Abstract
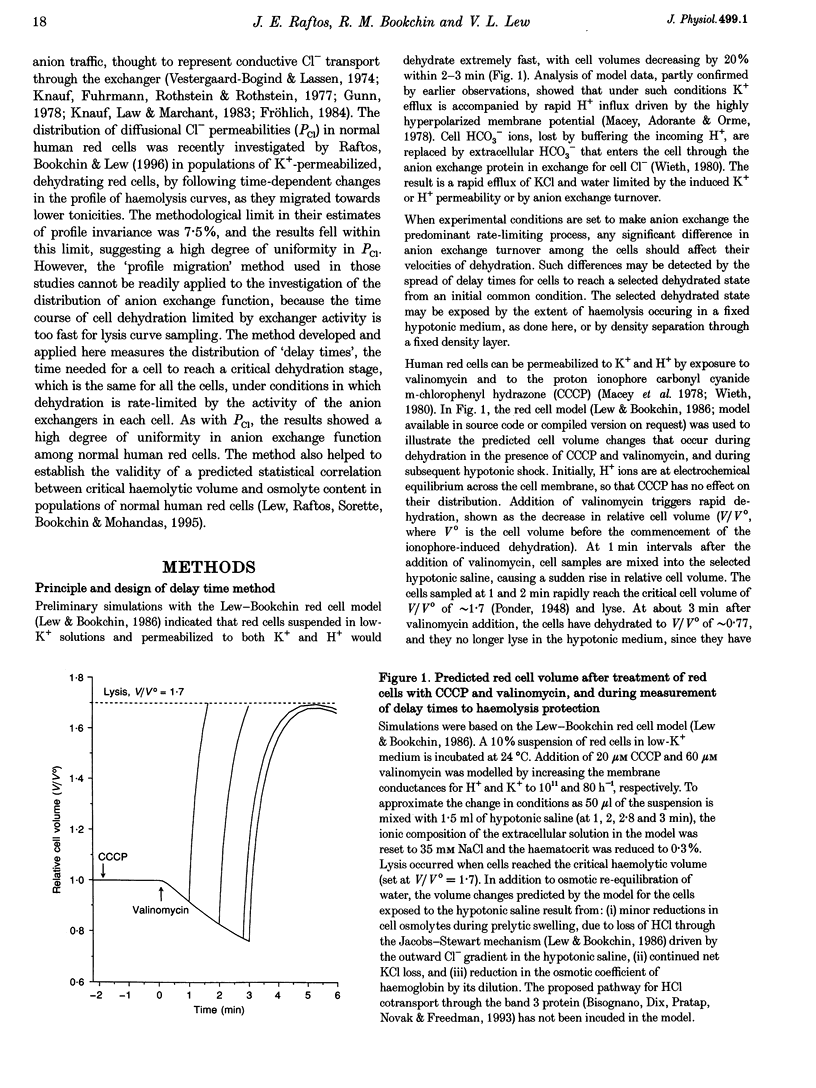
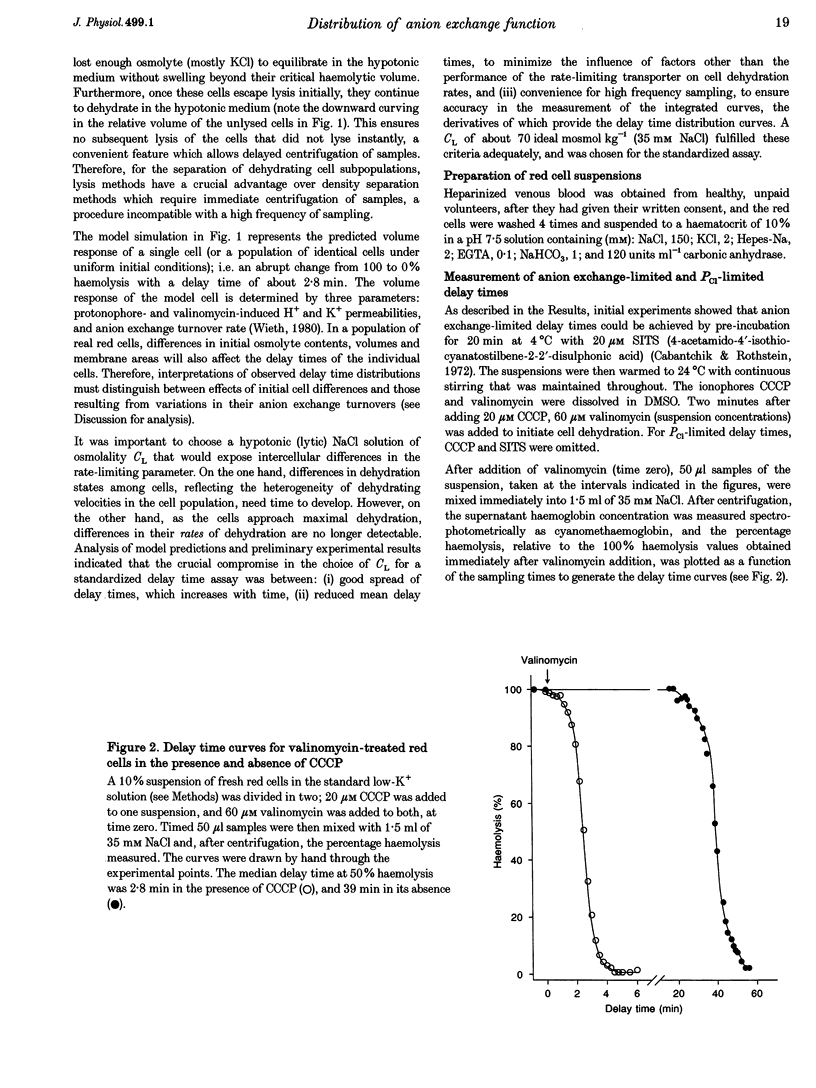
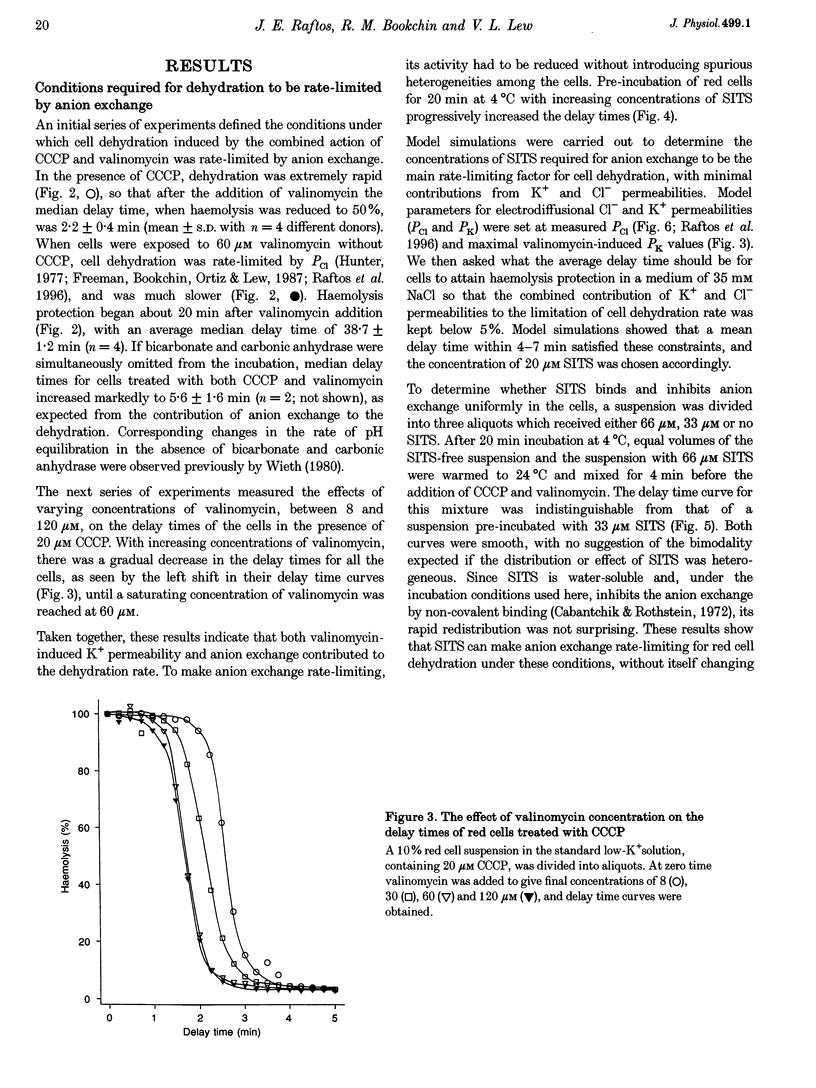
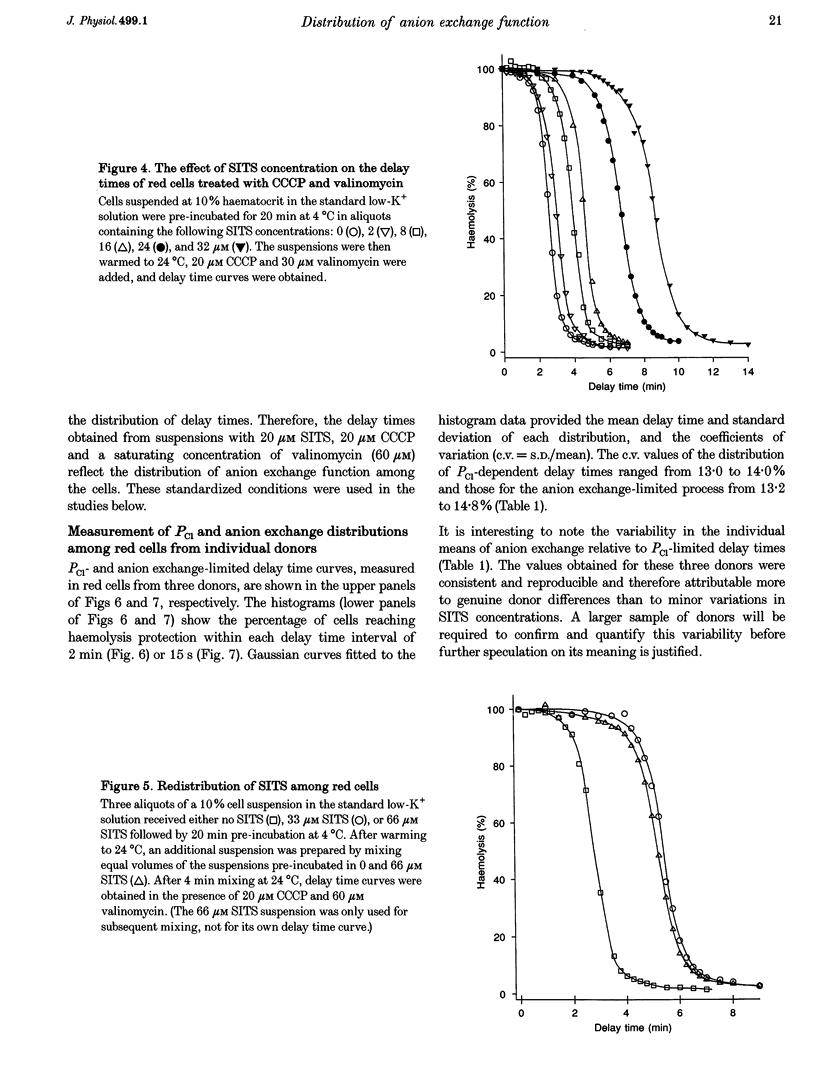
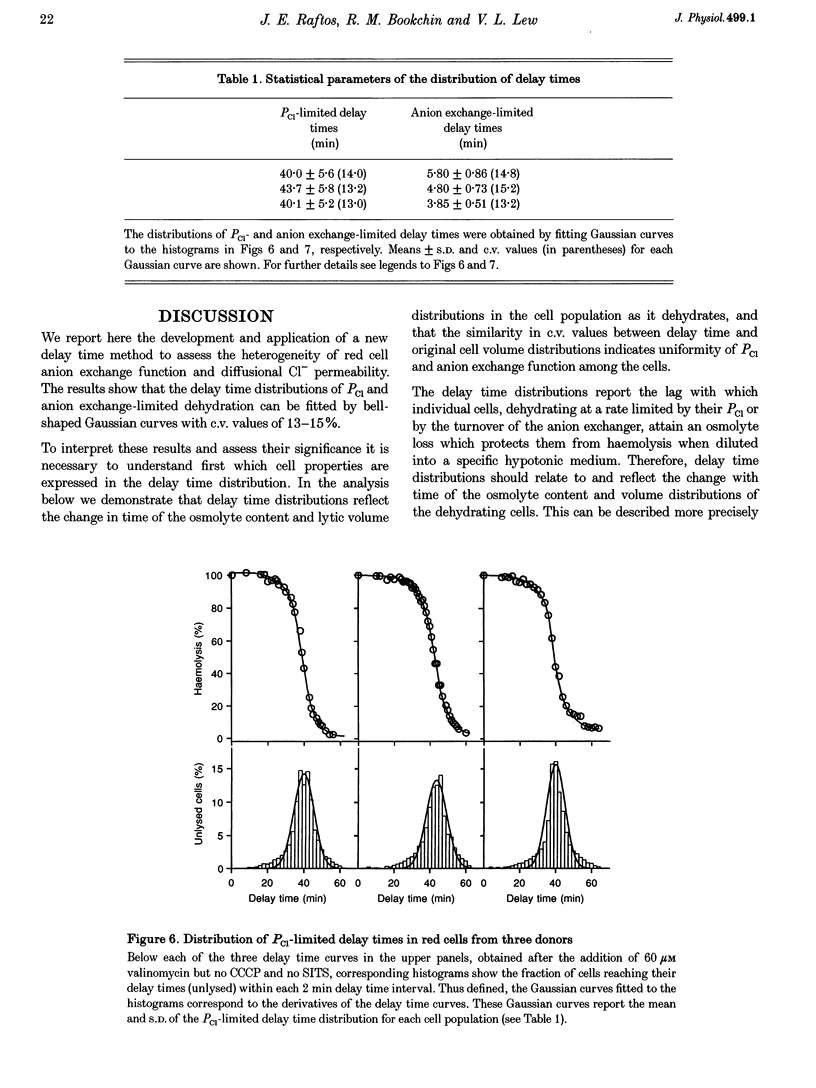
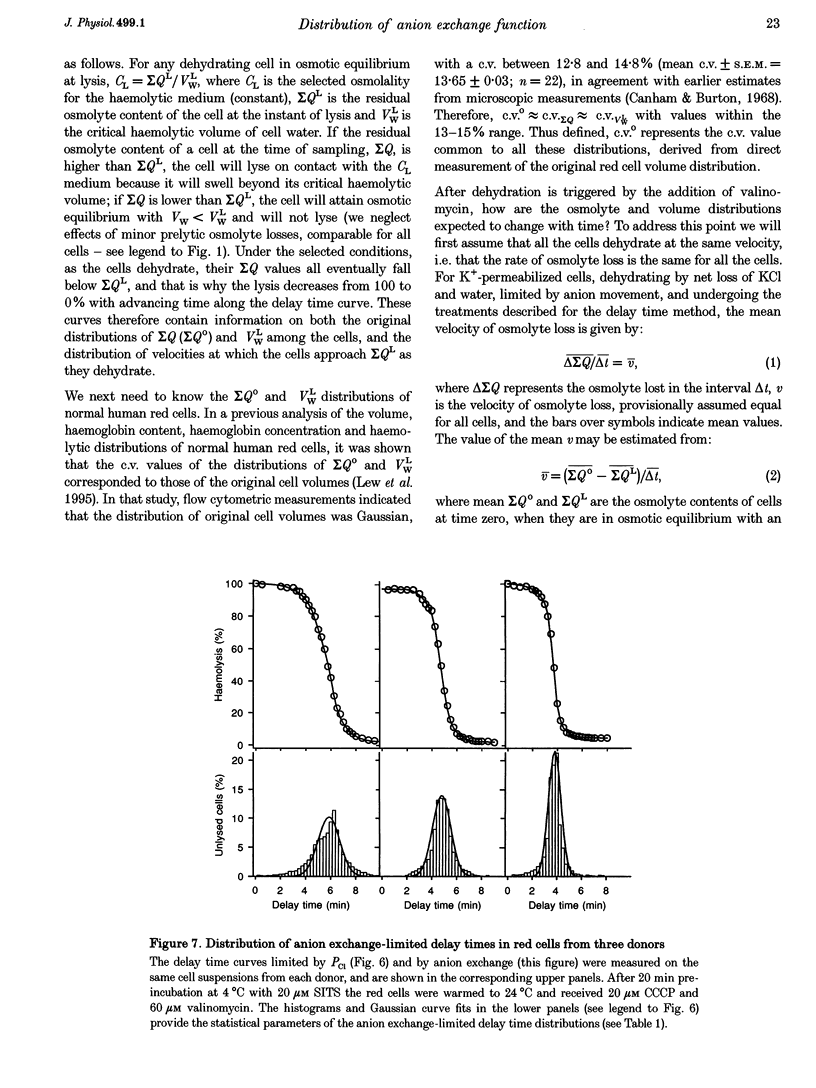
1. The aim of the present work was to investigate cell-to-cell variation in anion exchange turnover in normal human red cells. Red cells permeabilized to protons and K+ dehydrate extremely rapidly by processes that are rate-limited by the induced K+ permeability or by anion exchange turnover. Conditions were designed to render dehydration rate-limited by anion exchange turnover. Cell-to-cell variation in anion exchange function could then be measured from the distribution of delay times required for dehydrating cells to attain resistance to haemolysis in a selected hypotonic medium. 