Abstract
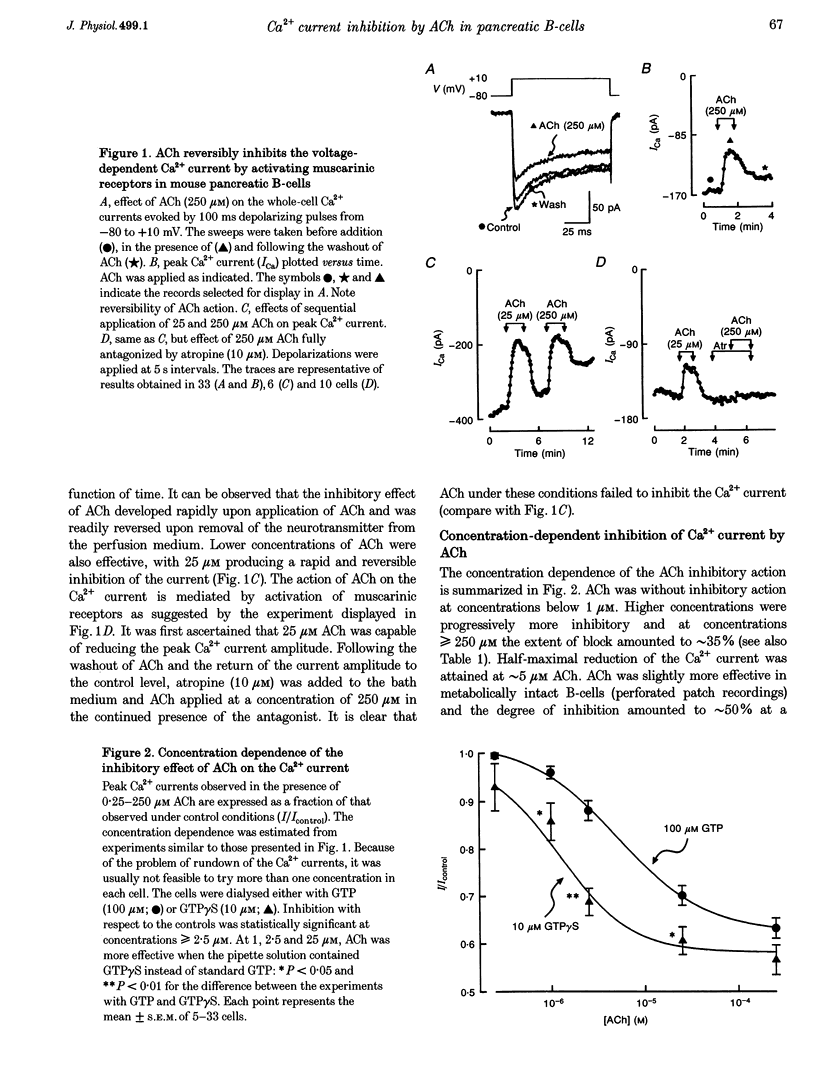
