Abstract
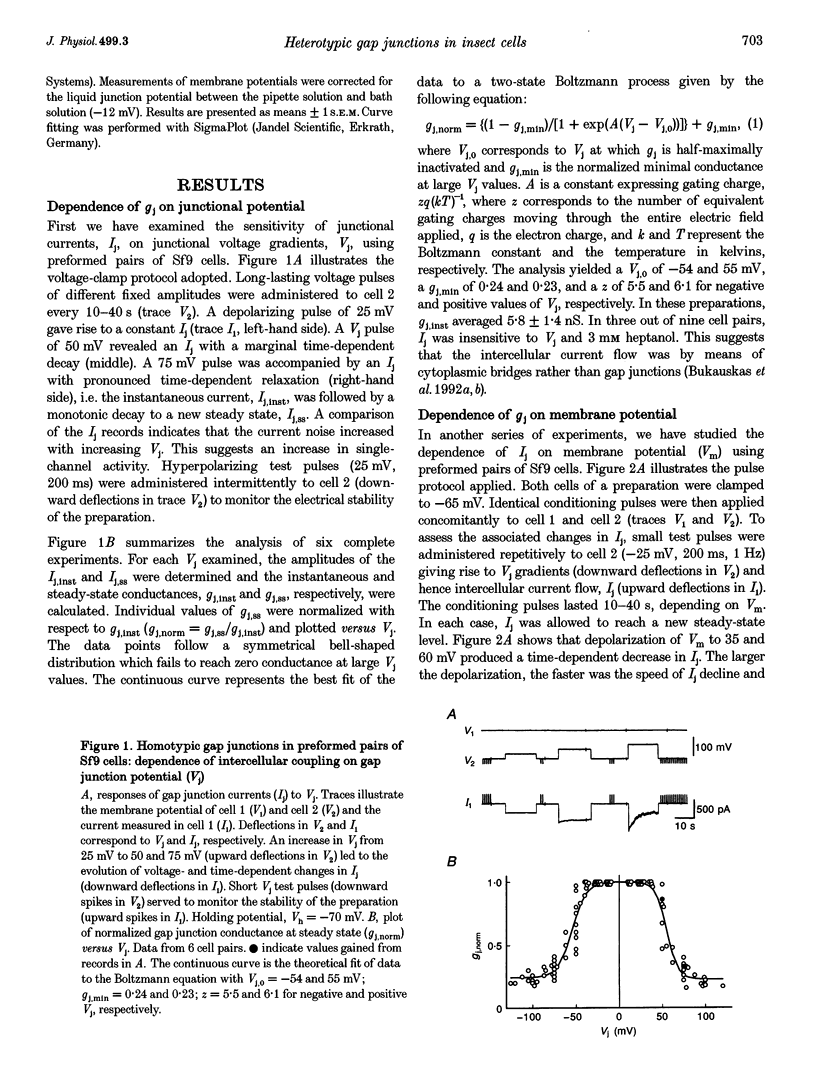
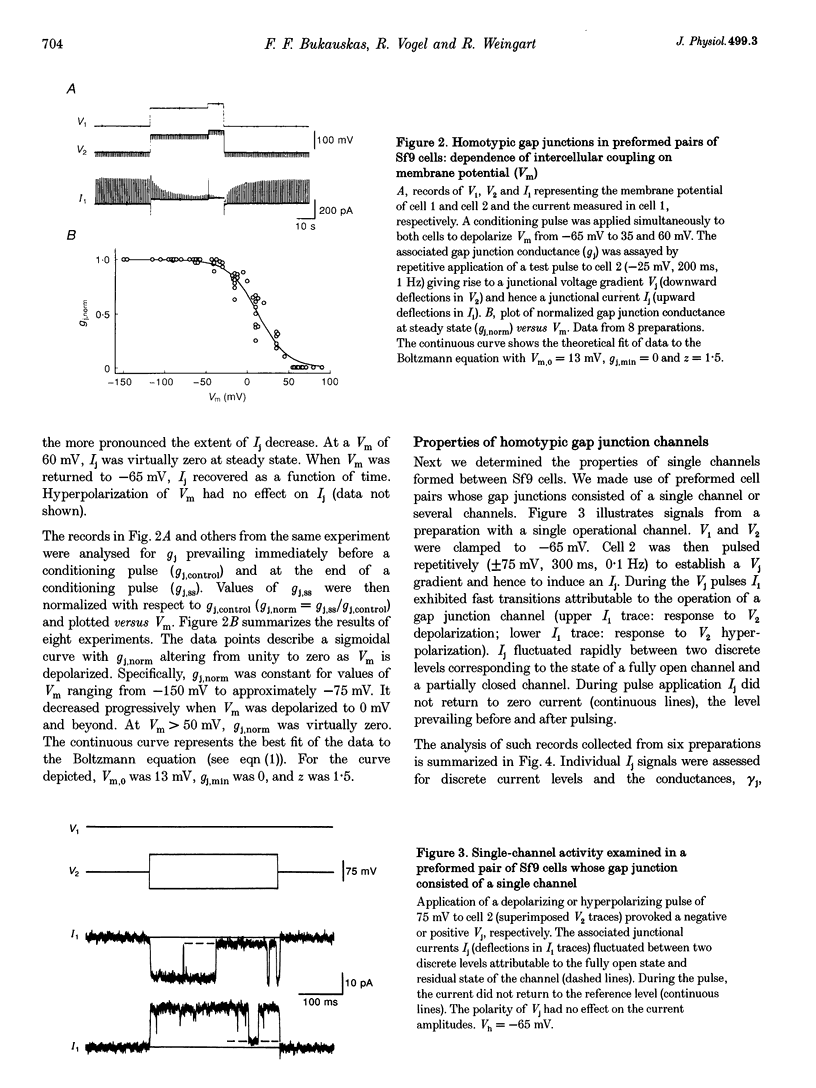
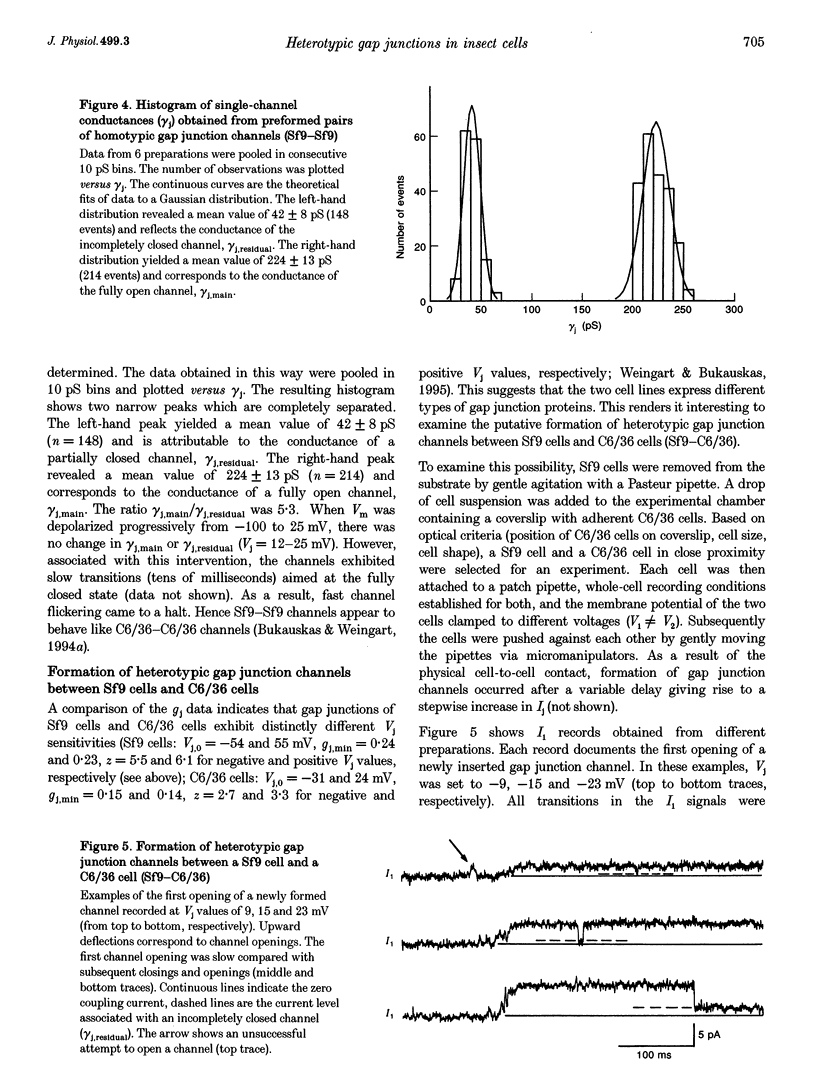
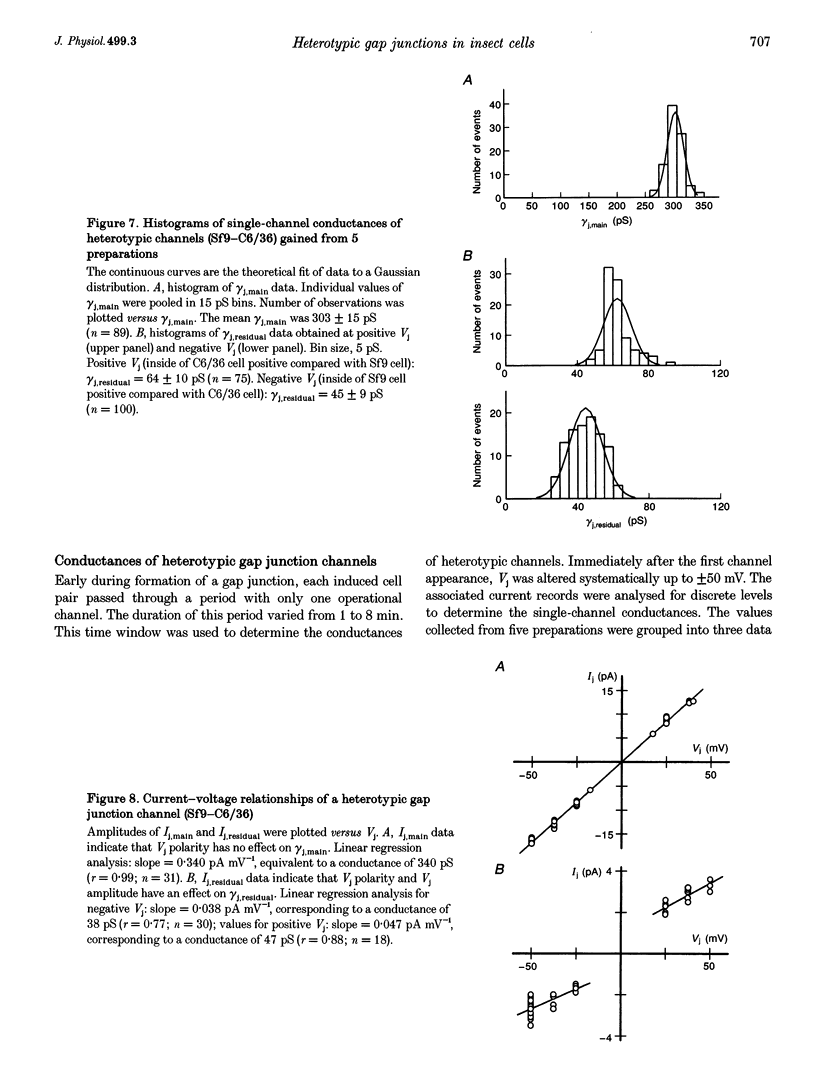
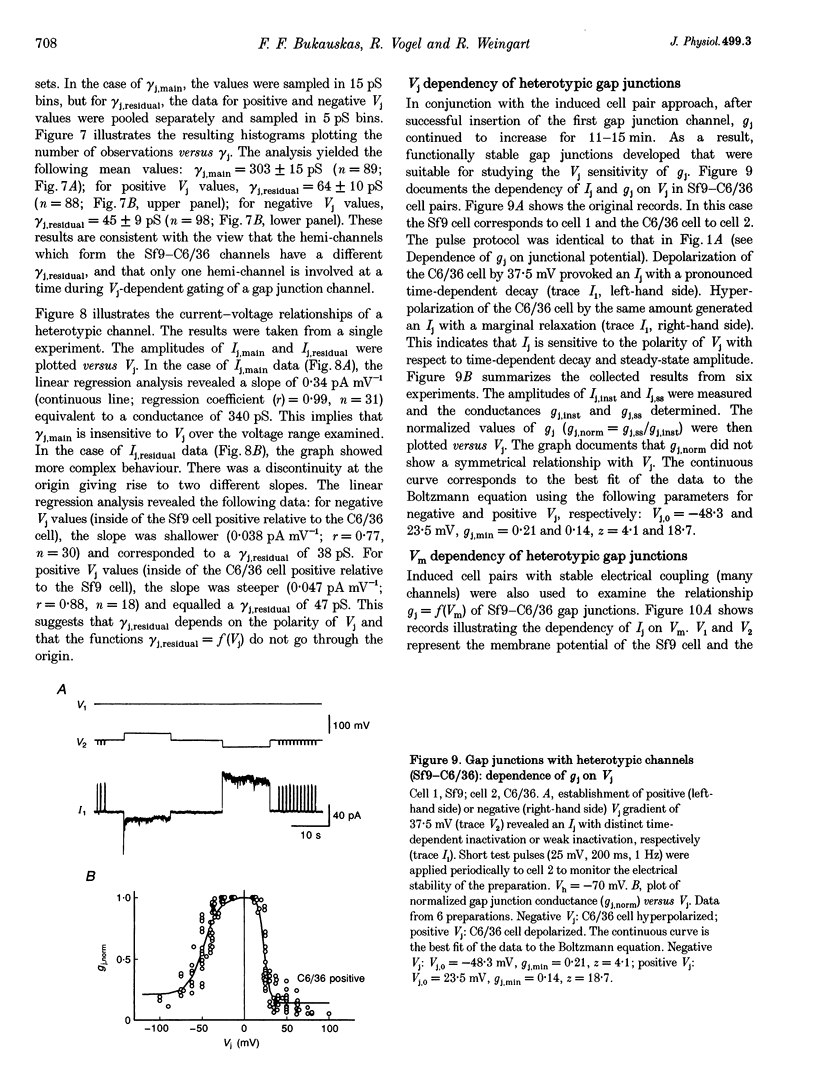
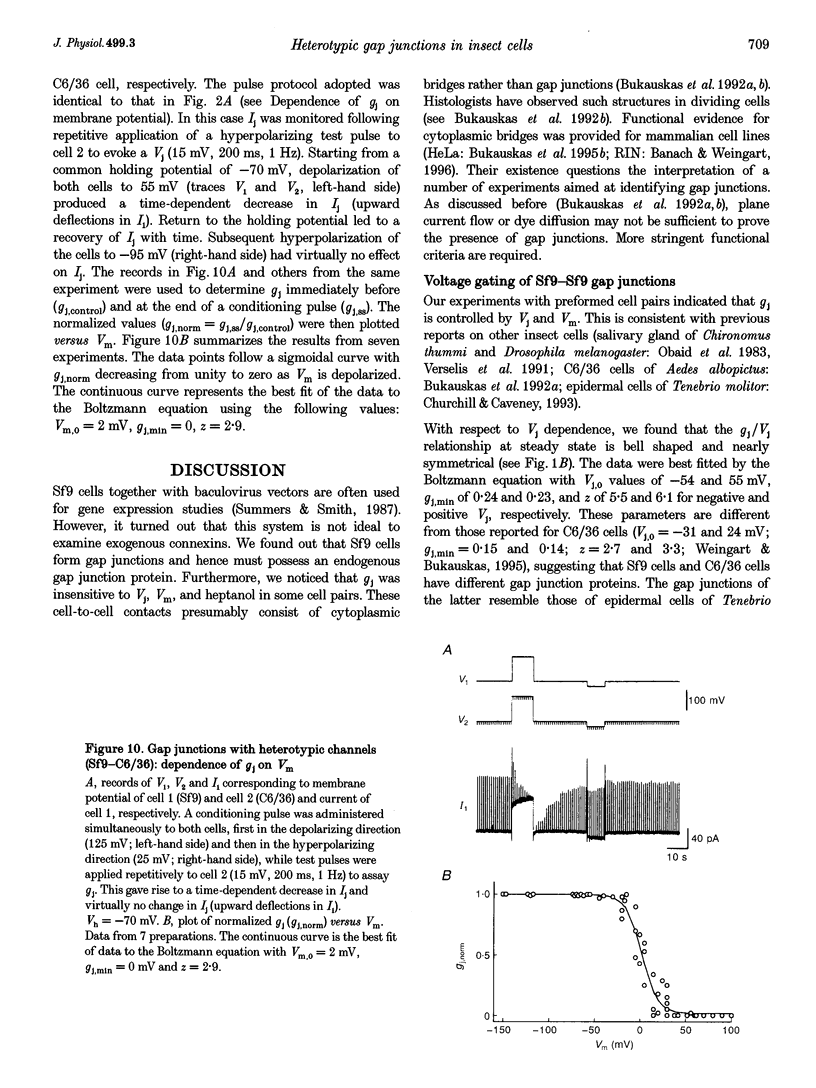
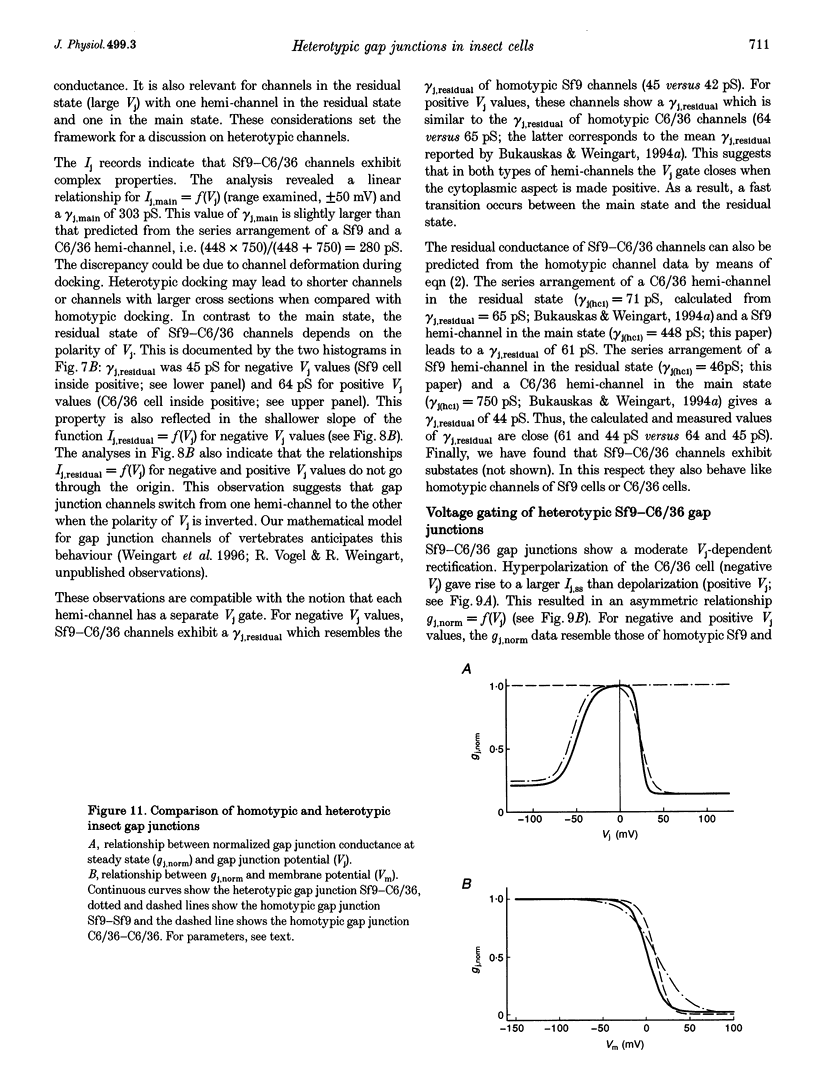
1. Cell pairs of the insect cell line Sf9 (Spodoptera frugiperda) were chosen to examine the electrical properties of gap junction channels. The dual voltage-clamp method was used to control the membrane potential of each cell (Vm,1 and Vm,2) and hence the junctional voltage gradient (Vj), and to measure intercellular current. 2. Studies with preformed pairs revealed that the gap junction conductance (gj) is controlled by a Vj- and a Vm-sensitive gate. At steady state, gj = f(Vj) was bell shaped and symmetrical (Boltzmann: Vj.0 = -54 and 55 mV, the normalized minimum conductance at large Vj values (gj,min) = 0.24 and 0.23, z = 5.5 and 6.1 for negative and positive Vj, respectively) and gj = f(Vm) was S shaped (Vm.0 = 13 mV, gj,min = 0, z = 1.5). 