Abstract
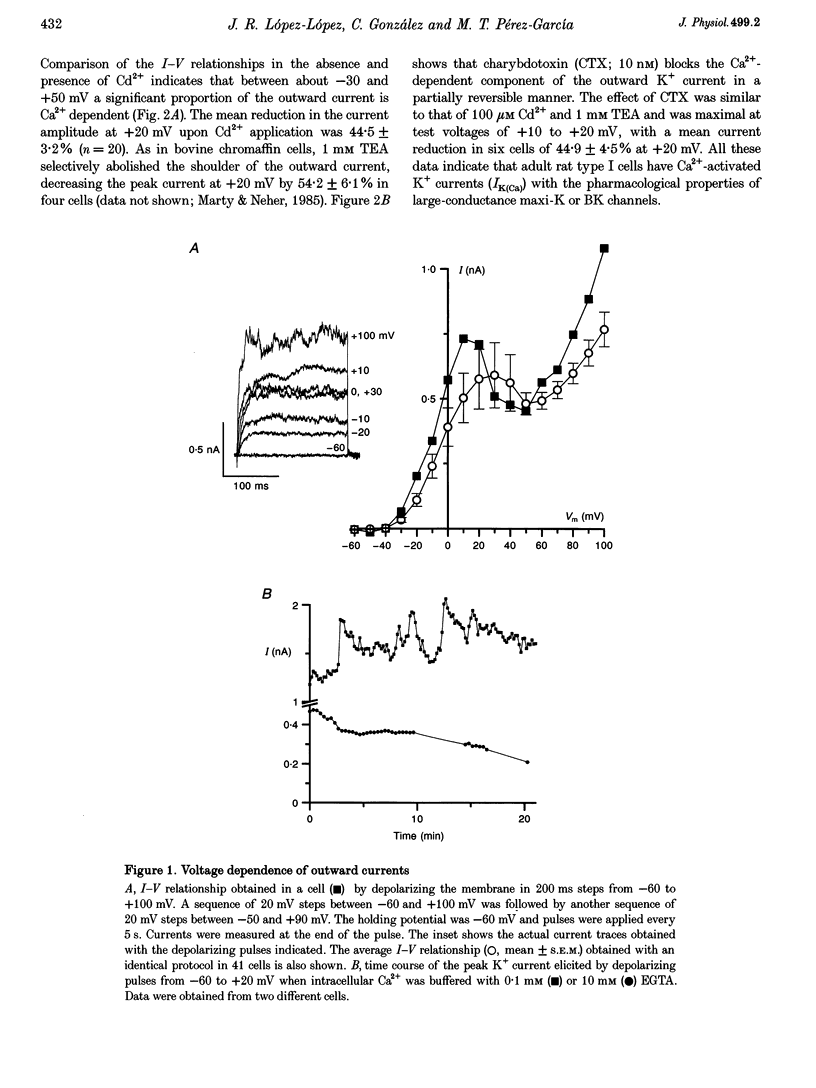
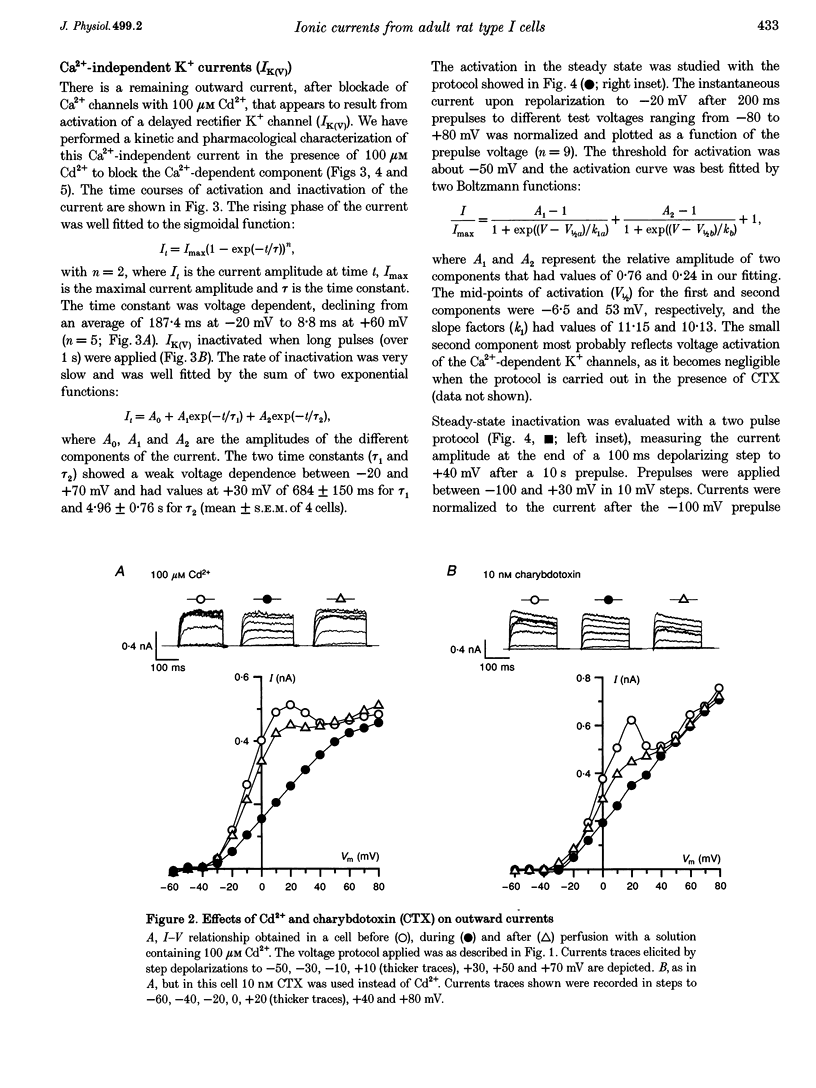
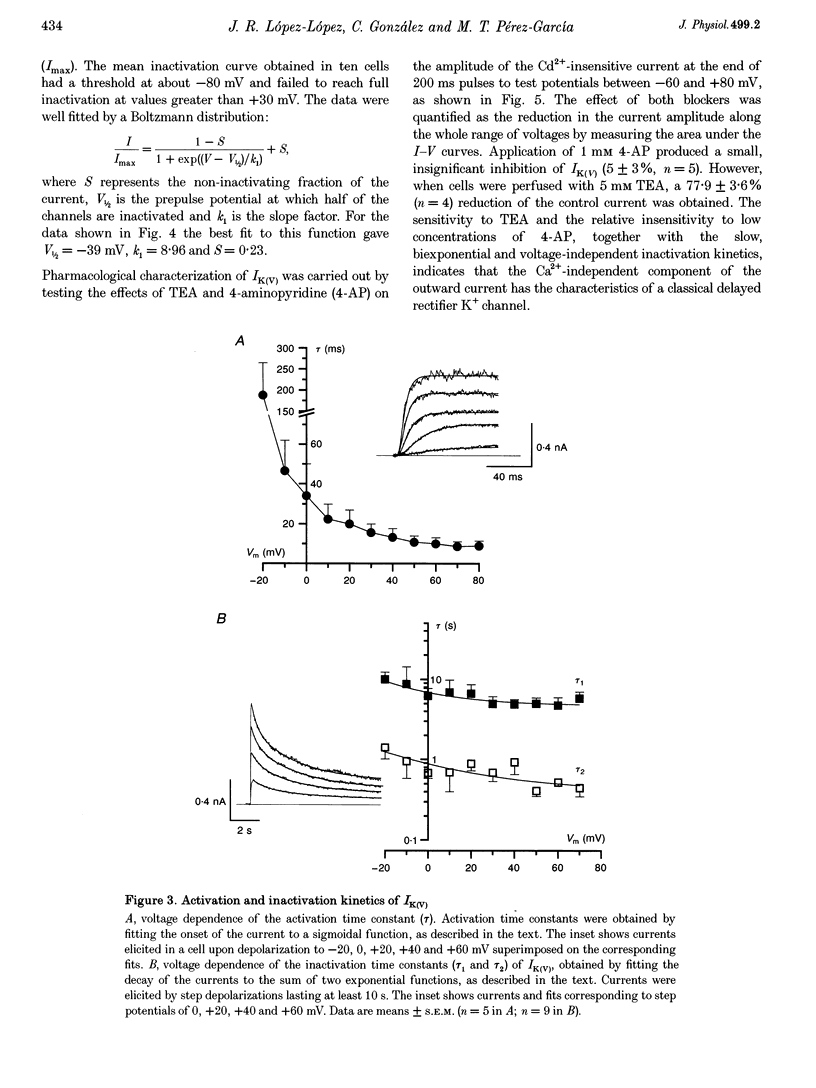
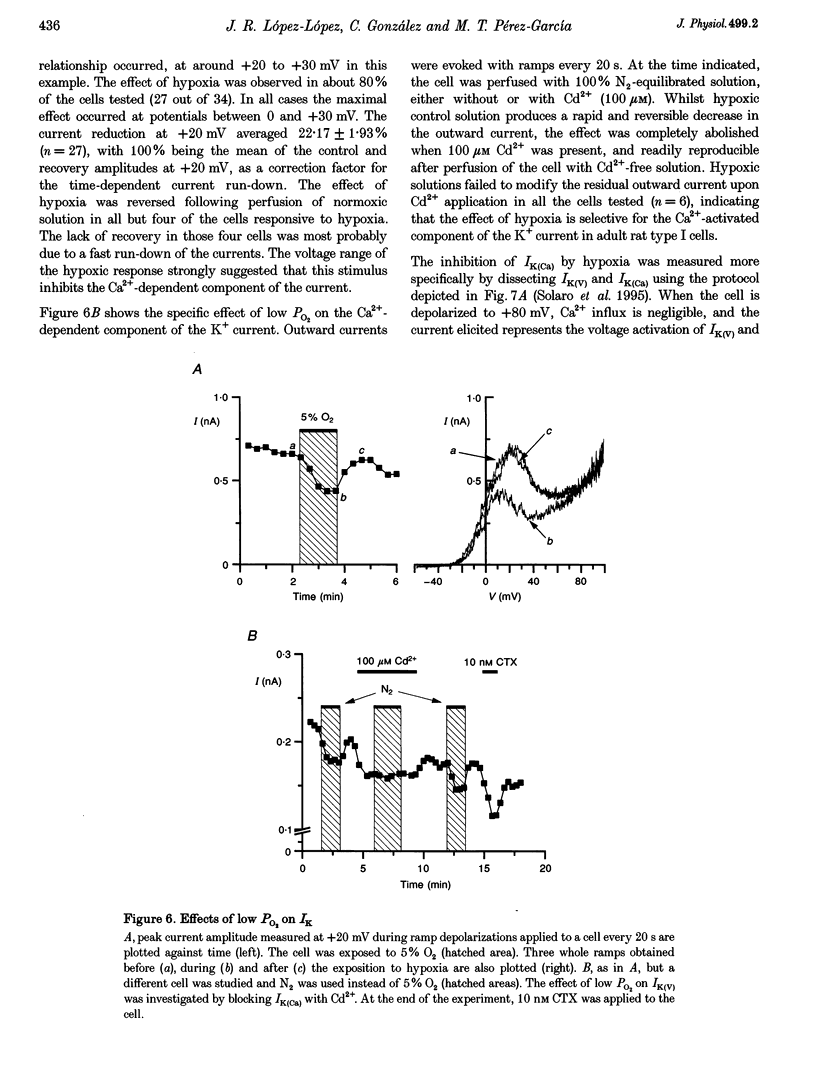
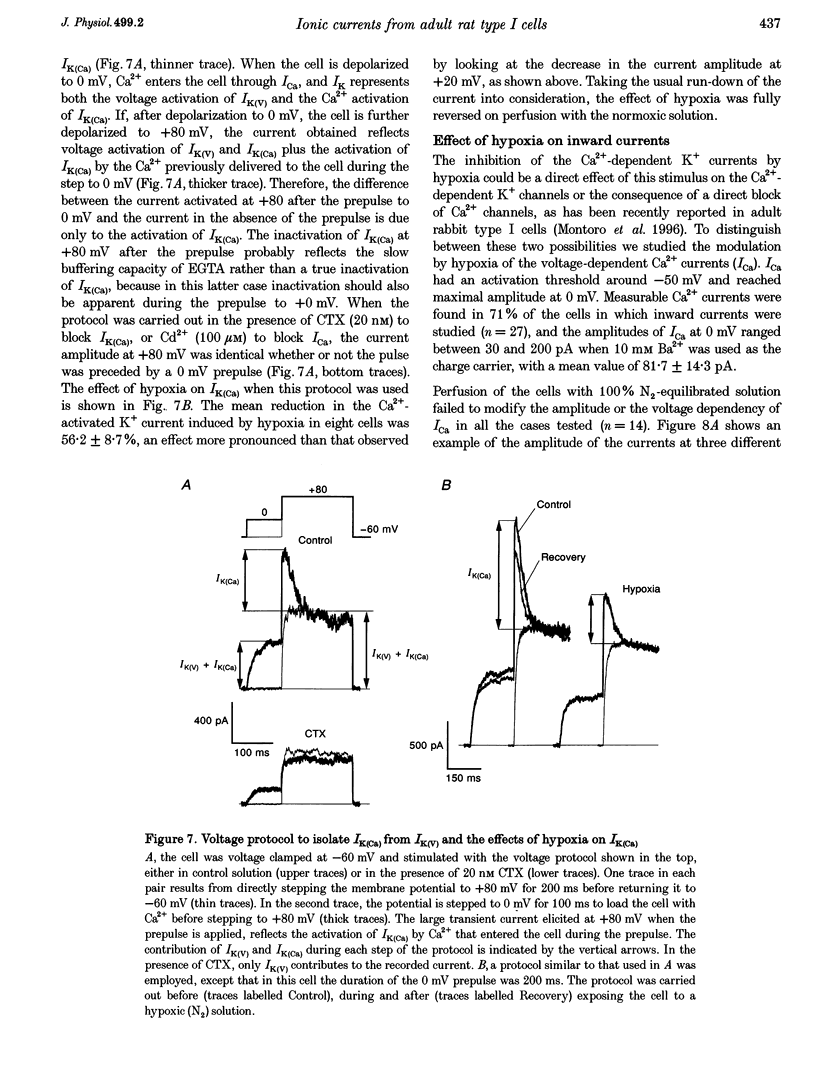
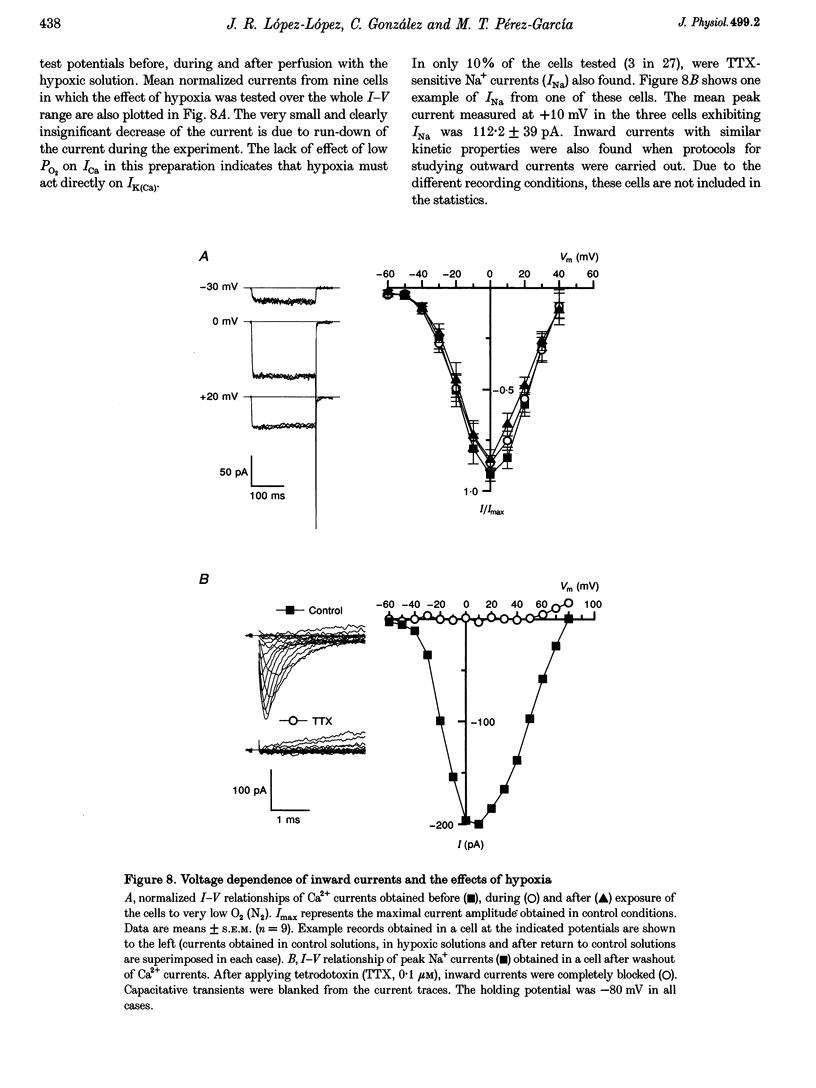
1. The electrical properties of chemoreceptor cells from neonatal rat and adult rabbit carotid bodies (CBs) are strikingly different. These differences have been suggested to be developmental and/or species related. To distinguish between the two possibilities, the whole-cell configuration of the patch-clamp technique was used to characterize the ionic currents present in isolated chemoreceptor cells from adult rat CBs. Since hypoxia-induced inhibition of O2-sensitive K+ currents is considered a crucial step in O2 chemoreception, the effect of hypoxia on the adult rat chemoreceptor cell currents was also studied. 2. Outward currents were carried mainly by K+, and two different components could be distinguished: a Ca(2+)-dependent K+ current (IK(Ca)) sensitive to Cd2+ and charybdotoxin (CTX), and a Ca(2+)-insensitive, voltage-dependent K+ current (IK(V)). IK(V) showed a slow voltage-dependent activation (time constant (tau) of 87.4 ms at -20 mV and 8.8 ms at +60 mV) and a very slow inactivation, described by the sum of two exponentials (tau 1 = 684 +/- 150 ms and tau 2 = 4.96 +/- 0.76 s at + 30 mV), that was almost voltage insensitive. The kinetic and pharmacological properties of IK(V) are typical of a delayed rectifier K+ channel. 