Abstract
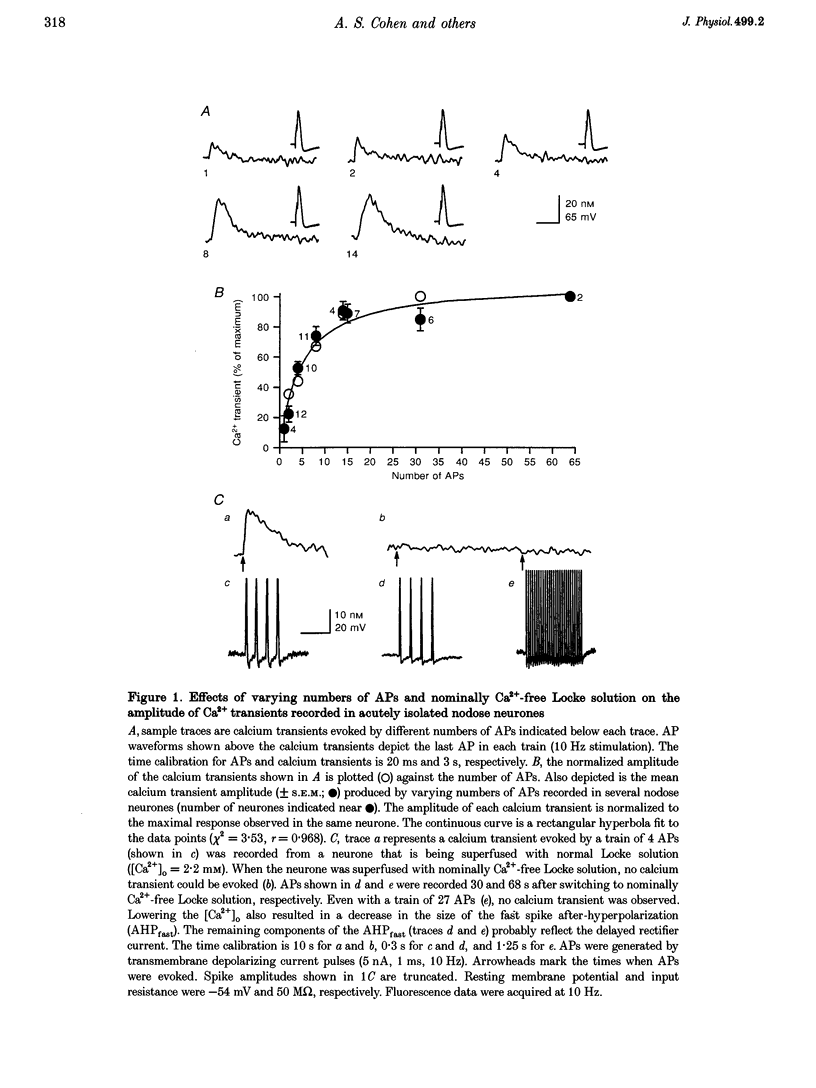
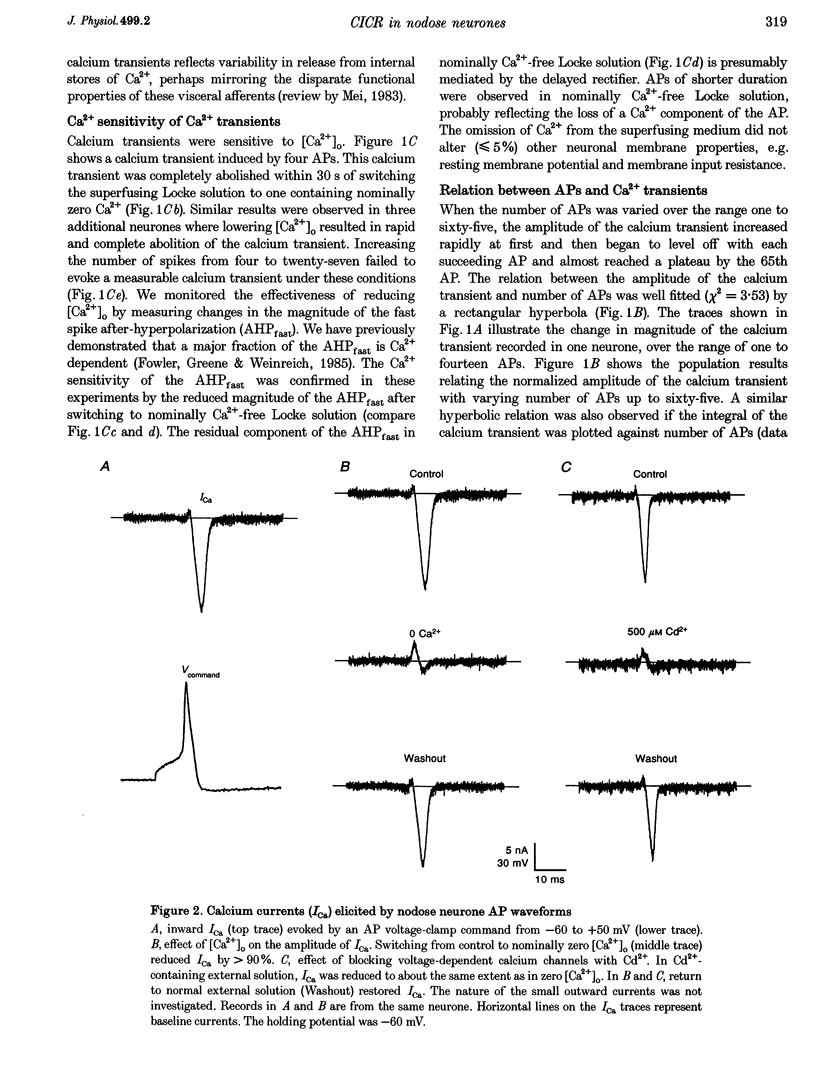
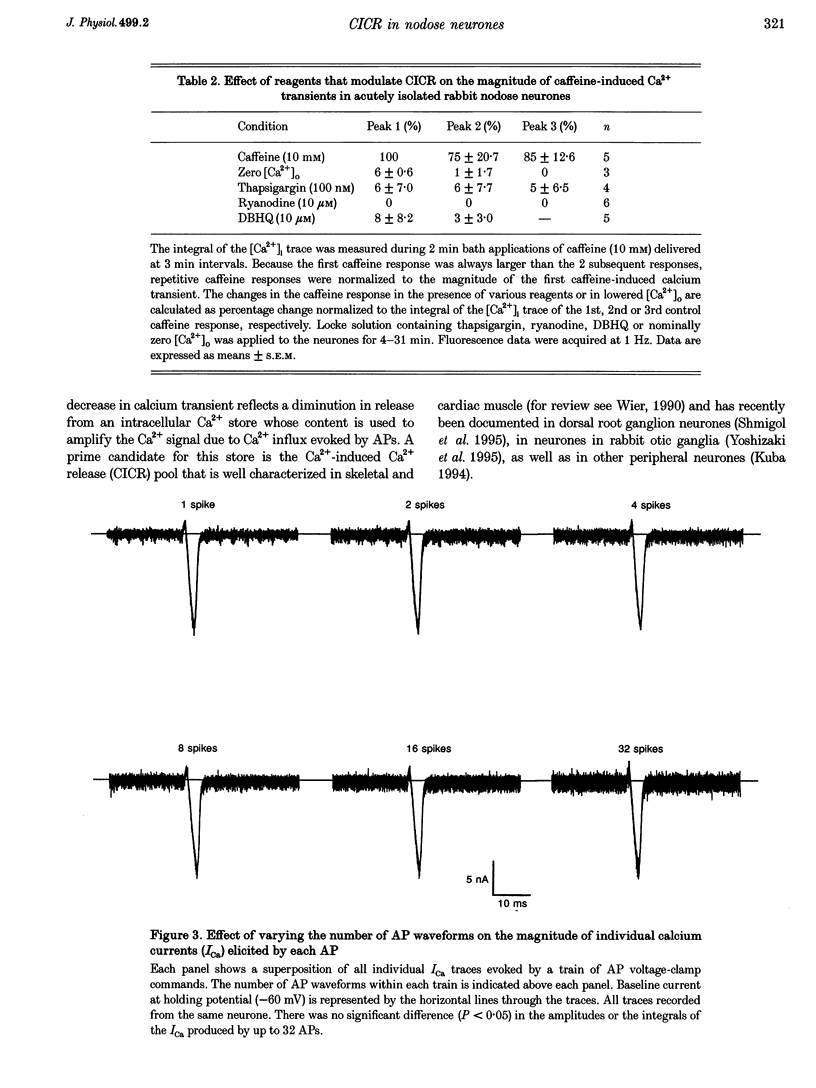
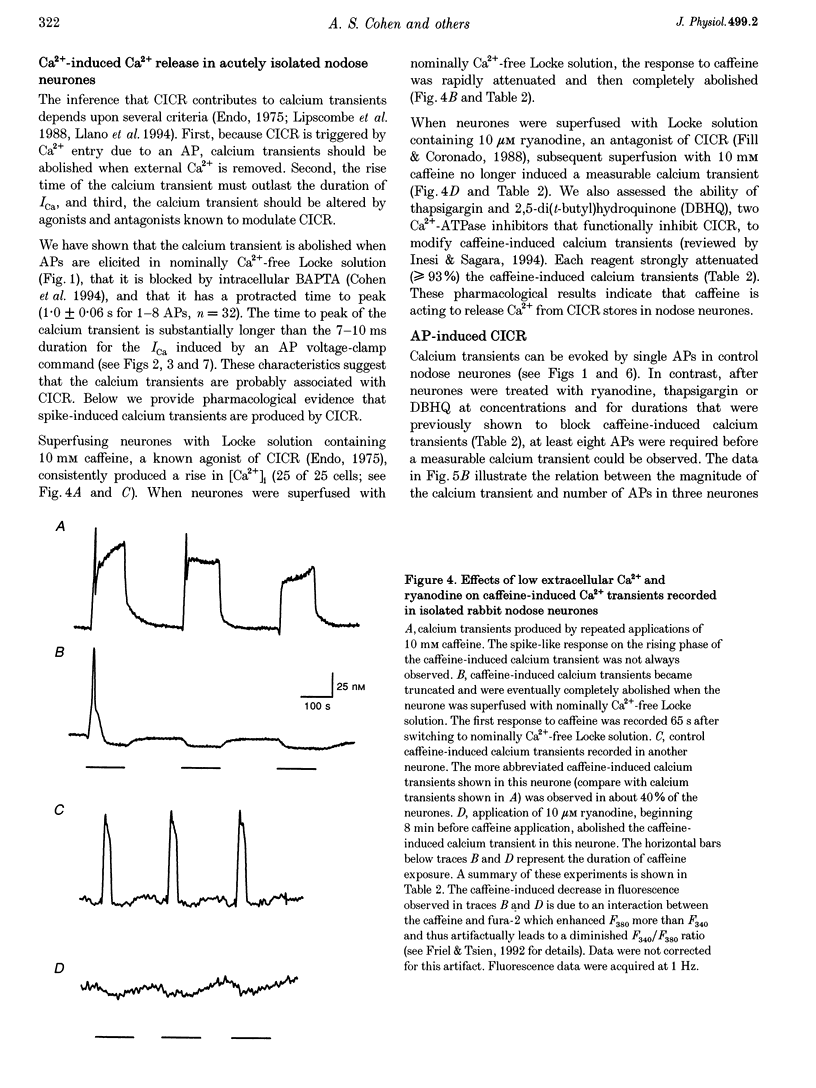
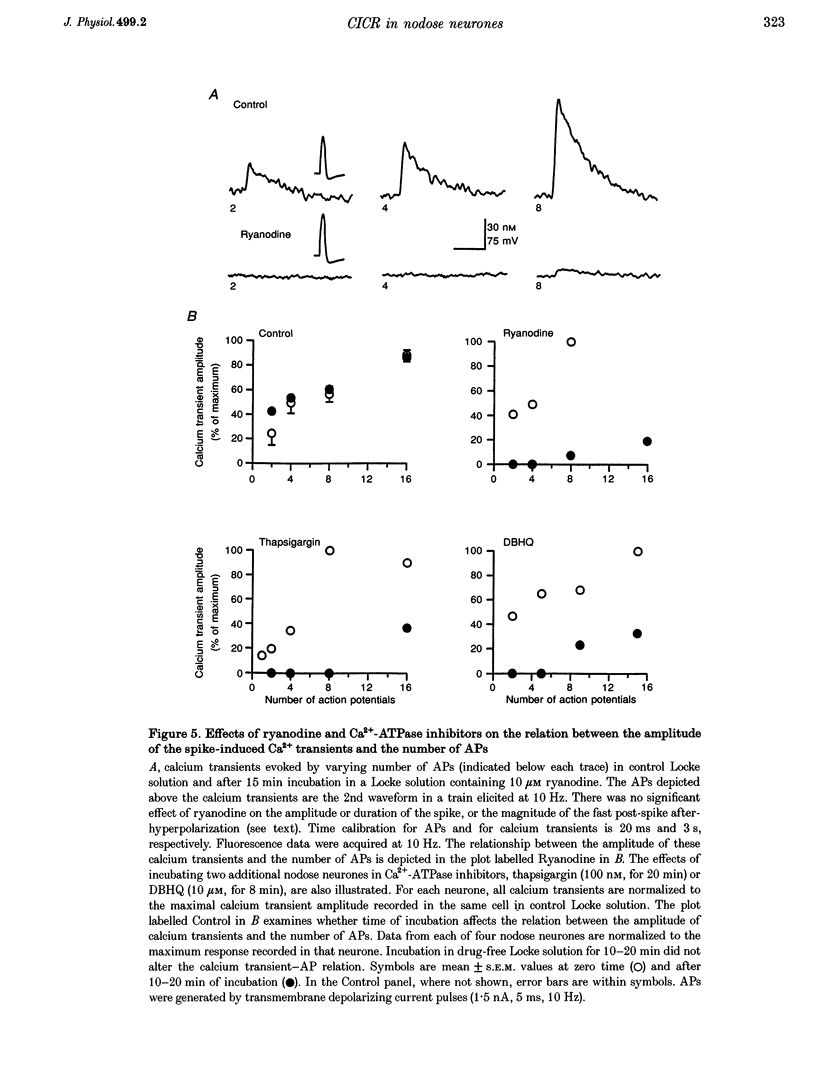
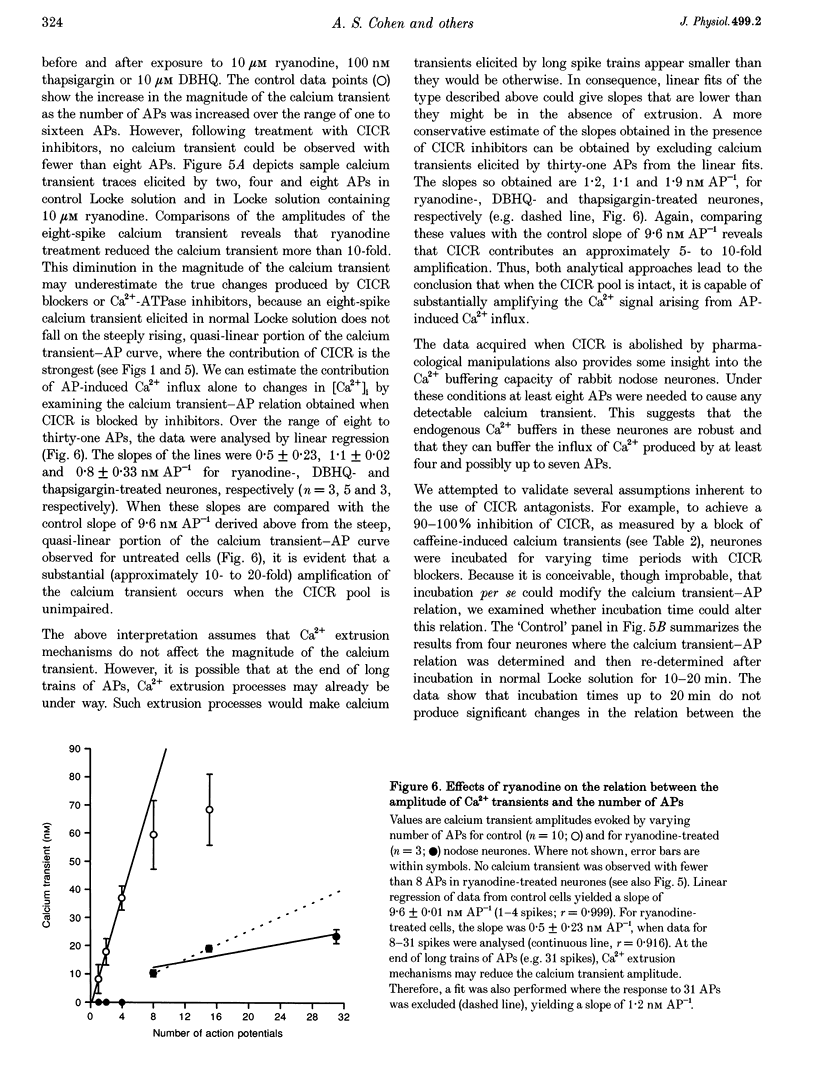
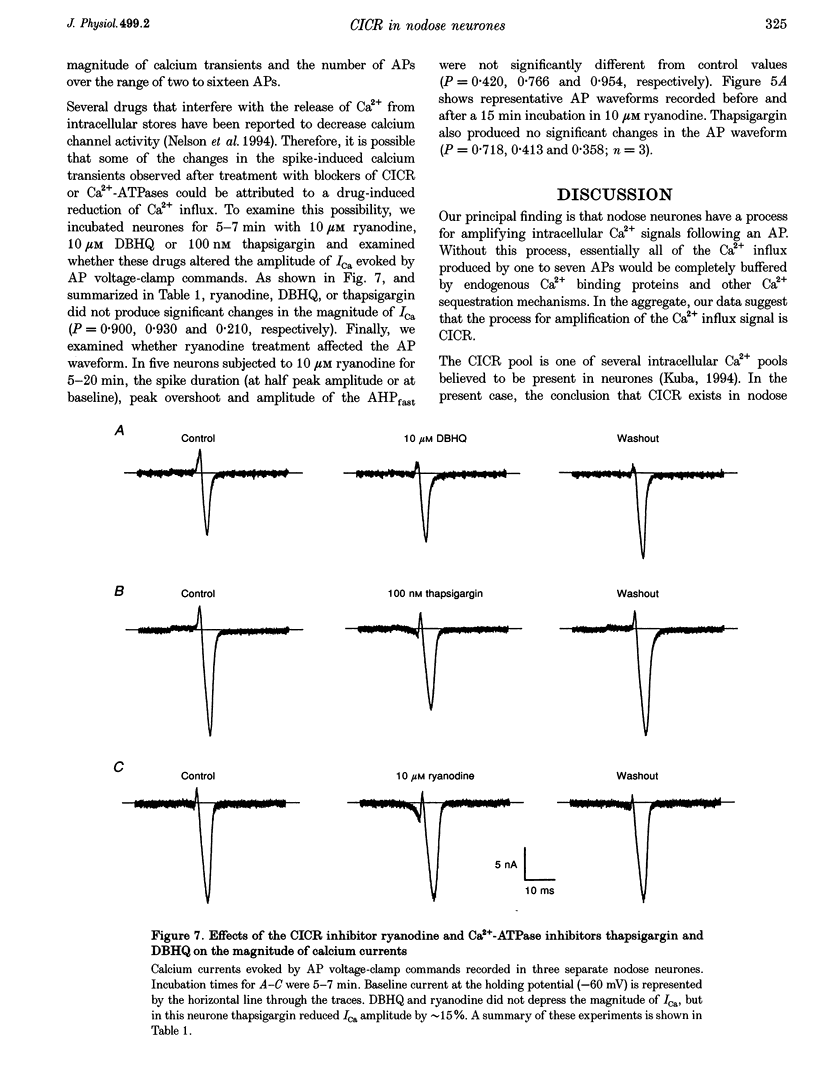
1. Standard intracellular recording techniques with 'sharp' micropipettes were used to evoke action potentials (APs) in acutely dissociated adult nodose neurones. 2. APs induced a transient increase in [Ca2+]i (a calcium transient), recorded with fura-2, that was dependent upon [Ca2+]o and the number of APs. Over the range of one to sixty-five APs, the relation between the amplitude of the calcium transient and the number of APs was well fitted by a rectangular hyperbola (chi 2 = 3.53, r = 0.968). From one to four APs, the calcium transient-AP relation can be described by a line with a slope of 9.6 nM AP-1 (r = 0.999). 3. Charge movement corresponding to Ca2+ influx evoked by a single AP was 39 +/- 2.8 pC (mean +/- S.E.M.) and did not change significantly during trains of one to thirty-one APs (P < 0.05). 4. Caffeine (10 mM), a known agonist of the ryanodine receptor, produced an increase in [Ca2+]i. The caffeine-induced rise in [Ca2+]i was attenuated (by > 90%) by lowering [Ca2+]o, and by ryanodine (10 microM), 2,5-di(t-butyl)hydroquinone (DBHQ, 10 microM), or thapsigargin (100 nM). 