3. Single channels exhibited two conductances, a main state (gamma j,main) of 224 pS and a residual state (gamma j,residual) of 42 pS. 4. We conclude that gap junctions in Sf9 cells behave similarly to those in the insect cell line C6/36 (Aedes albopictus). 5. An induced cell pair approach was used to examine heterotypic gap junction channels between Sf9 cells and C3/36 cells. 6. Heterotypic channels showed a gamma j,main of 303 pS and a gamma j,residual of 45 and 64 pS, depending on whether the Sf9 cell or C6/36 cell was positive inside. 7. In heterotypic gap junctions, gj = f(Vj) was bell shaped and asymmetrical (gj was more sensitive to Vj when the C6/36 cell was positive inside) and gj = f(Vm) was S shaped (Vm,0 = 2 mV, gj,min = 0, z = 2.9). 8. We conclude that heterotypic channels possess a Vj- and Vm-sensitive gating mechanism. Vj gating involves two gates, one located in each hemi-channel. Vj gates are operated independently and close when the cytoplasmic aspect is made positive. 9. A comparison of homo- and heterotypic channel data suggests that docking of hemi-channels may affect channel gating, but not channel conductance.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes T. M. OPUS: a growing family of gap junction proteins? Trends Genet. 1994 Sep;10(9):303–305. doi: 10.1016/0168-9525(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Barrio L. C., Suchyna T., Bargiello T., Xu L. X., Roginski R. S., Bennett M. V., Nicholson B. J. Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by applied voltage. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8410–8414. doi: 10.1073/pnas.88.19.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Barrio L. C., Bargiello T. A., Spray D. C., Hertzberg E., Sáez J. C. Gap junctions: new tools, new answers, new questions. Neuron. 1991 Mar;6(3):305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- Berdan R. C., Gilula N. B. The arthropod gap junction and pseudo-gap junction: isolation and preliminary biochemical analysis. Cell Tissue Res. 1988 Feb;251(2):257–274. doi: 10.1007/BF00215833. [DOI] [PubMed] [Google Scholar]
- Beyer E. C. Gap junctions. Int Rev Cytol. 1993;137C:1–37. [PubMed] [Google Scholar]