3. Voltage-dependent Ca2+ currents (ICa) were present in nineteen of twenty-seven cells. TTX-sensitive Na+ currents were also observed in about 10% of the cells. 4. Low PO2 (< 10 mmHg) reduced the whole outward current amplitude by 22.17 +/- 1.96% (n = 27) at +20 mV. This effect was absent in the presence of Cd2+. Since low PO2 did not affect ICa, we conclude that hypoxia selectively blocks IK(Ca). 5. The properties of the currents recorded in adult rat chemoreceptor cells, including the specific inhibition of IK(Ca) by hypoxia, are similar to those reported in neonatal rat CB cells, implying that the differences between rat and rabbit chemoreceptor cells are species related.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C. R., Bertrand D., Dupin E., Kato A. C. Development of electrical membrane properties in cultured avian neural crest. 1983 Oct 27-Nov 2Nature. 305(5937):808–810. doi: 10.1038/305808a0. [DOI] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. J Physiol. 1994 May 1;476(3):423–428. doi: 10.1113/jphysiol.1994.sp020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett P., Legendre P., Mason W. T. Characterization of three types of potassium current in cultured neurones of rat supraoptic nucleus area. J Physiol. 1989 Mar;410:443–462. doi: 10.1113/jphysiol.1989.sp017543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpiano M. A., Hescheler J. Evidence for a PO2-sensitive K+ channel in the type-I cell of the rabbit carotid body. FEBS Lett. 1989 Jun 5;249(2):195–198. doi: 10.1016/0014-5793(89)80623-4. [DOI] [PubMed] [Google Scholar]
