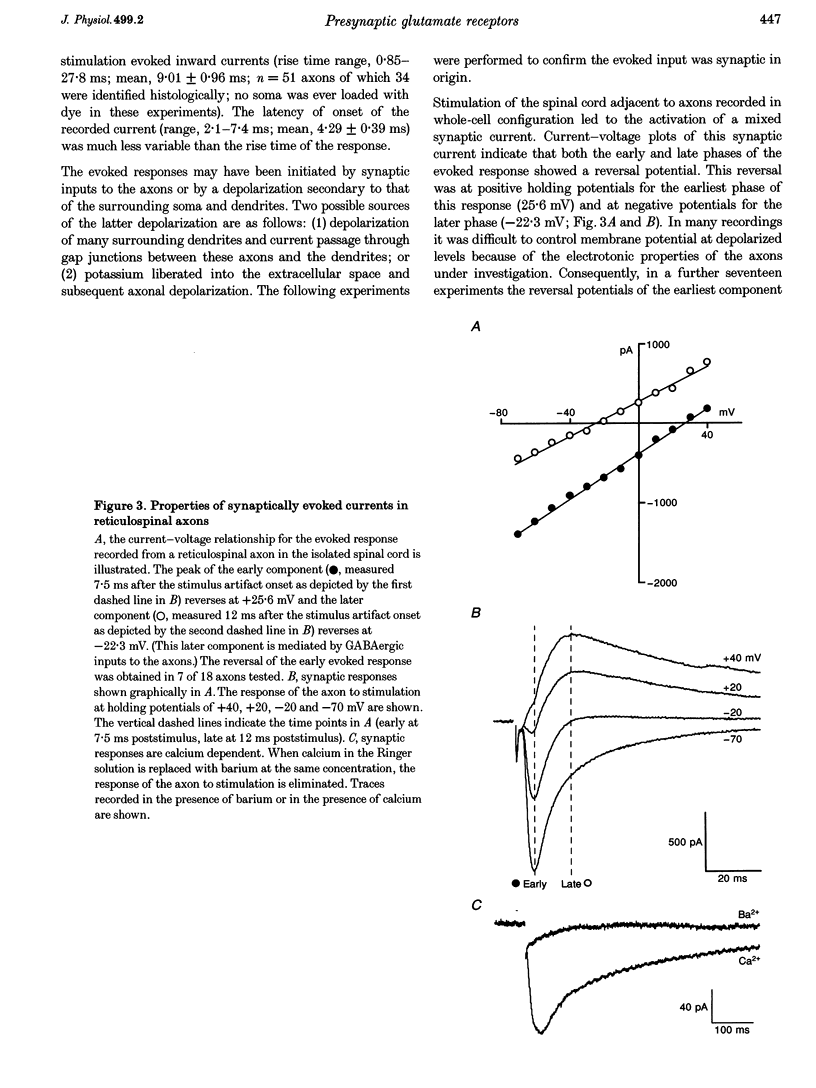
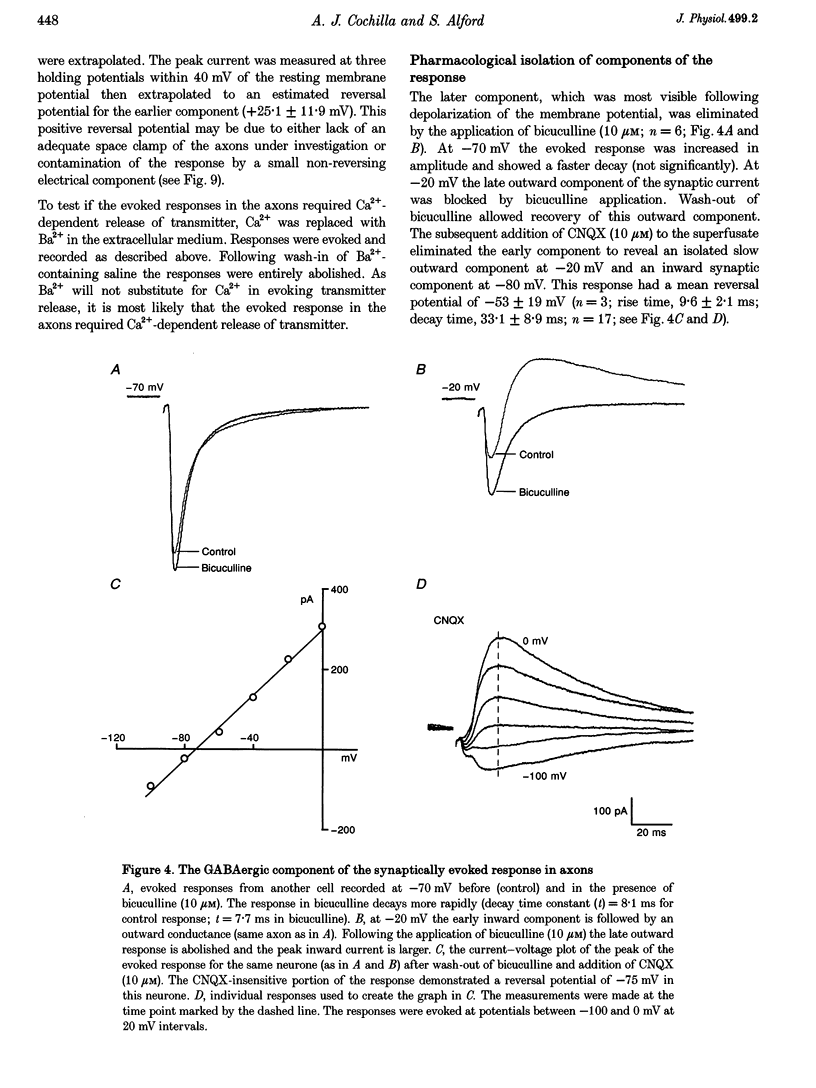
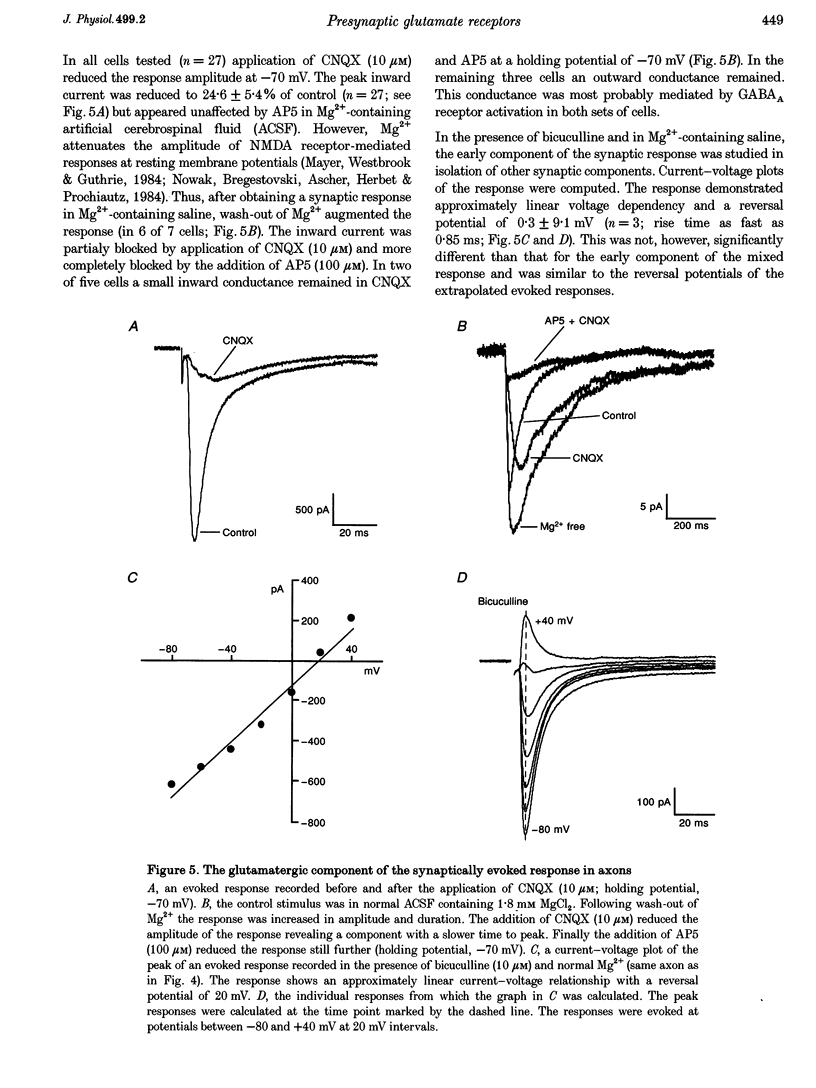
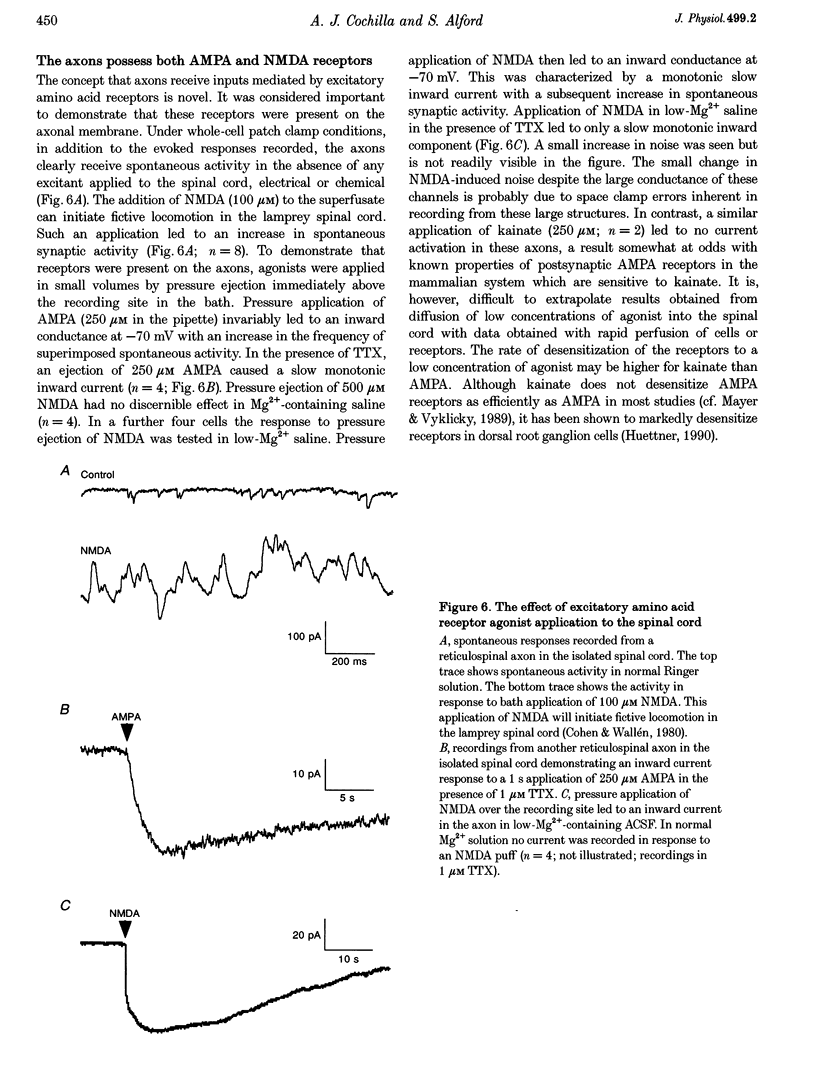
Abstract
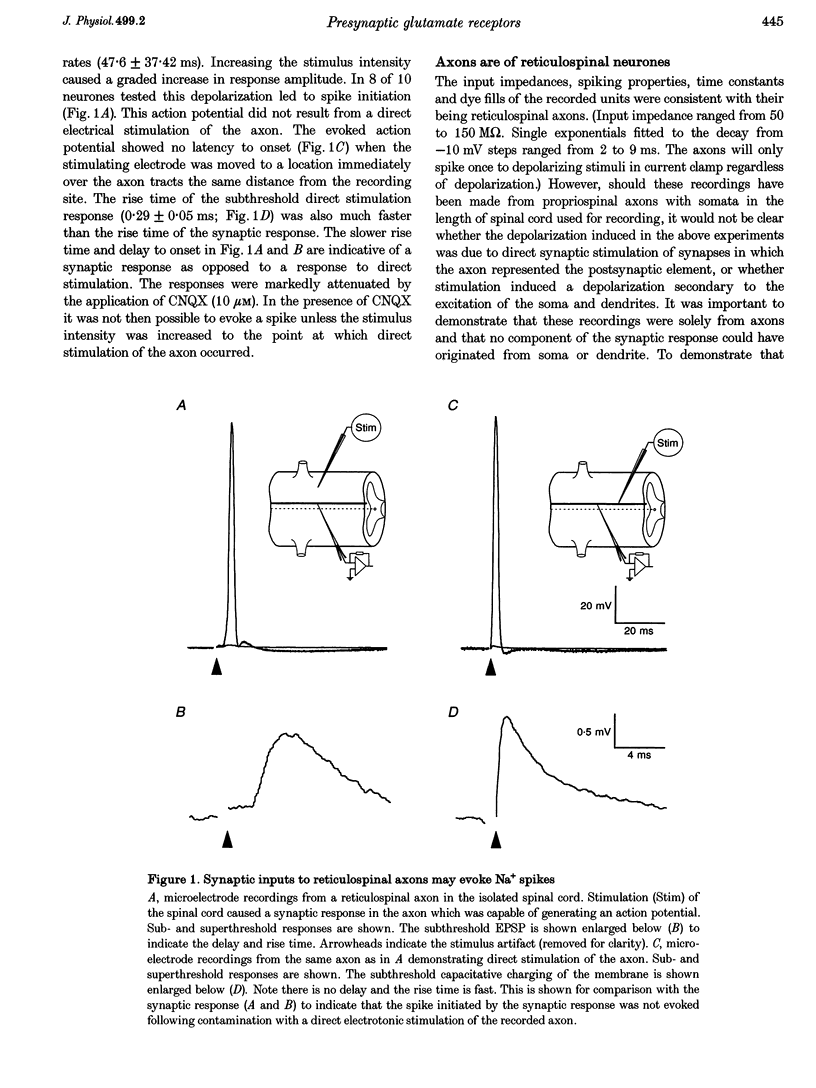
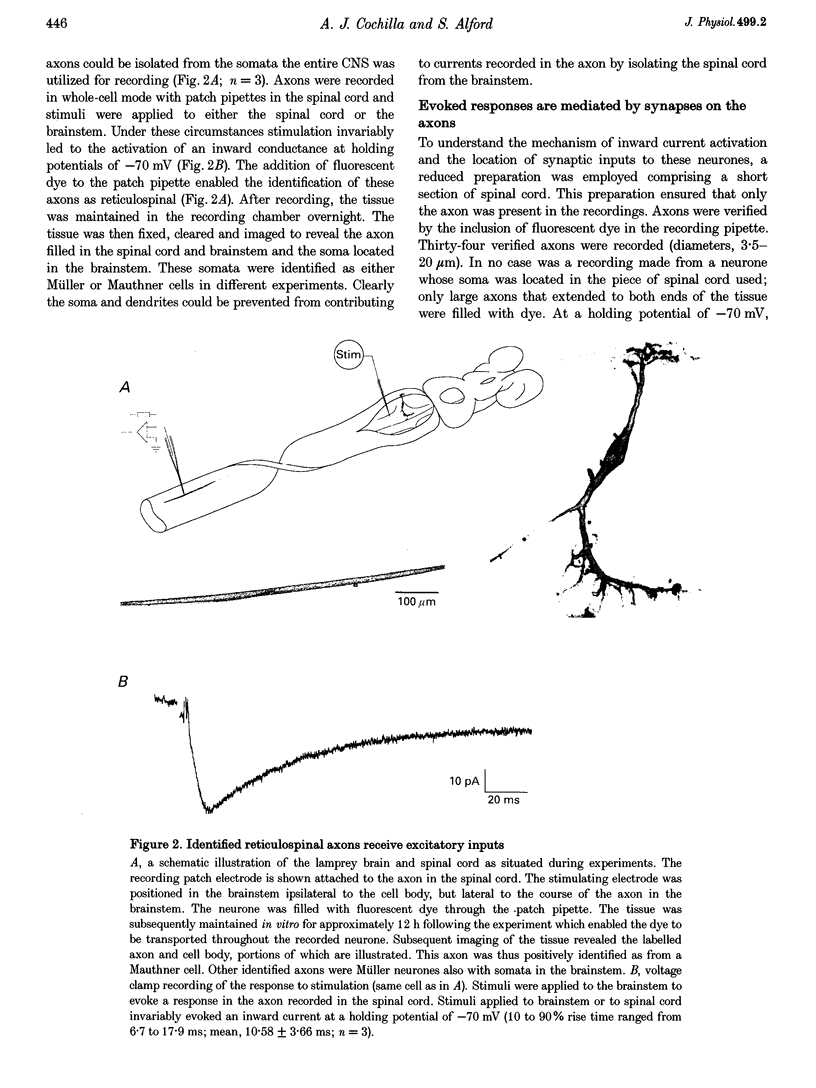
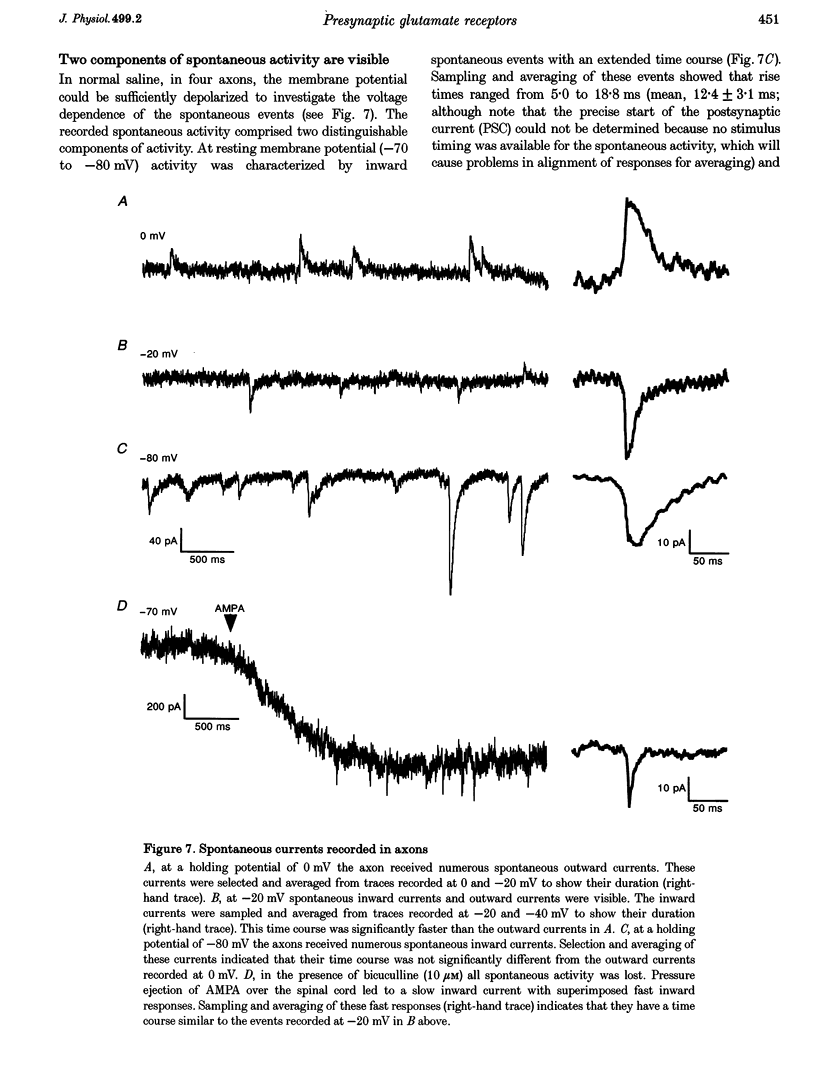
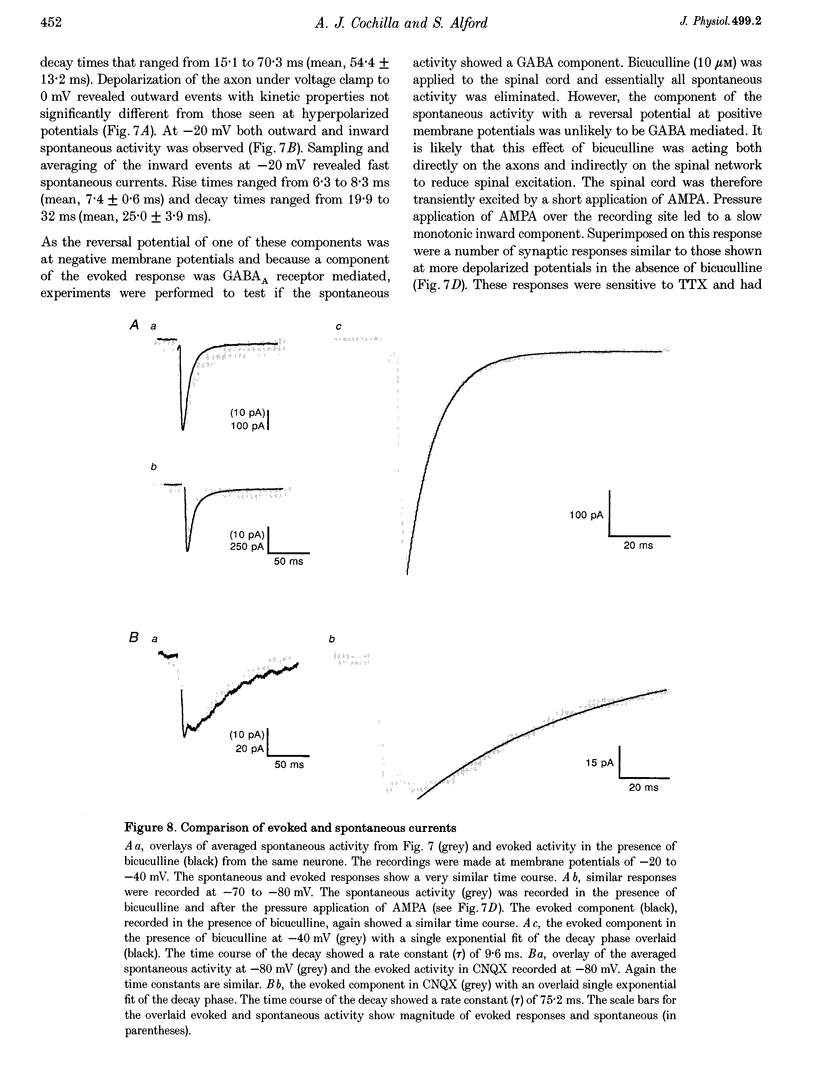
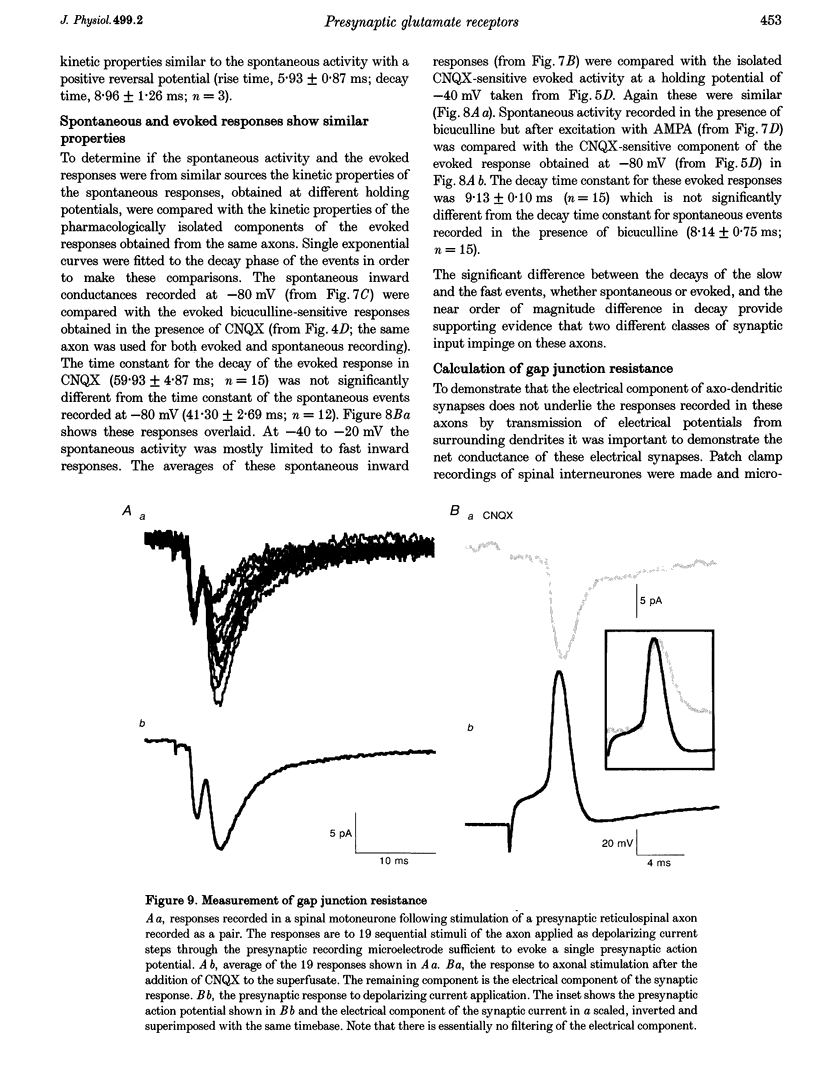
1. Spontaneous and evoked synaptic inputs were recorded in vitro in the axons of lamprey reticulospinal neurones. After isolation of the axon from its somata, synaptic inputs were recorded using microelectrode and whole-cell patch clamp recording techniques. 2. Single stimuli applied to the spinal cord elicited Ca(2+)-dependent synaptic potentials with short latencies in reticulospinal axons. These synaptic inputs are capable of summation and generate sufficient depolarization to raise the membrane potential beyond threshold to initiate action potentials. Action potential initiation in the absence of the cell body indicates that these axons show synaptic integration. 3. Both evoked and spontaneous responses comprise at least two components of synaptic drive: a slow component (rise time of 9.6 +/- 2.1 ms) with a reversal potential of -53 +/- 19 mV and a fast component (rise time as fast as 0.85 ms) with a reversal potential of 0.3 +/- 9.1 mV. The responses are Ca2+ dependent, and are blocked by the substitution of Ba2+ for Ca2+ in the saline solution. 