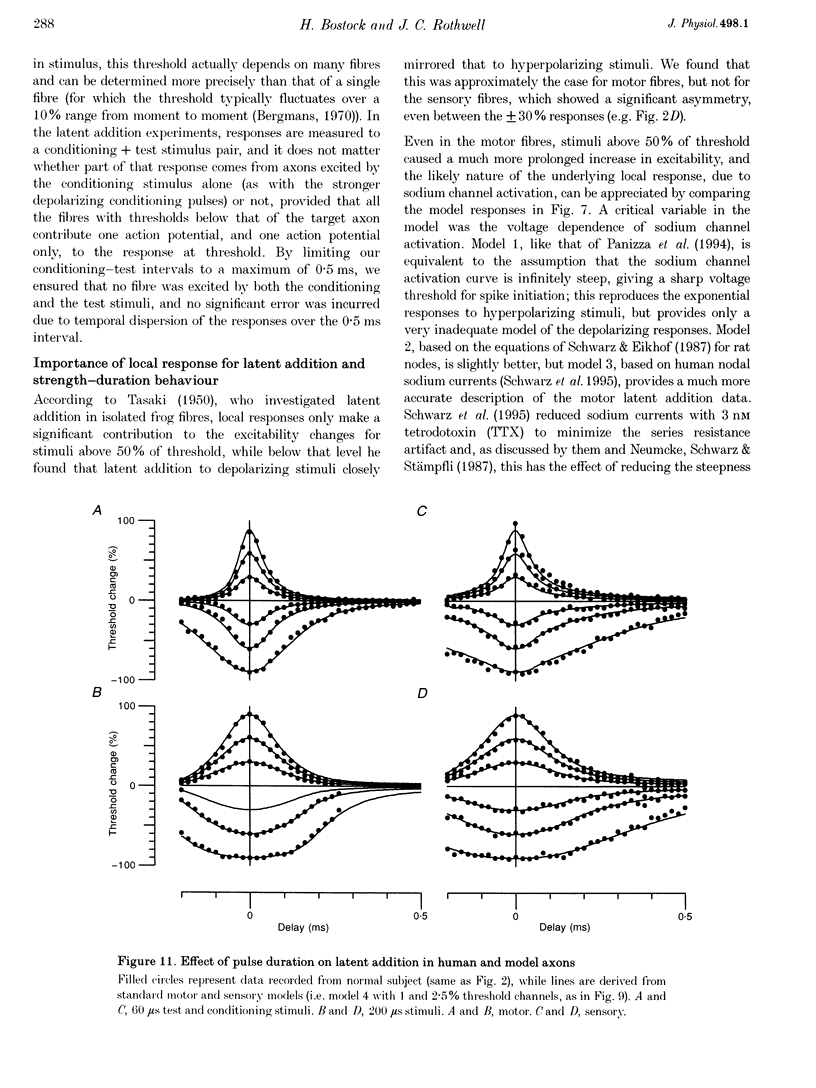
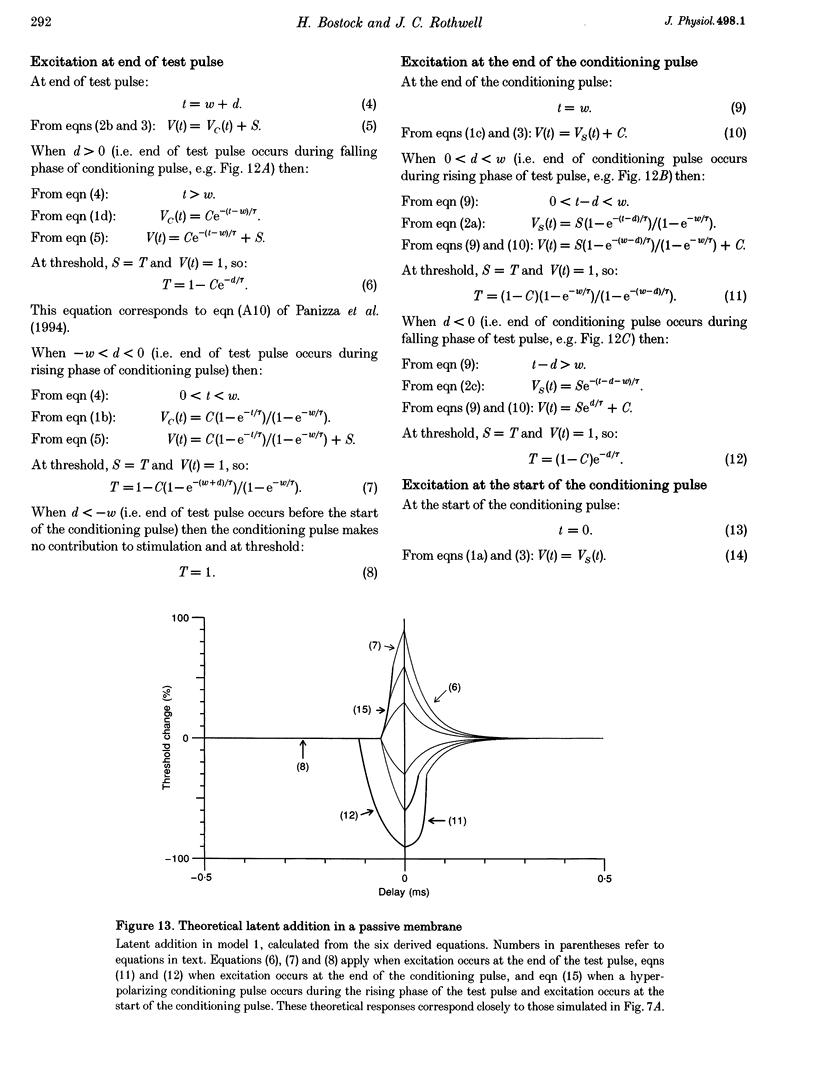
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alzheimer C., Schwindt P. C., Crill W. E. Modal gating of Na+ channels as a mechanism of persistent Na+ current in pyramidal neurons from rat and cat sensorimotor cortex. J Neurosci. 1993 Feb;13(2):660–673. doi: 10.1523/JNEUROSCI.13-02-00660.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Barrett J. N. Intracellular recording from vertebrate myelinated axons: mechanism of the depolarizing afterpotential. J Physiol. 1982 Feb;323:117–144. doi: 10.1113/jphysiol.1982.sp014064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H., Baker M., Reid G. Changes in excitability of human motor axons underlying post-ischaemic fasciculations: evidence for two stable states. J Physiol. 1991 Sep;441:537–557. doi: 10.1113/jphysiol.1991.sp018766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H., Bergmans J. Post-tetanic excitability changes and ectopic discharges in a human motor axon. Brain. 1994 Oct;117(Pt 5):913–928. doi: 10.1093/brain/117.5.913. [DOI] [PubMed] [Google Scholar]
- Bostock H., Burke D., Hales J. P. Differences in behaviour of sensory and motor axons following release of ischaemia. Brain. 1994 Apr;117(Pt 2):225–234. doi: 10.1093/brain/117.2.225. [DOI] [PubMed] [Google Scholar]
- Bostock H., Sears T. A., Sherratt R. M. The spatial distribution of excitability and membrane current in normal and demyelinated mammalian nerve fibres. J Physiol. 1983 Aug;341:41–58. doi: 10.1113/jphysiol.1983.sp014791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H., Sharief M. K., Reid G., Murray N. M. Axonal ion channel dysfunction in amyotrophic lateral sclerosis. Brain. 1995 Feb;118(Pt 1):217–225. doi: 10.1093/brain/118.1.217. [DOI] [PubMed] [Google Scholar]
- Bostock H. The strength-duration relationship for excitation of myelinated nerve: computed dependence on membrane parameters. J Physiol. 1983 Aug;341:59–74. doi: 10.1113/jphysiol.1983.sp014792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brismar T. Electrical properties of isolated demyelinated rat nerve fibres. Acta Physiol Scand. 1981 Oct;113(2):161–166. doi: 10.1111/j.1748-1716.1981.tb06877.x. [DOI] [PubMed] [Google Scholar]