- Donnelly D. F. Modulation of glomus cell membrane currents of intact rat carotid body. J Physiol. 1995 Dec 15;489(Pt 3):677–688. doi: 10.1113/jphysiol.1995.sp021082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen M. R., Caddy K. W., Kirby G. C., Patterson D. L., Ponte J., Biscoe T. J. Biophysical studies of the cellular elements of the rabbit carotid body. Neuroscience. 1988 Jul;26(1):291–311. doi: 10.1016/0306-4522(88)90146-7. [DOI] [PubMed] [Google Scholar]
- Eden G. J., Hanson M. A. Maturation of the respiratory response to acute hypoxia in the newborn rat. J Physiol. 1987 Nov;392:1–9. doi: 10.1113/jphysiol.1987.sp016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieber L. A., McCleskey E. W. L-type calcium channels in type I cells of the rat carotid body. J Neurophysiol. 1993 Oct;70(4):1378–1384. doi: 10.1152/jn.1993.70.4.1378. [DOI] [PubMed] [Google Scholar]
- Franco-Obregón A., Ureña J., López-Barneo J. Oxygen-sensitive calcium channels in vascular smooth muscle and their possible role in hypoxic arterial relaxation. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4715–4719. doi: 10.1073/pnas.92.10.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganfornina M. D., López-Barneo J. Single K+ channels in membrane patches of arterial chemoreceptor cells are modulated by O2 tension. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2927–2930. doi: 10.1073/pnas.88.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C., Almaraz L., Obeso A., Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994 Oct;74(4):829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron. 1991 Oct;7(4):547–556. doi: 10.1016/0896-6273(91)90367-9. [DOI] [PubMed] [Google Scholar]
