Abstract
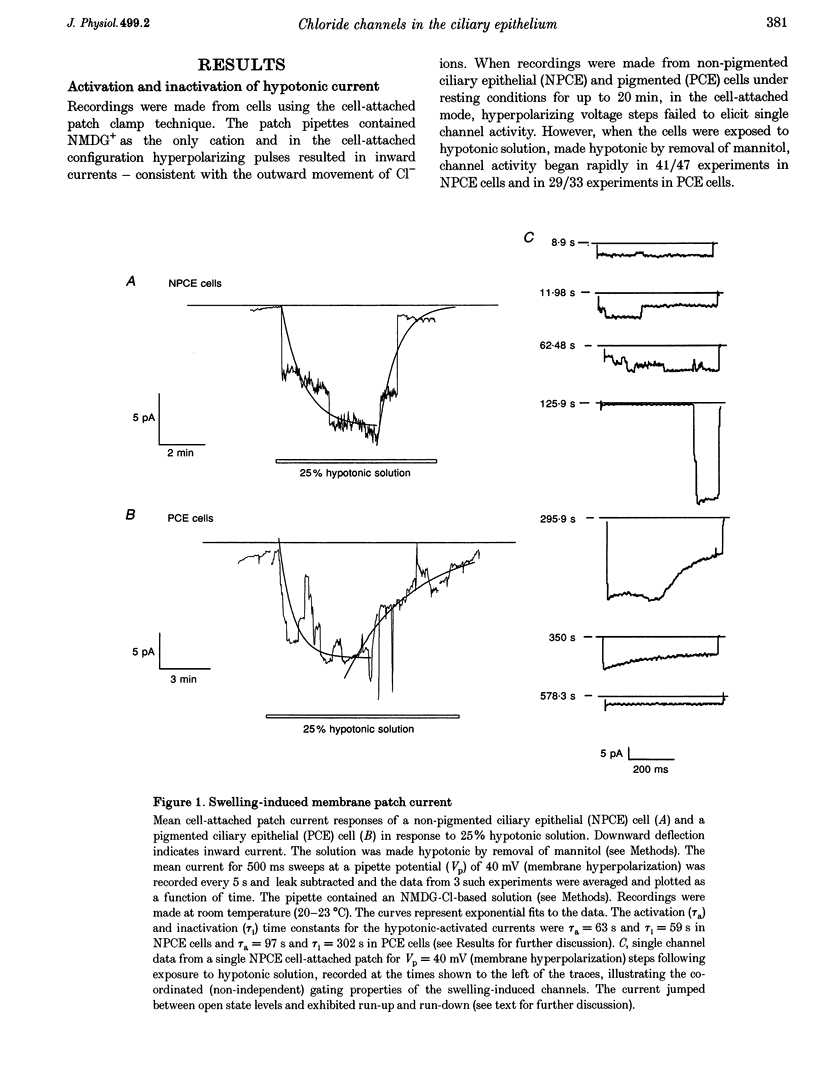
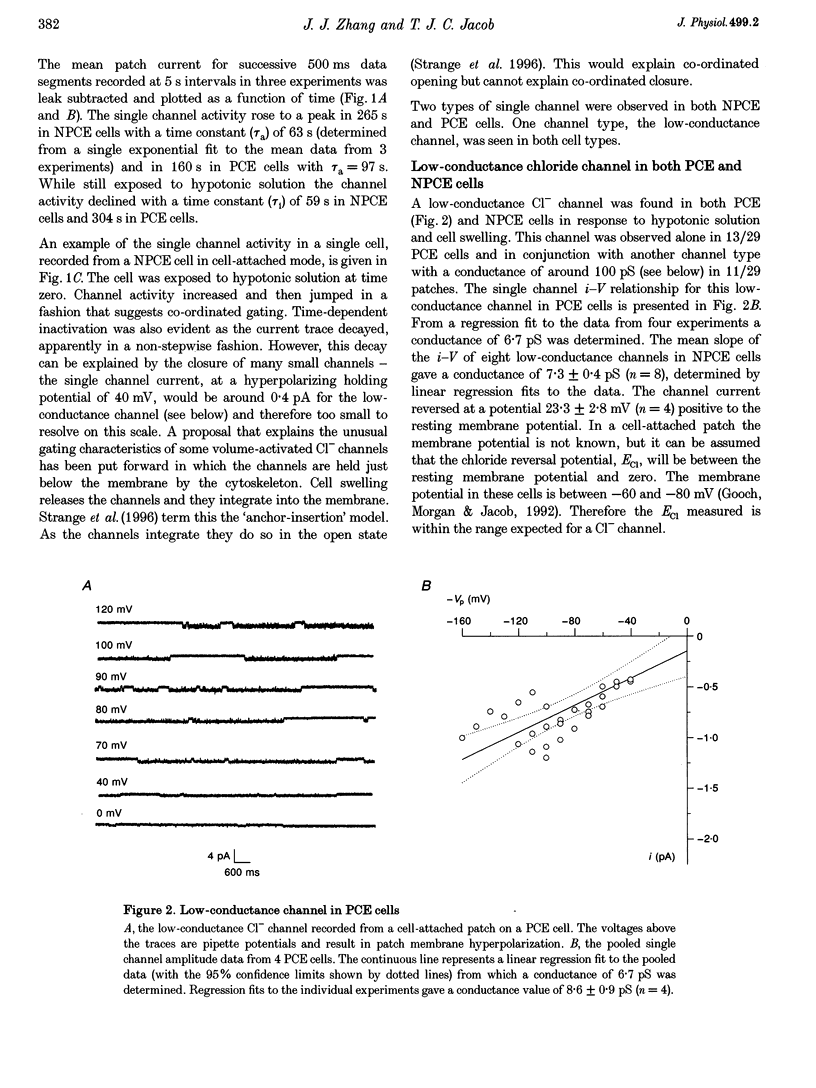
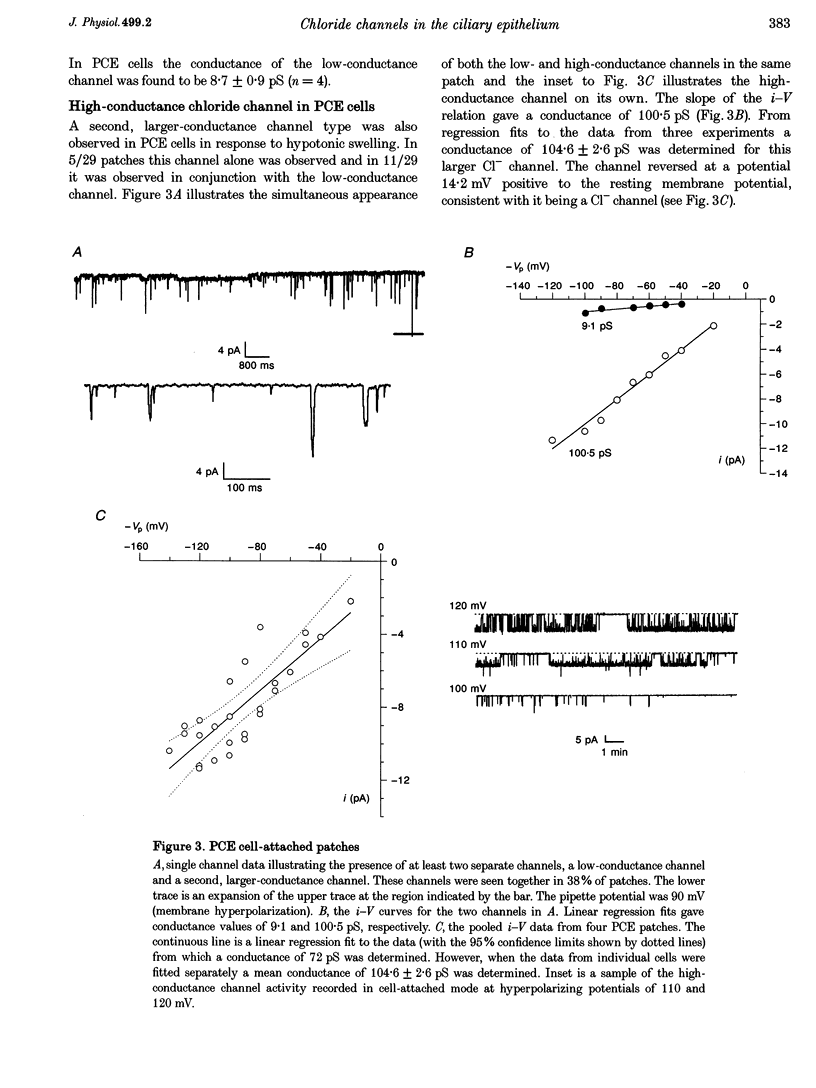
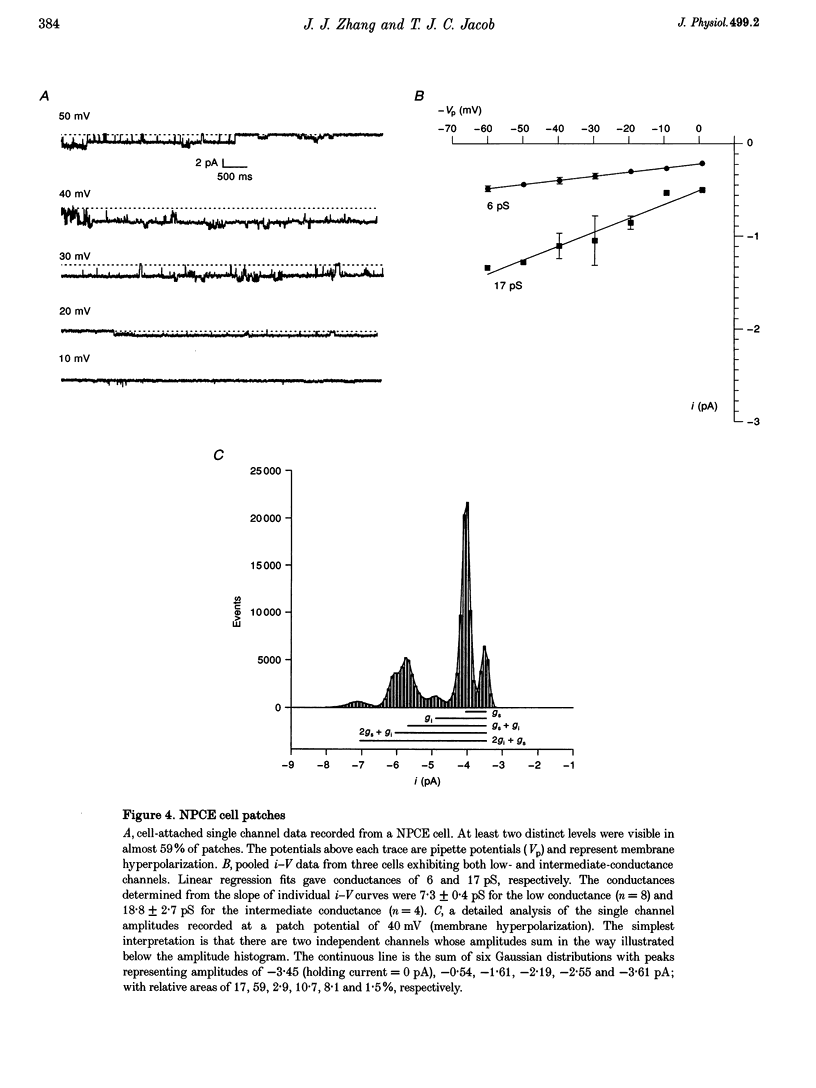
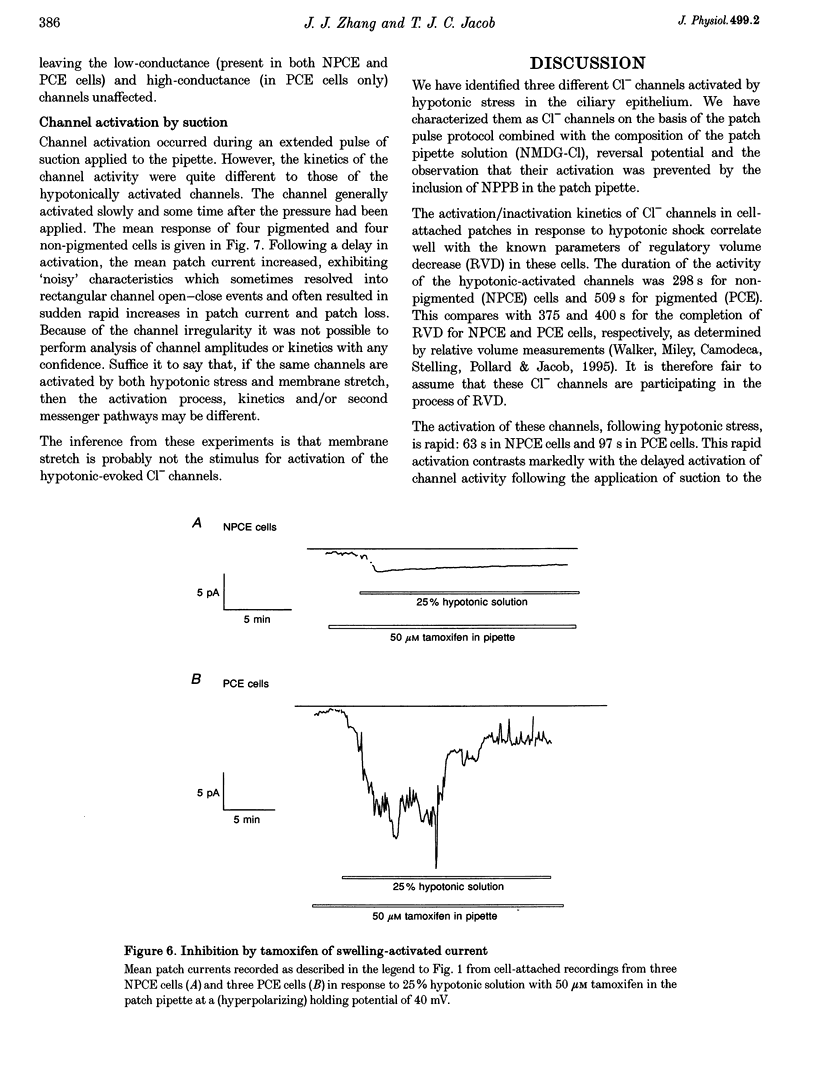
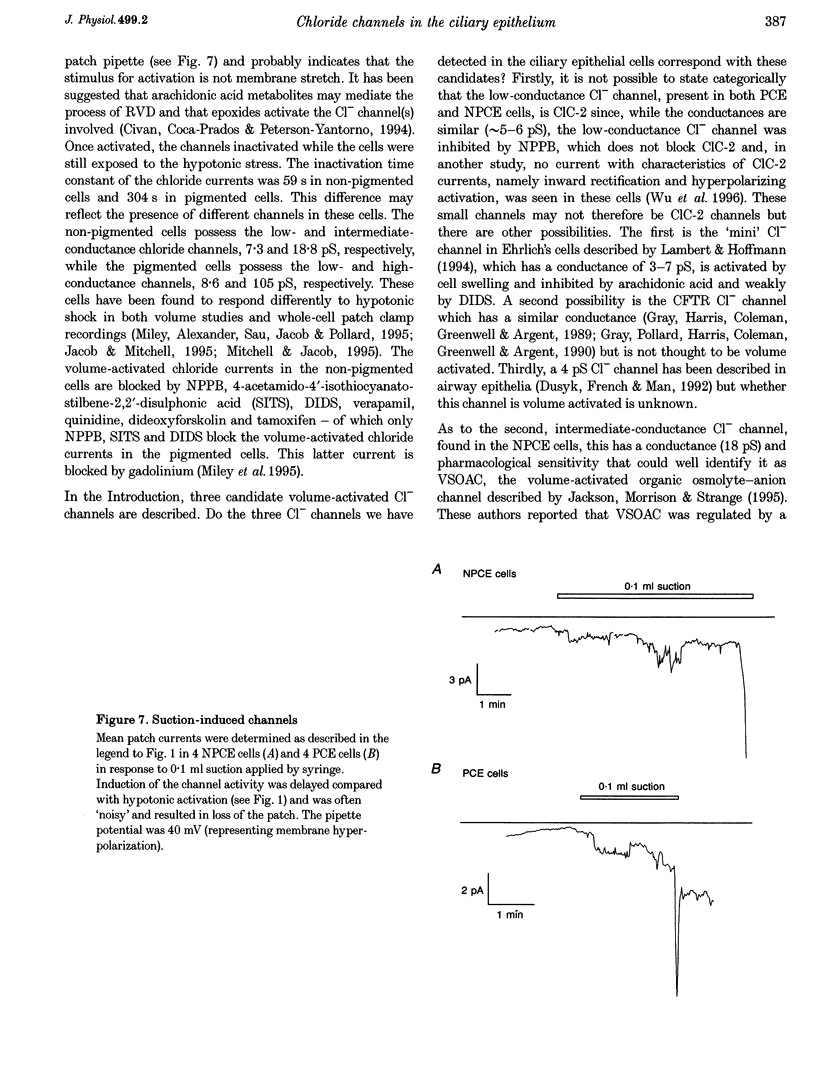
1. Hypotonic solution induced transient Cl- channel activity in both pigmented and non-pigmented ciliary epithelial cells in cell-attached patches. 2. The activation time constants for these currents were 63 and 97 s for non-pigmented and pigmented ciliary epithelial (NPCE and PCE) cells, respectively. The currents inactivated during the exposure to hypotonic solution with time constants of 59 and 304 s for NPCE and PCE cells, respectively. 3. Single channel analysis revealed the presence of a low-conductance Cl- channel in both the NPCE (7.3 +/- 0.4 pS) and PCE (8.6 +/- 0.9 pS) cells. In addition, an intermediate-conductance (18.8 pS) channel was found alone in the NPCE cells and a maxi Cl- channel (105 pS) was found in the PCE cells. The similarities between the 18.8 pS Cl- channel and the volume-activated organic osmolyte-anion channel (VSOAC) are discussed. 4. The hypotonic activation of all three Cl- channels was prevented by the inclusion of 100 microM 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) in the patch pipette. 5. Inclusion of tamoxifen in the patch pipette inhibited only the intermediate-conductance channel in the NPCE cells. This is consistent with the identification of the intermediate 18 pS Cl- channel, found only in NPCE cells, as VSOAC. 