5. Neurones incubated with ryanodine, DBHQ or thapsigargin required at least eight APs to evoke a detectable calcium transient. These reagents did not significantly affect Ca2+ influx (P < 0.05). In the presence of these inhibitors, the calcium transient-AP relation exhibited slopes of 1.2, 1.1 and 1.9 nM AP-1 for ryanodine, DBHQ and thapsigargin, respectively. When compared with the slope of 9.6 nM AP-1 in non-treated neurones, it appears that Ca2+ influx produced by a single AP is amplified by ca 5- to 10-fold.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Cheek T. R. Spatial aspects of calcium signalling. J Cell Sci. 1989 Jun;93(Pt 2):211–216. doi: 10.1242/jcs.93.2.211. [DOI] [PubMed] [Google Scholar]
- Christian E. P., Taylor G. E., Weinreich D. Serotonin increases excitability of rabbit C-fiber neurons by two distinct mechanisms. J Appl Physiol (1985) 1989 Aug;67(2):584–591. doi: 10.1152/jappl.1989.67.2.584. [DOI] [PubMed] [Google Scholar]
- Cohen A. S., Weinreich D., Kao J. P. Nitric oxide regulates spike frequency accommodation in nodose neurons of the rabbit. Neurosci Lett. 1994 May 23;173(1-2):17–20. doi: 10.1016/0304-3940(94)90140-6. [DOI] [PubMed] [Google Scholar]