- López-Barneo J., López-López J. R., Ureña J., González C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988 Jul 29;241(4865):580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- López-López J. R., De Luis D. A., Gonzalez C. Properties of a transient K+ current in chemoreceptor cells of rabbit carotid body. J Physiol. 1993 Jan;460:15–32. doi: 10.1113/jphysiol.1993.sp019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-López J., González C., Ureña J., López-Barneo J. Low pO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989 May;93(5):1001–1015. doi: 10.1085/jgp.93.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J Physiol. 1985 Oct;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., Moczydlowski E., Latorre R., Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985 Jan 24;313(6000):316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- Montoro R. J., Ureña J., Fernández-Chacón R., Alvarez de Toledo G., López-Barneo J. Oxygen sensing by ion channels and chemotransduction in single glomus cells. J Gen Physiol. 1996 Jan;107(1):133–143. doi: 10.1085/jgp.107.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke B., Ramza B. M., Marban E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science. 1994 Aug 12;265(5174):962–966. doi: 10.1126/science.8052856. [DOI] [PubMed] [Google Scholar]
- Pak M. D., Covarrubias M., Ratcliffe A., Salkoff L. A mouse brain homolog of the Drosophila Shab K+ channel with conserved delayed-rectifier properties. J Neurosci. 1991 Mar;11(3):869–880. doi: 10.1523/JNEUROSCI.11-03-00869.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M. K., Lee S. H., Ho W. K., Earm Y. E. Redox agents as a link between hypoxia and the responses of ionic channels in rabbit pulmonary vascular smooth muscle. Exp Physiol. 1995 Sep;80(5):835–842. doi: 10.1113/expphysiol.1995.sp003891. [DOI] [PubMed] [Google Scholar]
- Peers C., Buckler K. J. Transduction of chemostimuli by the type I carotid body cell. J Membr Biol. 1995 Mar;144(1):1–9. doi: 10.1007/BF00238411. [DOI] [PubMed] [Google Scholar]
- Peers C. Effect of lowered extracellular pH on Ca2(+)-dependent K+ currents in type I cells from the neonatal rat carotid body. J Physiol. 1990 Mar;422:381–395. doi: 10.1113/jphysiol.1990.sp017990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C., Green F. K. Inhibition of Ca(2+)-activated K+ currents by intracellular acidosis in isolated type I cells of the neonatal rat carotid body. J Physiol. 1991 Jun;437:589–602. doi: 10.1113/jphysiol.1991.sp018613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C. Hypoxic suppression of K+ currents in type I carotid body cells: selective effect on the Ca2(+)-activated K+ current. Neurosci Lett. 1990 Nov 13;119(2):253–256. doi: 10.1016/0304-3940(90)90846-2. [DOI] [PubMed] [Google Scholar]
- Peers C., O'Donnell J. Potassium currents recorded in type I carotid body cells from the neonatal rat and their modulation by chemoexcitatory agents. Brain Res. 1990 Jul 9;522(2):259–266. doi: 10.1016/0006-8993(90)91470-2. [DOI] [PubMed] [Google Scholar]