6. Negative pressure also evoked single Cl- channel activity but the activation kinetics were quite different, suggesting that, even if the channels are the same, the second messenger pathways involved are different. 7. The activation/inactivation kinetics of these Cl- channels correlate well with the time course of regulatory volume decrease (RVD) in these cells, suggesting that these channels may well participate in the process of RVD. 8. The asymmetrical distribution of Cl- channels in the ciliary epithelium may be significant for aqueous humour secretion. For instance, the presence of the VSOAC-like channel in the NPCE cells may allow one-way traffic of anions and organic osmolytes into the eye and, if these solutes were loaded by the PCE cells, then a vectorial flow from the blood into the eye would occur.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Civan M. M., Coca-Prados M., Peterson-Yantorno K. Pathways signaling the regulatory volume decrease of cultured nonpigmented ciliary epithelial cells. Invest Ophthalmol Vis Sci. 1994 May;35(6):2876–2886. [PubMed] [Google Scholar]
- Coca-Prados M., Sánchez-Torres J., Peterson-Yantorno K., Civan M. M. Association of ClC-3 channel with Cl- transport by human nonpigmented ciliary epithelial cells. J Membr Biol. 1996 Mar;150(2):197–208. doi: 10.1007/s002329900044. [DOI] [PubMed] [Google Scholar]