4. The slow component of synaptic input was blocked by the gamma-aminobutyric acidA (GABAA) receptor antagonist bicuculline (5 microM). The fast component was blocked by the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist 6-cyano-7-nitroqinoxaline-2,3-dione (CNQX; 10 microM) in Ringer solution containing physiological concentrations of Mg2+. Following removal of Mg2+ from the superfusate a further excitatory component was identified that was blocked by application of the N-methyl-D-aspartate (NMDA) receptor antagonist 2-amino-5-phosphonopentanoate (AP5; 100 microM). 5. Comparison of the kinetic properties and the voltage sensitivity of the isolated components of evoked and spontaneous synaptic activity indicate that these responses are mediated by similar synaptic inputs. 6. These results suggest that axons and presynaptic terminals receive excitatory and inhibitory ionotropic receptor-mediated inputs. Summation of these inputs is possible indicating that the axons act as sites of synaptic integration similar to the role previously attributed only to neuronal dendrites and somata.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S. G., Evans R. H. The primary afferent depolarizing action of kainate in the rat. Br J Pharmacol. 1986 Feb;87(2):345–355. doi: 10.1111/j.1476-5381.1986.tb10823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford S., Grillner S. The involvement of GABAB receptors and coupled G-proteins in spinal GABAergic presynaptic inhibition. J Neurosci. 1991 Dec;11(12):3718–3726. doi: 10.1523/JNEUROSCI.11-12-03718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford Simon, Christenson Johan, Grillner Sten. Presynaptic GABAA and GABAB Receptor-mediated Phasic Modulation in Axons of Spinal Motor Interneurons. Eur J Neurosci. 1991;3(2):107–117. doi: 10.1111/j.1460-9568.1991.tb00071.x. [DOI] [PubMed] [Google Scholar]
- Barnes J. M., Dev K. K., Henley J. M. Cyclothiazide unmasks AMPA-evoked stimulation of [3H]-L-glutamate release from rat hippocampal synaptosomes. Br J Pharmacol. 1994 Oct;113(2):339–341. doi: 10.1111/j.1476-5381.1994.tb16902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskys A., Malenka R. C. Agonists at metabotropic glutamate receptors presynaptically inhibit EPSCs in neonatal rat hippocampus. J Physiol. 1991 Dec;444:687–701. doi: 10.1113/jphysiol.1991.sp018901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton M. G., Lo Turco J. J., Kriegstein A. R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989 Dec;30(3):203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Brodin L., Christenson J., Grillner S. Single sensory neurons activate excitatory amino acid receptors in the lamprey spinal cord. Neurosci Lett. 1987 Mar 20;75(1):75–79. doi: 10.1016/0304-3940(87)90078-4. [DOI] [PubMed] [Google Scholar]
