Abstract
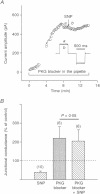
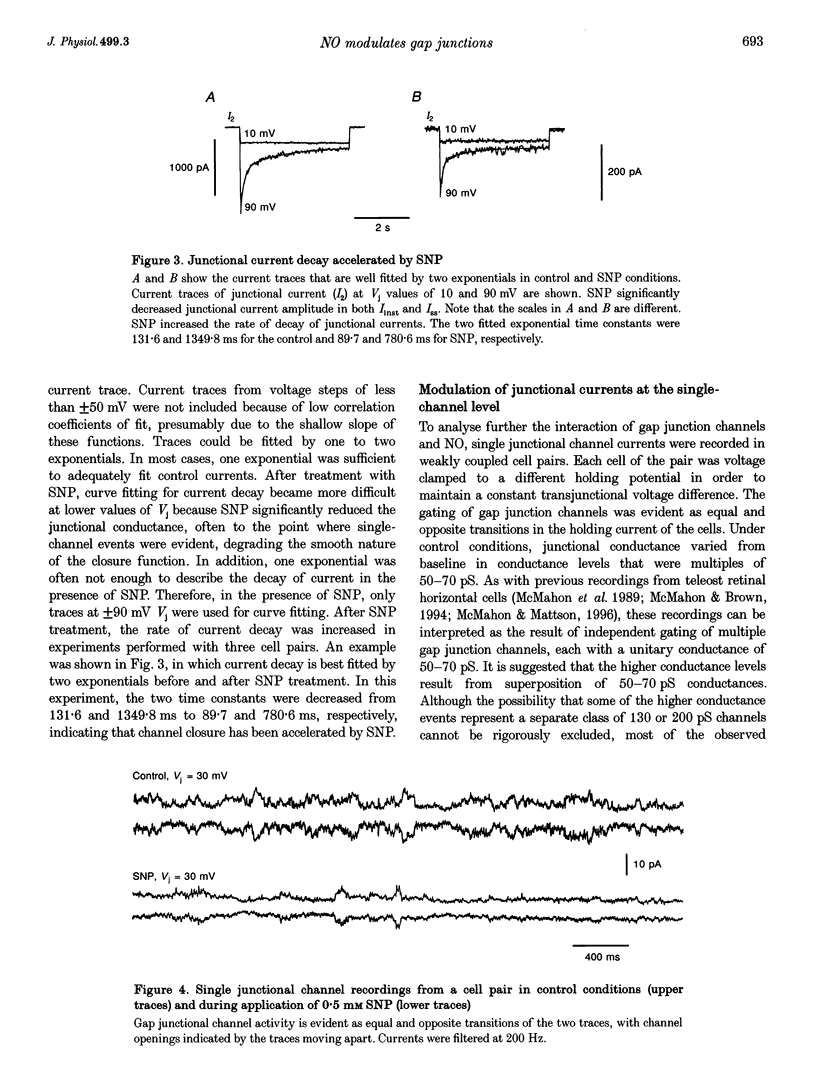
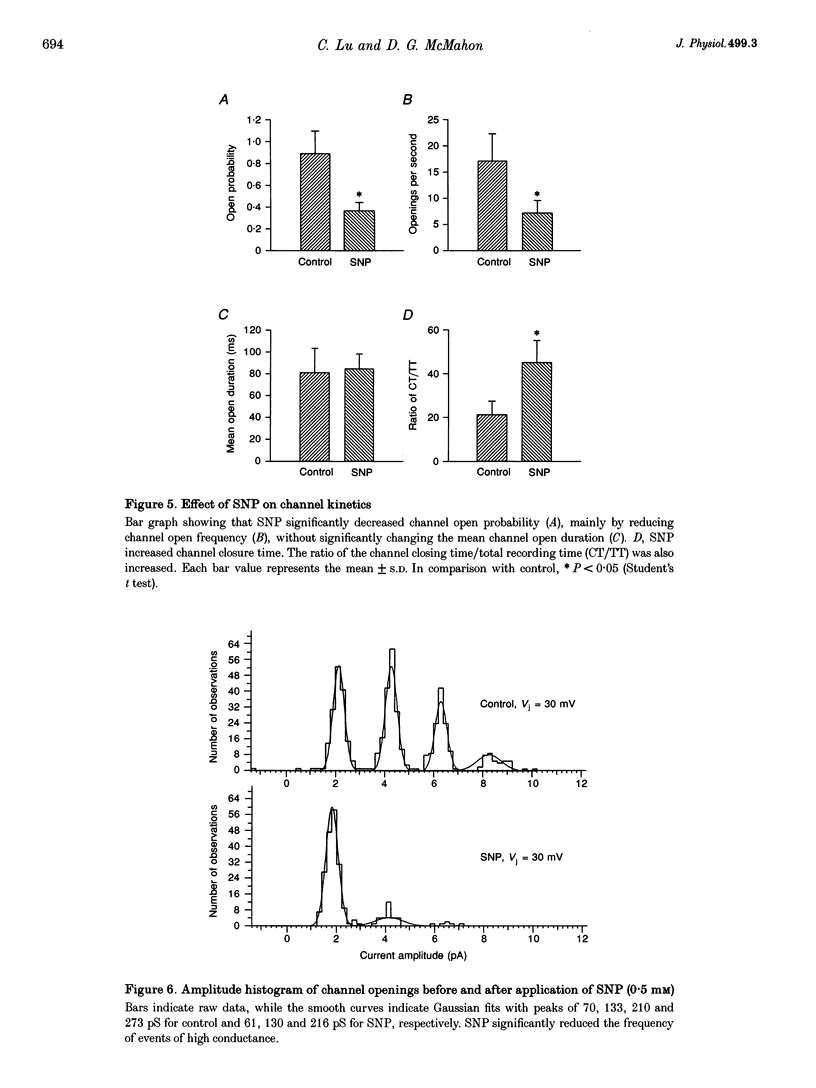
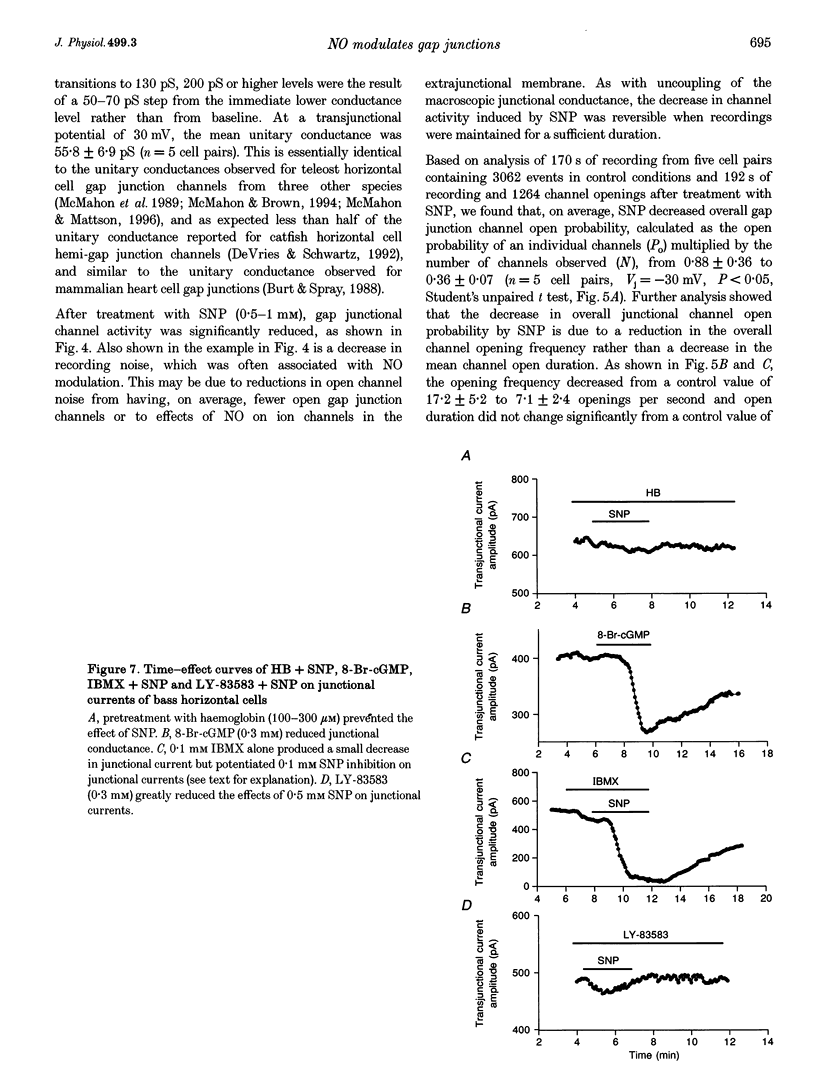
1. To elucidate the role of the nitric oxide (NO) transmitter system in the regulation of gap junctional channel gating, we have examined the effects of the NO donor sodium nitroprusside (SNP) on the electrical synapses of hybrid bass H2-type horizontal cells. 2. SNP reversibly reduced the macroscopic junctional conductance without significantly changing voltage sensitivity. 3. Kinetic analyses showed that SNP made the voltage-dependent decay of junctional currents more rapid. 4. Single-channel data showed that SNP reduced channel open probability by reducing channel open frequency. 5. The action of SNP can be prevented or largely reduced by the NO scavenger, haemoglobin. NO release by SNP solutions was detected directly by a NO sensor. 6. NO appears to modulate the gap junctional conductance by activating the cGMP-cGMP-dependent protein kinase G (PKG) pathway. A membrane-permeable cGMP analogue, 8-Br-cGMP, mimics the action of SNP. A soluble guanylate cyclase inhibitor (LY-83583) and a highly specific cGMP-dependent protein kinase inhibitor (RKRARKE) blocked the action of NO. 3-Isobutyl-1-methylxanthine (IBMX), a non-specific phosphodiesterase inhibitor, potentiated the effect of SNP. 7. [Ca2+]i image studies showed that NO donors did not change [Ca2+]i in horizontal cells, suggesting that the regulation of junctional channels by NO is [Ca2+]i independent.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad I., Leinders-Zufall T., Kocsis J. D., Shepherd G. M., Zufall F., Barnstable C. J. Retinal ganglion cells express a cGMP-gated cation conductance activatable by nitric oxide donors. Neuron. 