- Colbran R. J. Regulation and role of brain calcium/calmodulin-dependent protein kinase II. Neurochem Int. 1992 Dec;21(4):469–497. doi: 10.1016/0197-0186(92)90080-b. [DOI] [PubMed] [Google Scholar]
- Coleridge J. C., Coleridge H. M. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Snyder S. H. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994 Sep;14(9):5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fill M., Coronado R. Ryanodine receptor channel of sarcoplasmic reticulum. Trends Neurosci. 1988 Oct;11(10):453–457. doi: 10.1016/0166-2236(88)90198-1. [DOI] [PubMed] [Google Scholar]
- Fowler J. C., Greene R., Weinreich D. Two calcium-sensitive spike after-hyperpolarizations in visceral sensory neurones of the rabbit. J Physiol. 1985 Aug;365:59–75. doi: 10.1113/jphysiol.1985.sp015759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel D. D., Tsien R. W. A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurones modulates effects of Ca2+ entry on [Ca2+]i. J Physiol. 1992 May;450:217–246. doi: 10.1113/jphysiol.1992.sp019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hua S. Y., Nohmi M., Kuba K. Characteristics of Ca2+ release induced by Ca2+ influx in cultured bullfrog sympathetic neurones. J Physiol. 1993 May;464:245–272. doi: 10.1113/jphysiol.1993.sp019633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inesi G., Sagara Y. Specific inhibitors of intracellular Ca2+ transport ATPases. J Membr Biol. 1994 Jul;141(1):1–6. doi: 10.1007/BF00232868. [DOI] [PubMed] [Google Scholar]
- Irving A. J., Collingridge G. L., Schofield J. G. Interactions between Ca2+ mobilizing mechanisms in cultured rat cerebellar granule cells. J Physiol. 1992 Oct;456:667–680. doi: 10.1113/jphysiol.1992.sp019360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao J. P. Practical aspects of measuring [Ca2+] with fluorescent indicators. Methods Cell Biol. 1994;40:155–181. doi: 10.1016/s0091-679x(08)61114-0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The timing of calcium action during neuromuscular transmission. J Physiol. 1967 Apr;189(3):535–544. doi: 10.1113/jphysiol.1967.sp008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K. Ca(2+)-induced Ca2+ release in neurones. Jpn J Physiol. 1994;44(6):613–650. doi: 10.2170/jjphysiol.44.613. [DOI] [PubMed] [Google Scholar]
- Kuba K., Morita K., Nohmi M. Origin of calcium ions involved in the generation of a slow afterhyperpolarization in bullfrog sympathetic neurones. Pflugers Arch. 1983 Nov;399(3):194–202. doi: 10.1007/BF00656714. [DOI] [PubMed] [Google Scholar]