- Brodin L., Dale N., Christenson J., Storm-Mathisen J., Hökfelt T., Grillner S. Three types of GABA-immunoreactive cells in the lamprey spinal cord. Brain Res. 1990 Jan 29;508(1):172–175. doi: 10.1016/0006-8993(90)91134-3. [DOI] [PubMed] [Google Scholar]
- Buchanan J. T., Brodin L., Dale N., Grillner S. Reticulospinal neurones activate excitatory amino acid receptors. Brain Res. 1987 Apr 7;408(1-2):321–325. doi: 10.1016/0006-8993(87)90397-0. [DOI] [PubMed] [Google Scholar]
- Buchanan J. T., Grillner S. Newly identified 'glutamate interneurons' and their role in locomotion in the lamprey spinal cord. Science. 1987 Apr 17;236(4799):312–314. doi: 10.1126/science.3563512. [DOI] [PubMed] [Google Scholar]
- Buchanan J. T. Identification of interneurons with contralateral, caudal axons in the lamprey spinal cord: synaptic interactions and morphology. J Neurophysiol. 1982 May;47(5):961–975. doi: 10.1152/jn.1982.47.5.961. [DOI] [PubMed] [Google Scholar]
- Christenson J., Bongianni F., Grillner S., Hökfelt T. Putative GABAergic input to axons of spinal interneurons and primary sensory neurons in the lamprey spinal cord as shown by intracellular Lucifer yellow and GABA immunohistochemistry. Brain Res. 1991 Jan 11;538(2):313–318. doi: 10.1016/0006-8993(91)90446-3. [DOI] [PubMed] [Google Scholar]