- Duszyk M., French A. S., Man S. F. Noise analysis and single-channel observations of 4 pS chloride channels in human airway epithelia. Biophys J. 1992 Feb;61(2):583–587. doi: 10.1016/S0006-3495(92)81861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke L. C., Misler S. Activity of ion channels during volume regulation by clonal N1E115 neuroblastoma cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3919–3923. doi: 10.1073/pnas.86.10.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahbakhsh N. A., Fain G. L. Volume regulation of non-pigmented cells from ciliary epithelium. Invest Ophthalmol Vis Sci. 1987 Jun;28(6):934–944. [PubMed] [Google Scholar]
- Gooch A. J., Morgan J., Jacob T. J. Adrenergic stimulation of bovine non-pigmented ciliary epithelial cells raises cAMP but has no effect on K+ or Cl- currents. Curr Eye Res. 1992 Oct;11(10):1019–1029. doi: 10.3109/02713689209033500. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Harris A., Coleman L., Greenwell J. R., Argent B. E. Two types of chloride channel on duct cells cultured from human fetal pancreas. Am J Physiol. 1989 Aug;257(2 Pt 1):C240–C251. doi: 10.1152/ajpcell.1989.257.2.C240. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Pollard C. E., Harris A., Coleman L., Greenwell J. R., Argent B. E. Anion selectivity and block of the small-conductance chloride channel on pancreatic duct cells. Am J Physiol. 1990 Nov;259(5 Pt 1):C752–C761. doi: 10.1152/ajpcell.1990.259.5.C752. [DOI] [PubMed] [Google Scholar]