- Leal-Cardoso H., Koschorke G. M., Taylor G., Weinreich D. Electrophysiological properties and chemosensitivity of acutely isolated nodose ganglion neurons of the rabbit. J Auton Nerv Syst. 1993 Oct;45(1):29–39. doi: 10.1016/0165-1838(93)90359-3. [DOI] [PubMed] [Google Scholar]
- Lipscombe D., Madison D. V., Poenie M., Reuter H., Tsien R. W., Tsien R. Y. Imaging of cytosolic Ca2+ transients arising from Ca2+ stores and Ca2+ channels in sympathetic neurons. Neuron. 1988 Jul;1(5):355–365. doi: 10.1016/0896-6273(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Llano I., DiPolo R., Marty A. Calcium-induced calcium release in cerebellar Purkinje cells. Neuron. 1994 Mar;12(3):663–673. doi: 10.1016/0896-6273(94)90221-6. [DOI] [PubMed] [Google Scholar]
- Lüscher C., Lipp P., Lüscher H. R., Niggli E. Control of action potential propagation by intracellular Ca2+ in cultured rat dorsal root ganglion cells. J Physiol. 1996 Jan 15;490(Pt 2):319–324. doi: 10.1113/jphysiol.1996.sp021146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee J. C., Schofield G. G. Room temperature culture extends the useful life of adult neurons for voltage-clamp experiments. J Neurosci Methods. 1991 Jul;38(2-3):201–208. doi: 10.1016/0165-0270(91)90170-5. [DOI] [PubMed] [Google Scholar]