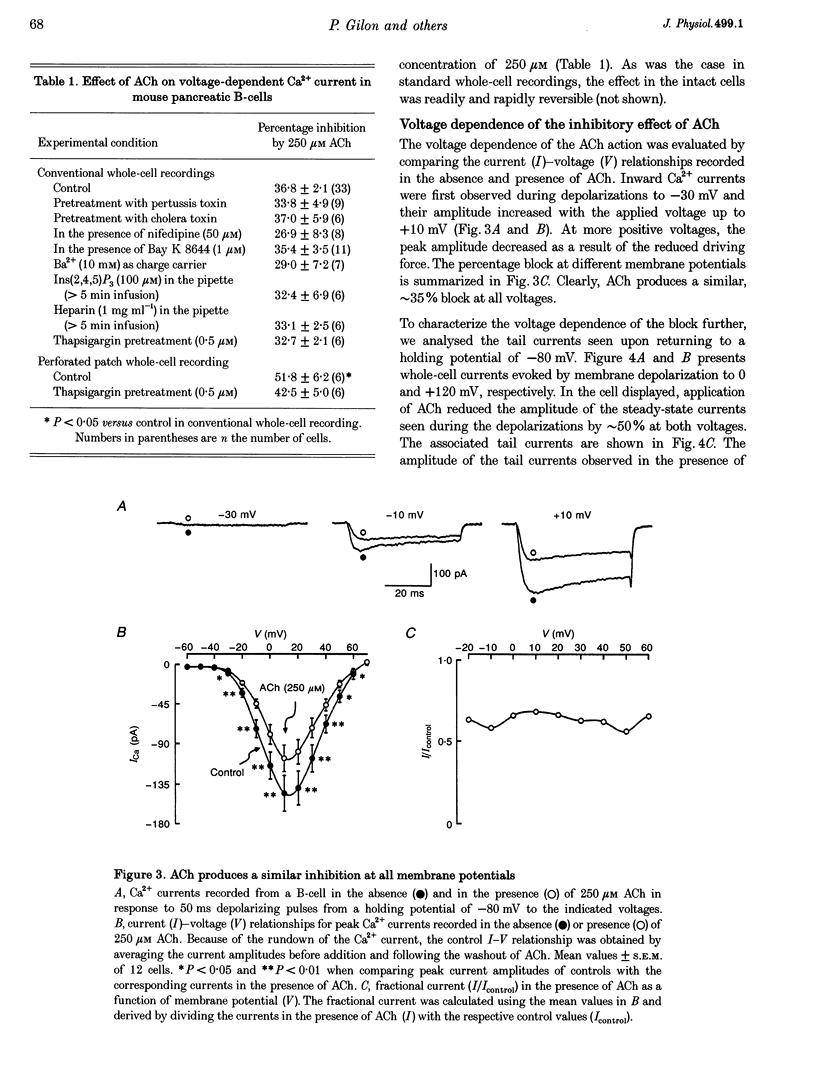
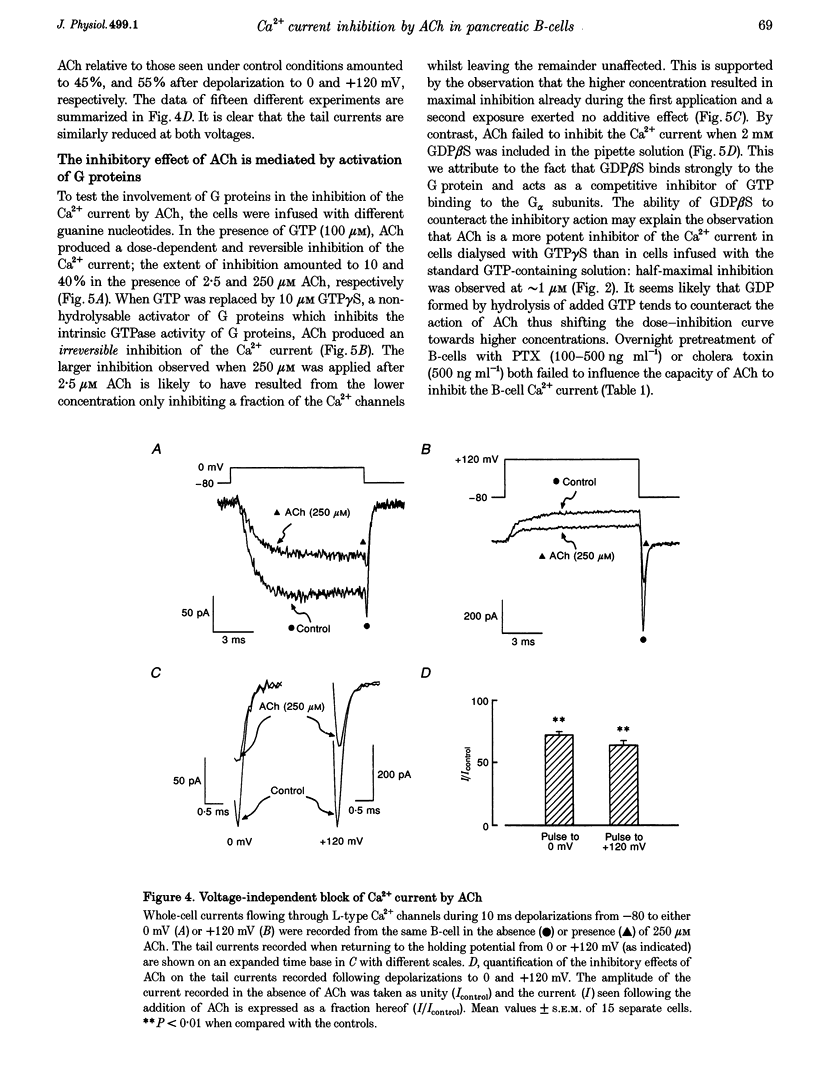