- De Schutter E., Bower J. M. An active membrane model of the cerebellar Purkinje cell. I. Simulation of current clamps in slice. J Neurophysiol. 1994 Jan;71(1):375–400. doi: 10.1152/jn.1994.71.1.375. [DOI] [PubMed] [Google Scholar]
- Dubois J. M., Bergman C. Late sodium current in the node of Ranvier. Pflugers Arch. 1975;357(1-2):145–148. doi: 10.1007/BF00584552. [DOI] [PubMed] [Google Scholar]
- French C. R., Sah P., Buckett K. J., Gage P. W. A voltage-dependent persistent sodium current in mammalian hippocampal neurons. J Gen Physiol. 1990 Jun;95(6):1139–1157. doi: 10.1085/jgp.95.6.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Lamb G. D., Wakefield B. T. Transient and persistent sodium currents in normal and denervated mammalian skeletal muscle. J Physiol. 1989 Nov;418:427–439. doi: 10.1113/jphysiol.1989.sp017850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilly W. F., Armstrong C. M. Threshold channels--a novel type of sodium channel in squid giant axon. 1984 May 31-Jun 6Nature. 309(5967):448–450. doi: 10.1038/309448a0. [DOI] [PubMed] [Google Scholar]
- Grafe P., Bostock H., Schneider U. The effects of hyperglycaemic hypoxia on rectification in rat dorsal root axons. J Physiol. 1994 Oct 15;480(Pt 2):297–307. doi: 10.1113/jphysiol.1994.sp020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogyoros I., Kiernan M. C., Burke D. Strength-duration properties of human peripheral nerve. Brain. 1996 Apr;119(Pt 2):439–447. doi: 10.1093/brain/119.2.439. [DOI] [PubMed] [Google Scholar]
- Neumcke B., Schwarz J. R., Stämpfli R. A comparison of sodium currents in rat and frog myelinated nerve: normal and modified sodium inactivation. J Physiol. 1987 Jan;382:175–191. doi: 10.1113/jphysiol.1987.sp016362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizza M., Nilsson J., Hallett M. Optimal stimulus duration for the H reflex. Muscle Nerve. 1989 Jul;12(7):576–579. doi: 10.1002/mus.880120708. [DOI] [PubMed] [Google Scholar]
- Panizza M., Nilsson J., Roth B. J., Basser P. J., Hallett M. Relevance of stimulus duration for activation of motor and sensory fibers: implications for the study of H-reflexes and magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1992 Feb;85(1):22–29. doi: 10.1016/0168-5597(92)90097-u. [DOI] [PubMed] [Google Scholar]