- Cohen A. H., Wallén P. The neuronal correlate of locomotion in fish. "Fictive swimming" induced in an in vitro preparation of the lamprey spinal cord. Exp Brain Res. 1980;41(1):11–18. doi: 10.1007/BF00236674. [DOI] [PubMed] [Google Scholar]
- Diamond J. S., Jahr C. E. Asynchronous release of synaptic vesicles determines the time course of the AMPA receptor-mediated EPSC. Neuron. 1995 Nov;15(5):1097–1107. doi: 10.1016/0896-6273(95)90098-5. [DOI] [PubMed] [Google Scholar]
- Dubuc R., Cabelguen J. M., Rossignol S. Rhythmic fluctuations of dorsal root potentials and antidromic discharges of primary afferents during fictive locomotion in the cat. J Neurophysiol. 1988 Dec;60(6):2014–2036. doi: 10.1152/jn.1988.60.6.2014. [DOI] [PubMed] [Google Scholar]
- Dubuc R., Rossignol S., Lamarre Y. The effects of 4-aminopyridine on the spinal cord: rhythmic discharges recorded from the peripheral nerves. Brain Res. 1986 Mar 26;369(1-2):243–259. doi: 10.1016/0006-8993(86)90533-0. [DOI] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. A physiological role for GABAB receptors in the central nervous system. Nature. 1988 Mar 10;332(6160):156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Gossard J. P., Cabelguen J. M., Rossignol S. Intra-axonal recordings of cutaneous primary afferents during fictive locomotion in the cat. J Neurophysiol. 1989 Nov;62(5):1177–1188. doi: 10.1152/jn.1989.62.5.1177. [DOI] [PubMed] [Google Scholar]