- Jackson P. S., Morrison R., Strange K. The volume-sensitive organic osmolyte-anion channel VSOAC is regulated by nonhydrolytic ATP binding. Am J Physiol. 1994 Nov;267(5 Pt 1):C1203–C1209. doi: 10.1152/ajpcell.1994.267.5.C1203. [DOI] [PubMed] [Google Scholar]
- Jalonen T. Single-channel characteristics of the large-conductance anion channel in rat cortical astrocytes in primary culture. Glia. 1993 Nov;9(3):227–237. doi: 10.1002/glia.440090308. [DOI] [PubMed] [Google Scholar]
- Kawasaki M., Uchida S., Monkawa T., Miyawaki A., Mikoshiba K., Marumo F., Sasaki S. Cloning and expression of a protein kinase C-regulated chloride channel abundantly expressed in rat brain neuronal cells. Neuron. 1994 Mar;12(3):597–604. doi: 10.1016/0896-6273(94)90215-1. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K., Goderie S. K., Higman S., Pang S., Waniewski R. A. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990 May;10(5):1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk K., Ellory J. C., Young J. D. Transport of organic substrates via a volume-activated channel. J Biol Chem. 1992 Nov 25;267(33):23475–23478. [PubMed] [Google Scholar]
- Lambert I. H., Hoffmann E. K. Cell swelling activates separate taurine and chloride channels in Ehrlich mouse ascites tumor cells. J Membr Biol. 1994 Dec;142(3):289–298. doi: 10.1007/BF00233436. [DOI] [PubMed] [Google Scholar]