2. Red cells were suspended at 10% haematocrit in a low-K+ solution and, after a brief preincubation with 20 microM SITS at 4 degrees C, were warmed to 24 degrees C, and the protonophore CCCP was added (20 microM) followed 2 min later by valinomycin (60 microM). Delay times for cells to become resistant to lysis were measured from the instant of valinomycin addition by sampling suspension aliquots into thirty volumes of 35 mM NaCl. After centrifugation the per cent lysis was estimated by measuring the haemoglobin concentration in the supernatant. Typical median delay times with this standardized method were 4-5 min. 3. The statistical parameters of the delay time distributions report the population spread in the transport function that was limiting to dehydration. In the absence of SITS and CCCP, dehydration was limited by the diffusional Cl- permeability (PCl). Delay time distributions for PCl- and anion exchange-limited dehydration were measured in red cells from three normal donors. For both distributions, the coefficients of variation ranged between 13.0 and 15.2%, indicating a high degree of uniformity in PCl and anion exchange function among individual red cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisognano J. D., Dix J. A., Pratap P. R., Novak T. S., Freedman J. C. Proton (or hydroxide) fluxes and the biphasic osmotic response of human red blood cells. J Gen Physiol. 1993 Jul;102(1):99–123. doi: 10.1085/jgp.102.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookchin R. M., Ortiz O. E., Lew V. L. Evidence for a direct reticulocyte origin of dense red cells in sickle cell anemia. J Clin Invest. 1991 Jan;87(1):113–124. doi: 10.1172/JCI114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. The nature of the membrane sites controlling anion permeability of human red blood cells as determined by studies with disulfonic stilbene derivatives. J Membr Biol. 1972 Dec 29;10(3):311–330. doi: 10.1007/BF01867863. [DOI] [PubMed] [Google Scholar]
- Canham P. B., Burton A. C. Distribution of size and shape in populations of normal human red cells. Circ Res. 1968 Mar;22(3):405–422. doi: 10.1161/01.res.22.3.405. [DOI] [PubMed] [Google Scholar]