1994 Jan;12(1):155–165. doi: 10.1016/0896-6273(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- Burt J. M., Spray D. C. Single-channel events and gating behavior of the cardiac gap junction channel. Proc Natl Acad Sci U S A. 1988 May;85(10):3431–3434. doi: 10.1073/pnas.85.10.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries S. H., Schwartz E. A. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J Physiol. 1992 Jan;445:201–230. doi: 10.1113/jphysiol.1992.sp018920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries S. H., Schwartz E. A. Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. J Physiol. 1989 Jul;414:351–375. doi: 10.1113/jphysiol.1989.sp017692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Pak M. W., Lasater E. M. White perch horizontal cells in culture: methods, morphology and process growth. Brain Res. 1985 Dec 23;360(1-2):331–338. doi: 10.1016/0006-8993(85)91250-8. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991 Feb;14(2):60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Geyer O., Almog J., Lupu-Meiri M., Lazar M., Oron Y. Nitric oxide synthase inhibitors protect rat retina against ischemic injury. FEBS Lett. 1995 Nov 6;374(3):399–402. doi: 10.1016/0014-5793(95)01147-7. [DOI] [PubMed] [Google Scholar]
- Glass D. B. Differential responses of cyclic GMP-dependent and cyclic AMP-dependent protein kinases to synthetic peptide inhibitors. Biochem J. 1983 Jul 1;213(1):159–164. doi: 10.1042/bj2130159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstreet E. H., Djamgoz M. B. Nitric oxide induces light-adaptive morphological changes in retinal neurones. Neuroreport. 1994 Dec 30;6(1):109–112. doi: 10.1097/00001756-199412300-00029. [DOI] [PubMed] [Google Scholar]