- Okada Y., Petersen C. C., Kubo M., Morishima S., Tominaga M. Osmotic swelling activates intermediate-conductance Cl- channels in human intestinal epithelial cells. Jpn J Physiol. 1994;44(4):403–409. doi: 10.2170/jjphysiol.44.403. [DOI] [PubMed] [Google Scholar]
- Paulmichl M., Li Y., Wickman K., Ackerman M., Peralta E., Clapham D. New mammalian chloride channel identified by expression cloning. Nature. 1992 Mar 19;356(6366):238–241. doi: 10.1038/356238a0. [DOI] [PubMed] [Google Scholar]
- Pusch M., Jentsch T. J. Molecular physiology of voltage-gated chloride channels. Physiol Rev. 1994 Oct;74(4):813–827. doi: 10.1152/physrev.1994.74.4.813. [DOI] [PubMed] [Google Scholar]
- Schwiebert E. M., Mills J. W., Stanton B. A. Actin-based cytoskeleton regulates a chloride channel and cell volume in a renal cortical collecting duct cell line. J Biol Chem. 1994 Mar 11;269(10):7081–7089. [PubMed] [Google Scholar]
- Solc C. K., Wine J. J. Swelling-induced and depolarization-induced C1-channels in normal and cystic fibrosis epithelial cells. Am J Physiol. 1991 Oct;261(4 Pt 1):C658–C674. doi: 10.1152/ajpcell.1991.261.4.C658. [DOI] [PubMed] [Google Scholar]