- Bukauskas F. F., Elfgang C., Willecke K., Weingart R. Biophysical properties of gap junction channels formed by mouse connexin40 in induced pairs of transfected human HeLa cells. Biophys J. 1995 Jun;68(6):2289–2298. doi: 10.1016/S0006-3495(95)80411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas F. F., Elfgang C., Willecke K., Weingart R. Heterotypic gap junction channels (connexin26-connexin32) violate the paradigm of unitary conductance. Pflugers Arch. 1995 Apr;429(6):870–872. doi: 10.1007/BF00374812. [DOI] [PubMed] [Google Scholar]
- Bukauskas F. F., Kempf C., Weingart R. Cytoplasmic bridges and gap junctions in an insect cell line (Aedes albopictus). Exp Physiol. 1992 Nov;77(6):903–911. doi: 10.1113/expphysiol.1992.sp003657. [DOI] [PubMed] [Google Scholar]
- Bukauskas F. F., Weingart R. Voltage-dependent gating of single gap junction channels in an insect cell line. Biophys J. 1994 Aug;67(2):613–625. doi: 10.1016/S0006-3495(94)80521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas F., Kempf C., Weingart R. Electrical coupling between cells of the insect Aedes albopictus. J Physiol. 1992 Mar;448:321–337. doi: 10.1113/jphysiol.1992.sp019044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill D., Caveney S. Double whole-cell patch-clamp characterization of gap junctional channels in isolated insect epidermal cell pairs. J Membr Biol. 1993 Aug;135(2):165–180. doi: 10.1007/BF00231442. [DOI] [PubMed] [Google Scholar]
- Dahl G., Werner R., Levine E., Rabadan-Diehl C. Mutational analysis of gap junction formation. Biophys J. 1992 Apr;62(1):172–182. doi: 10.1016/S0006-3495(92)81803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi A. Isolation of a Singh's Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J Gen Virol. 1978 Sep;40(3):531–544. doi: 10.1099/0022-1317-40-3-531. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Laird D. W., Revel J. P., Johnson R. G. Inhibition of gap junction and adherens junction assembly by connexin and A-CAM antibodies. J Cell Biol. 1992 Oct;119(1):179–189. doi: 10.1083/jcb.119.