- Post J. M., Gelband C. H., Hume J. R. [Ca2+]i inhibition of K+ channels in canine pulmonary artery. Novel mechanism for hypoxia-induced membrane depolarization. Circ Res. 1995 Jul;77(1):131–139. doi: 10.1161/01.res.77.1.131. [DOI] [PubMed] [Google Scholar]
- Pérez-García M. T., Obeso A., López-López J. R., Herreros B., González C. Characterization of cultured chemoreceptor cells dissociated from adult rabbit carotid body. Am J Physiol. 1992 Dec;263(6 Pt 1):C1152–C1159. doi: 10.1152/ajpcell.1992.263.6.C1152. [DOI] [PubMed] [Google Scholar]
- Ritchie A. K. Two distinct calcium-activated potassium currents in a rat anterior pituitary cell line. J Physiol. 1987 Apr;385:591–609. doi: 10.1113/jphysiol.1987.sp016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: potassium conductances. J Neurophysiol. 1984 Jun;51(6):1409–1433. doi: 10.1152/jn.1984.51.6.1409. [DOI] [PubMed] [Google Scholar]
- Solaro C. R., Prakriya M., Ding J. P., Lingle C. J. Inactivating and noninactivating Ca(2+)- and voltage-dependent K+ current in rat adrenal chromaffin cells. J Neurosci. 1995 Sep;15(9):6110–6123. doi: 10.1523/JNEUROSCI.15-09-06110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stea A., Jackson A., Macintyre L., Nurse C. A. Long-term modulation of inward currents in O2 chemoreceptors by chronic hypoxia and cyclic AMP in vitro. J Neurosci. 1995 Mar;15(3 Pt 2):2192–2202. doi: 10.1523/JNEUROSCI.15-03-02192.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stea A., Nurse C. A. Whole-cell and perforated-patch recordings from O2-sensitive rat carotid body cells grown in short- and long-term culture. Pflugers Arch. 1991 Mar;418(1-2):93–101. doi: 10.1007/BF00370457. [DOI] [PubMed] [Google Scholar]