- Huettner J. E. Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron. 1990 Sep;5(3):255–266. doi: 10.1016/0896-6273(90)90163-a. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Calcium conductances in Purkinje cell dendrites: their role in development and integration. Prog Brain Res. 1979;51:323–334. doi: 10.1016/S0079-6123(08)61312-6. [DOI] [PubMed] [Google Scholar]
- Matthews G., Wickelgren W. O. Evoked depolarizing and hyperpolarizing potentials in reticulospinal axons of lamprey. J Physiol. 1978 Jun;279:551–567. doi: 10.1113/jphysiol.1978.sp012361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G., Wickelgren W. O. Glutamate and synaptic excitation of reticulospinal neurones of lamprey. J Physiol. 1979 Aug;293:417–433. doi: 10.1113/jphysiol.1979.sp012897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr Concanavalin A selectively reduces desensitization of mammalian neuronal quisqualate receptors. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1411–1415. doi: 10.1073/pnas.86.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. Presynaptic inhibition: transmitter and ionic mechanisms. Int Rev Neurobiol. 1979;21:217–258. doi: 10.1016/s0074-7742(08)60639-x. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Pereda A. E., Bell T. D., Faber D. S. Retrograde synaptic communication via gap junctions coupling auditory afferents to the Mauthner cell. J Neurosci. 