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaid A. L., Socolar S. J., Rose B. Cell-to-cell channels with two independently regulated gates in series: analysis of junctional conductance modulation by membrane potential, calcium, and pH. J Membr Biol. 1983;73(1):69–89. doi: 10.1007/BF01870342. [DOI] [PubMed] [Google Scholar]
- Rubin J. B., Verselis V. K., Bennett M. V., Bargiello T. A. Molecular analysis of voltage dependence of heterotypic gap junctions formed by connexins 26 and 32. Biophys J. 1992 Apr;62(1):183–195. doi: 10.1016/S0006-3495(92)81804-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Ju G., Ericson B. L., Moschera J., Lahm H. W., Chizzonite R., Summers M. D. Modification and secretion of human interleukin 2 produced in insect cells by a baculovirus expression vector. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8404–8408. doi: 10.1073/pnas.82.24.8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson K. I., Jordan J. R., Beyer E. C., Paul D. L. Formation of gap junctions by expression of connexins in Xenopus oocyte pairs. Cell. 1989 Apr 7;57(1):145–155. doi: 10.1016/0092-8674(89)90180-3. [DOI] [PubMed] [Google Scholar]
- Verselis V. K., Bennett M. V., Bargiello T. A. A voltage-dependent gap junction in Drosophila melanogaster. Biophys J. 1991 Jan;59(1):114–126. doi: 10.1016/S0006-3495(91)82204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verselis V. K., Ginter C. S., Bargiello T. A. Opposite voltage gating polarities of two closely related connexins. Nature. 1994 Mar 24;368(6469):348–351. doi: 10.1038/368348a0. [DOI] [PubMed] [Google Scholar]
- Werner R., Levine E., Rabadan-Diehl C., Dahl G. Formation of hybrid cell-cell channels. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5380–5384. doi: 10.1073/pnas.86.14.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. W., Bruzzone R., Wolfram S., Paul D. L., Goodenough D. A. Selective interactions among the multiple connexin proteins expressed in the vertebrate lens: the second extracellular domain is a determinant of compatibility between connexins. J Cell Biol. 1994 May;125(4):879–892. doi: 10.1083/jcb.125.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]