- Marty A. The physiological role of calcium-dependent channels. Trends Neurosci. 1989 Nov;12(11):420–424. doi: 10.1016/0166-2236(89)90090-8. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Nelson E. J., Li C. C., Bangalore R., Benson T., Kass R. S., Hinkle P. M. Inhibition of L-type calcium-channel activity by thapsigargin and 2,5-t-butylhydroquinone, but not by cyclopiazonic acid. Biochem J. 1994 Aug 15;302(Pt 1):147–154. doi: 10.1042/bj3020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P. Ca(2+)-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996 Apr;19(4):150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- Scroggs R. S., Fox A. P. Multiple Ca2+ currents elicited by action potential waveforms in acutely isolated adult rat dorsal root ganglion neurons. J Neurosci. 1992 May;12(5):1789–1801. doi: 10.1523/JNEUROSCI.12-05-01789.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmigol A., Verkhratsky A., Isenberg G. Calcium-induced calcium release in rat sensory neurons. J Physiol. 1995 Dec 15;489(Pt 3):627–636. doi: 10.1113/jphysiol.1995.sp021078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi H., Hirai K., Katayama Y. Measurement of the intracellular calcium concentration in guinea-pig myenteric neurons by using fura-2. Brain Res. 1988 Jun 7;451(1-2):371–375. doi: 10.1016/0006-8993(88)90787-1. [DOI] [PubMed] [Google Scholar]
- Thayer S. A., Perney T. M., Miller R. J. Regulation of calcium homeostasis in sensory neurons by bradykinin. J Neurosci. 1988 Nov;8(11):4089–4097. doi: 10.1523/JNEUROSCI.08-11-04089.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich D., Wonderlin W. F. Inhibition of calcium-dependent spike after-hyperpolarization increases excitability of rabbit visceral sensory neurones. J Physiol. 1987 Dec;394:415–427. doi: 10.1113/jphysiol.1987.sp016878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier W. G. Cytoplasmic [Ca2+] in mammalian ventricle: dynamic control by cellular processes. Annu Rev Physiol. 1990;52:467–485. doi: 10.1146/annurev.ph.52.030190.002343. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K., Hoshino T., Sato M., Koyano H., Nohmi M., Hua S. Y., Kuba K. Ca(2+)-induced Ca2+ release and its activation in response to a single action potential in rabbit otic ganglion cells. J Physiol. 1995 Jul 1;486(Pt 1):177–187. doi: 10.1113/jphysiol.1995.sp020801. [DOI] [PMC free article] [PubMed] [Google Scholar]