1995 Sep;15(9):5943–5955. doi: 10.1523/JNEUROSCI.15-09-05943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell J. S., Jankowska E., Eide E. Depolarization of group II muscle afferents by stimuli applied in the locus coeruleus and raphe nuclei of the cat. J Physiol. 1993 Feb;461:723–741. doi: 10.1113/jphysiol.1993.sp019538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringham G. L. Localization and electrical characteristics of a giant synapse in the spinal cord of the lamprey. J Physiol. 1975 Oct;251(2):395–407. doi: 10.1113/jphysiol.1975.sp011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N., Duan S. M., Hori T., Oomura Y. Glutamate modulates dopamine release in the striatum as measured by brain microdialysis. Brain Res Bull. 1990 Jul;25(1):99–102. doi: 10.1016/0361-9230(90)90258-2. [DOI] [PubMed] [Google Scholar]
- Shupliakov O., Brodin L., Cullheim S., Ottersen O. P., Storm-Mathisen J. Immunogold quantification of glutamate in two types of excitatory synapse with different firing patterns. J Neurosci. 1992 Oct;12(10):3789–3803. doi: 10.1523/JNEUROSCI.12-10-03789.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N., Jaffe D. B., Williams S. H., Johnston D. Voltage- and space-clamp errors associated with the measurement of electrotonically remote synaptic events. J Neurophysiol. 1993 Aug;70(2):781–802. doi: 10.1152/jn.1993.70.2.781. [DOI] [PubMed] [Google Scholar]