- Freeman C. J., Bookchin R. M., Ortiz O. E., Lew V. L. K-permeabilized human red cells lose an alkaline, hypertonic fluid containing excess K over diffusible anions. J Membr Biol. 1987;96(3):235–241. doi: 10.1007/BF01869305. [DOI] [PubMed] [Google Scholar]
- Fröhlich O. Relative contributions of the slippage and tunneling mechanisms to anion net efflux from human erythrocytes. J Gen Physiol. 1984 Dec;84(6):877–893. doi: 10.1085/jgp.84.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sancho J., Lew V. L. Detection and separation of human red cells with different calcium contents following uniform calcium permeabilization. J Physiol. 1988 Dec;407:505–522. doi: 10.1113/jphysiol.1988.sp017428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn R. B., Dalmark M., Tosteson D. C., Wieth J. O. Characteristics of chloride transport in human red blood cells. J Gen Physiol. 1973 Feb;61(2):185–206. doi: 10.1085/jgp.61.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter M. J. Human erythrocyte anion permeabilities measured under conditions of net charge transfer. J Physiol. 1977 Jun;268(1):35–49. doi: 10.1113/jphysiol.1977.sp011845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf P. A., Fuhrmann G. F., Rothstein S., Rothstein A. The relationship between anion exchange and net anion flow across the human red blood cell membrane. J Gen Physiol. 1977 Mar;69(3):363–386. doi: 10.1085/jgp.69.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf P. A., Law F. Y., Marchant P. J. Relationship of net chloride flow across the human erythrocyte membrane to the anion exchange mechanism. J Gen Physiol. 1983 Jan;81(1):95–126. doi: 10.1085/jgp.81.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew V. L., Bookchin R. M. Role of reticulocyte transport heterogeneity in the generation of mature sickle cells with different volumes. Biochem Soc Trans. 1992 Nov;20(4):797–800. doi: 10.1042/bst0200797. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Bookchin R. M. Volume, pH, and ion-content regulation in human red cells: analysis of transient behavior with an integrated model. J Membr Biol. 1986;92(1):57–74. doi: 10.1007/BF01869016. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Raftos J. E., Sorette M., Bookchin R. M., Mohandas N. Generation of normal human red cell volume, hemoglobin content, and membrane area distributions by "birth" or regulation? Blood. 1995 Jul 1;86(1):334–341. [PubMed] [Google Scholar]
- Macey R. I., Adorante J. S., Orme F. W. Erythrocyte membrane potentials determined by hydrogen ion distribution. Biochim Biophys Acta. 1978 Sep 22;512(2):284–295. doi: 10.1016/0005-2736(78)90253-5. [DOI] [PubMed] [Google Scholar]
- Raftos J. E., Bookchin R. M., Lew V. L. Distribution of chloride permeabilities in normal human red cells. J Physiol. 1996 Mar 15;491(Pt 3):773–777. doi: 10.1113/jphysiol.1996.sp021256. [DOI] [PMC free article] [PubMed] [Google Scholar]