- Panizza M., Nilsson J., Roth B. J., Rothwell J., Hallett M. The time constants of motor and sensory peripheral nerve fibers measured with the method of latent addition. Electroencephalogr Clin Neurophysiol. 1994 Apr;93(2):147–154. doi: 10.1016/0168-5597(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Saint D. A., Ju Y. K., Gage P. W. A persistent sodium current in rat ventricular myocytes. J Physiol. 1992;453:219–231. doi: 10.1113/jphysiol.1992.sp019225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. R., Eikhof G. Na currents and action potentials in rat myelinated nerve fibres at 20 and 37 degrees C. Pflugers Arch. 1987 Aug;409(6):569–577. doi: 10.1007/BF00584655. [DOI] [PubMed] [Google Scholar]
- Schwarz J. R., Reid G., Bostock H. Action potentials and membrane currents in the human node of Ranvier. Pflugers Arch. 1995 Jun;430(2):283–292. doi: 10.1007/BF00374660. [DOI] [PubMed] [Google Scholar]
- Stafstrom C. E., Schwindt P. C., Chubb M. C., Crill W. E. Properties of persistent sodium conductance and calcium conductance of layer V neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1985 Jan;53(1):153–170. doi: 10.1152/jn.1985.53.1.153. [DOI] [PubMed] [Google Scholar]
- Stafstrom C. E., Schwindt P. C., Crill W. E. Negative slope conductance due to a persistent subthreshold sodium current in cat neocortical neurons in vitro. Brain Res. 1982 Mar 18;236(1):221–226. doi: 10.1016/0006-8993(82)90050-6. [DOI] [PubMed] [Google Scholar]
- Stys P. K., Sontheimer H., Ransom B. R., Waxman S. G. Noninactivating, tetrodotoxin-sensitive Na+ conductance in rat optic nerve axons. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):6976–6980. doi: 10.1073/pnas.90.15.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. P. Na+ currents that fail to inactivate. Trends Neurosci. 1993 Nov;16(11):455–460. doi: 10.1016/0166-2236(93)90077-y. [DOI] [PubMed] [Google Scholar]
- Veale J. L., Mark R. F., Rees S. Differential sensitivity of motor and sensory fibres in human ulnar nerve. J Neurol Neurosurg Psychiatry. 1973 Feb;36(1):75–86. doi: 10.1136/jnnp.36.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]