- Spruston N., Schiller Y., Stuart G., Sakmann B. Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites. Science. 1995 Apr 14;268(5208):297–300. doi: 10.1126/science.7716524. [DOI] [PubMed] [Google Scholar]
- Starke K. Influence of extracellular noradrenaline on the stimulation-evoked secretion of noradrenaline from sympathetic nerves: evidence for an -receptor-mediated feed-back inhibition of noradrenaline release. Naunyn Schmiedebergs Arch Pharmacol. 1972;275(1):11–23. doi: 10.1007/BF00505064. [DOI] [PubMed] [Google Scholar]
- Sugimori M., Llinás R. R. Real-time imaging of calcium influx in mammalian cerebellar Purkinje cells in vitro. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5084–5088. doi: 10.1073/pnas.87.13.5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallén P., Grafe P., Grillner S. Phasic variations of extracellular potassium during fictive swimming in the lamprey spinal cord in vitro. Acta Physiol Scand. 1984 Mar;120(3):457–463. doi: 10.1111/j.1748-1716.1984.tb07406.x. [DOI] [PubMed] [Google Scholar]
- Wickelgren W. O. Physiological and anatomical characteristics of reticulospinalneurones in lamprey. J Physiol. 1977 Aug;270(1):89–114. doi: 10.1113/jphysiol.1977.sp011940. [DOI] [PMC free article] [PubMed] [Google Scholar]