- Knapp A. G., Dowling J. E. Dopamine enhances excitatory amino acid-gated conductances in cultured retinal horizontal cells. 1987 Jan 29-Feb 4Nature. 325(6103):437–439. doi: 10.1038/325437a0. [DOI] [PubMed] [Google Scholar]
- Lasater E. M., Dowling J. E. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3025–3029. doi: 10.1073/pnas.82.9.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E. M. Retinal horizontal cell gap junctional conductance is modulated by dopamine through a cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7319–7323. doi: 10.1073/pnas.84.20.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- McMahon D. G., Knapp A. G., Dowling J. E. Horizontal cell gap junctions: single-channel conductance and modulation by dopamine. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7639–7643. doi: 10.1073/pnas.86.19.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon D. G., Mattson M. P. Horizontal cell electrical coupling in the giant danio: synaptic modulation by dopamine and synaptic maintenance by calcium. Brain Res. 1996 Apr 29;718(1-2):89–96. doi: 10.1016/0006-8993(96)00043-1. [DOI] [PubMed] [Google Scholar]
- McMahon D. G., Ponomareva L. V. Nitric oxide and cGMP modulate retinal glutamate receptors. J Neurophysiol. 1996 Oct;76(4):2307–2315. doi: 10.1152/jn.1996.76.4.2307. [DOI] [PubMed] [Google Scholar]
- Miyachi E., Murakami M., Nakaki T. Arginine blocks gap junctions between retinal horizontal cells. Neuroreport. 1990 Oct;1(2):107–110. doi: 10.1097/00001756-199010000-00006. [DOI] [PubMed] [Google Scholar]
- Neyton J., Trautmann A. Single-channel currents of an intercellular junction. 1985 Sep 26-Oct 2Nature. 317(6035):331–335. doi: 10.1038/317331a0. [DOI] [PubMed] [Google Scholar]