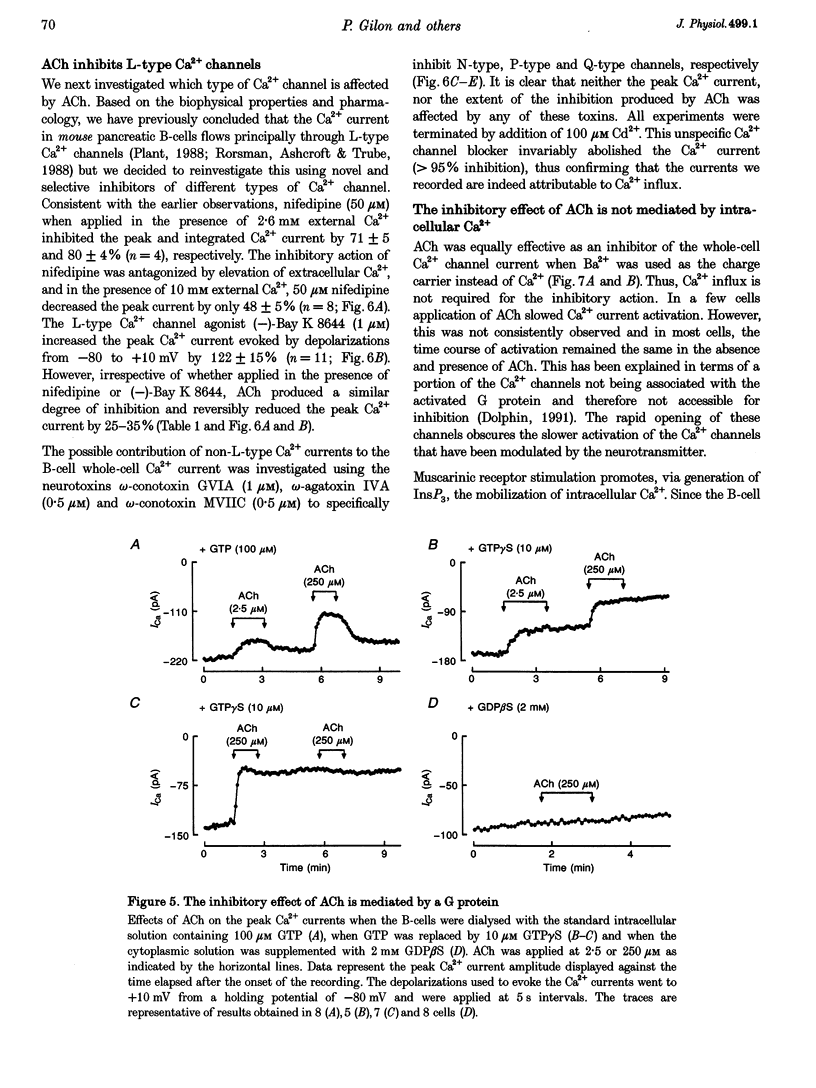