- Strange K., Emma F., Jackson P. S. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol. 1996 Mar;270(3 Pt 1):C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Strange K., Jackson P. S. Swelling-activated organic osmolyte efflux: a new role for anion channels. Kidney Int. 1995 Oct;48(4):994–1003. doi: 10.1038/ki.1995.381. [DOI] [PubMed] [Google Scholar]
- Sánchez-Olea R., Peña C., Morán J., Pasantes-Morales H. Inhibition of volume regulation and efflux of osmoregulatory amino acids by blockers of Cl- transport in cultured astrocytes. Neurosci Lett. 1993 Jun 25;156(1-2):141–144. doi: 10.1016/0304-3940(93)90458-w. [DOI] [PubMed] [Google Scholar]
- Thiemann A., Gründer S., Pusch M., Jentsch T. J. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature. 1992 Mar 5;356(6364):57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- Worrell R. T., Butt A. G., Cliff W. H., Frizzell R. A. A volume-sensitive chloride conductance in human colonic cell line T84. Am J Physiol. 1989 Jun;256(6 Pt 1):C1111–C1119. doi: 10.1152/ajpcell.1989.256.6.C1111. [DOI] [PubMed] [Google Scholar]
- Wu J., Zhang J. J., Koppel H., Jacob T. J. P-glycoprotein regulates a volume-activated chloride current in bovine non-pigmented ciliary epithelial cells. J Physiol. 1996 Mar 15;491(Pt 3):743–755. doi: 10.1113/jphysiol.1996.sp021254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. J., Jacob T. J. ATP-activated chloride channel inhibited by an antibody to P glycoprotein. Am J Physiol. 1994 Oct;267(4 Pt 1):C1095–C1102. doi: 10.1152/ajpcell.1994.267.4.C1095. [DOI] [PubMed] [Google Scholar]