- Schuman E. M., Madison D. V. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991 Dec 6;254(5037):1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- Shiells R., Falk G. Retinal on-bipolar cells contain a nitric oxide-sensitive guanylate cyclase. Neuroreport. 1992 Oct;3(10):845–848. doi: 10.1097/00001756-199210000-00006. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Bennett M. V. Physiology and pharmacology of gap junctions. Annu Rev Physiol. 1985;47:281–303. doi: 10.1146/annurev.ph.47.030185.001433. [DOI] [PubMed] [Google Scholar]
- Umino O., Lee Y., Dowling J. E. Effects of light stimuli on the release of dopamine from interplexiform cells in the white perch retina. Vis Neurosci. 1991 Nov;7(5):451–458. doi: 10.1017/s0952523800009743. [DOI] [PubMed] [Google Scholar]
- Vaney D. I. Many diverse types of retinal neurons show tracer coupling when injected with biocytin or Neurobiotin. Neurosci Lett. 1991 Apr 29;125(2):187–190. doi: 10.1016/0304-3940(91)90024-n. [DOI] [PubMed] [Google Scholar]
- Weiler R., Kewitz B. The marker for nitric oxide synthase, NADPH-diaphorase, co-localizes with GABA in horizontal cells and cells of the inner retina in the carp retina. Neurosci Lett. 1993 Aug 20;158(2):151–154. doi: 10.1016/0304-3940(93)90251-f. [DOI] [PubMed] [Google Scholar]
- Yang X. L., Fan T. X., Shen W. Effects of prolonged darkness on light responsiveness and spectral sensitivity of cone horizontal cells in carp retina in vivo. J Neurosci. 1994 Jan;14(1):326–334. doi: 10.1523/JNEUROSCI.14-01-00326.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Dawson V. L., Dawson T. M., Snyder S. H. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science. 1994 Feb 4;263(5147):687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]