- Ureña J., López-López J., González C., López-Barneo J. Ionic currents in dispersed chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989 May;93(5):979–999. doi: 10.1085/jgp.93.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde M. A., Sheppard D. N., Represa J., Giraldez F. Development of Na(+)- and K(+)-currents in the cochlear ganglion of the chick embryo. Neuroscience. 1992 Dec;51(3):621–630. doi: 10.1016/0306-4522(92)90301-h. [DOI] [PubMed] [Google Scholar]
- Wyatt C. N., Peers C. Ca(2+)-activated K+ channels in isolated type I cells of the neonatal rat carotid body. J Physiol. 1995 Mar 15;483(Pt 3):559–565. doi: 10.1113/jphysiol.1995.sp020606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngson C., Nurse C., Yeger H., Cutz E. Oxygen sensing in airway chemoreceptors. Nature. 1993 Sep 9;365(6442):153–155. doi: 10.1038/365153a0. [DOI] [PubMed] [Google Scholar]
- Yuan X. J., Goldman W. F., Tod M. L., Rubin L. J., Blaustein M. P. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. Am J Physiol. 1993 Feb;264(2 Pt 1):L116–L123. doi: 10.1152/ajplung.1993.264.2.L116. [DOI] [PubMed] [Google Scholar]
- e Silva M. J., Lewis D. L. L- and N-type Ca2+ channels in adult rat carotid body chemoreceptor type I cells. J Physiol. 1995 Dec 15;489(Pt 3):689–699. doi: 10.1113/jphysiol.1995.sp021083. [DOI] [PMC free article] [PubMed] [Google Scholar]