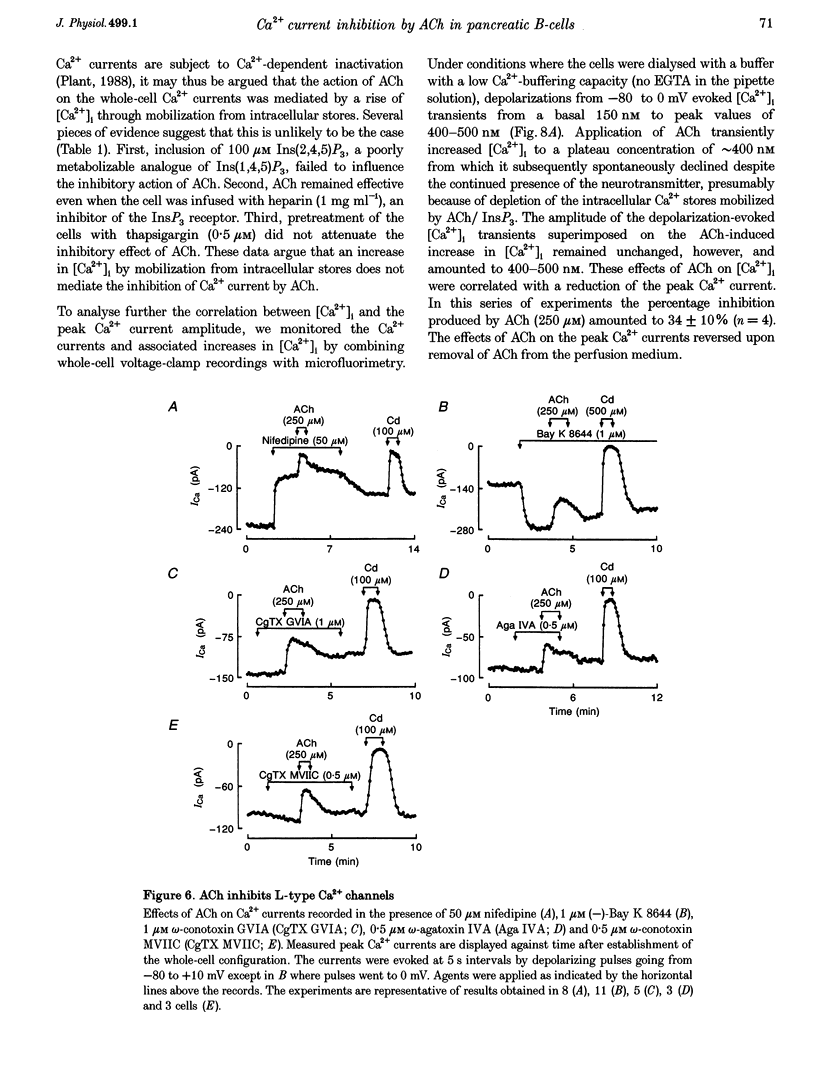
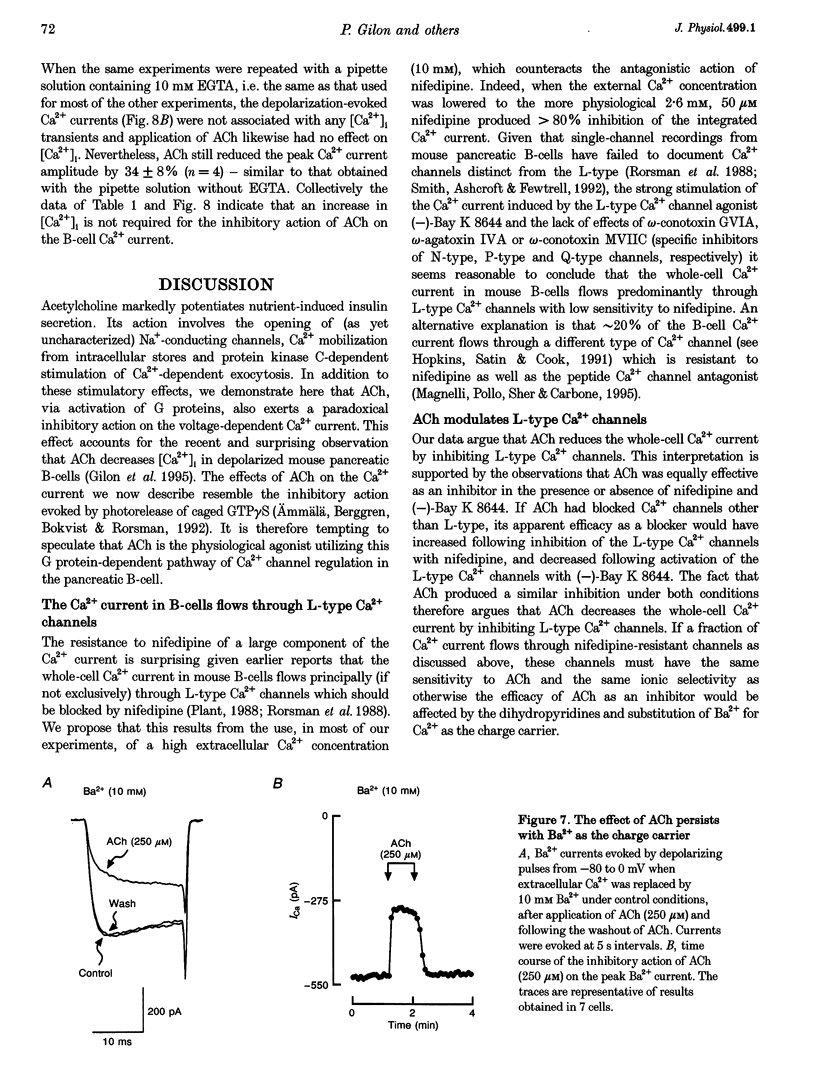
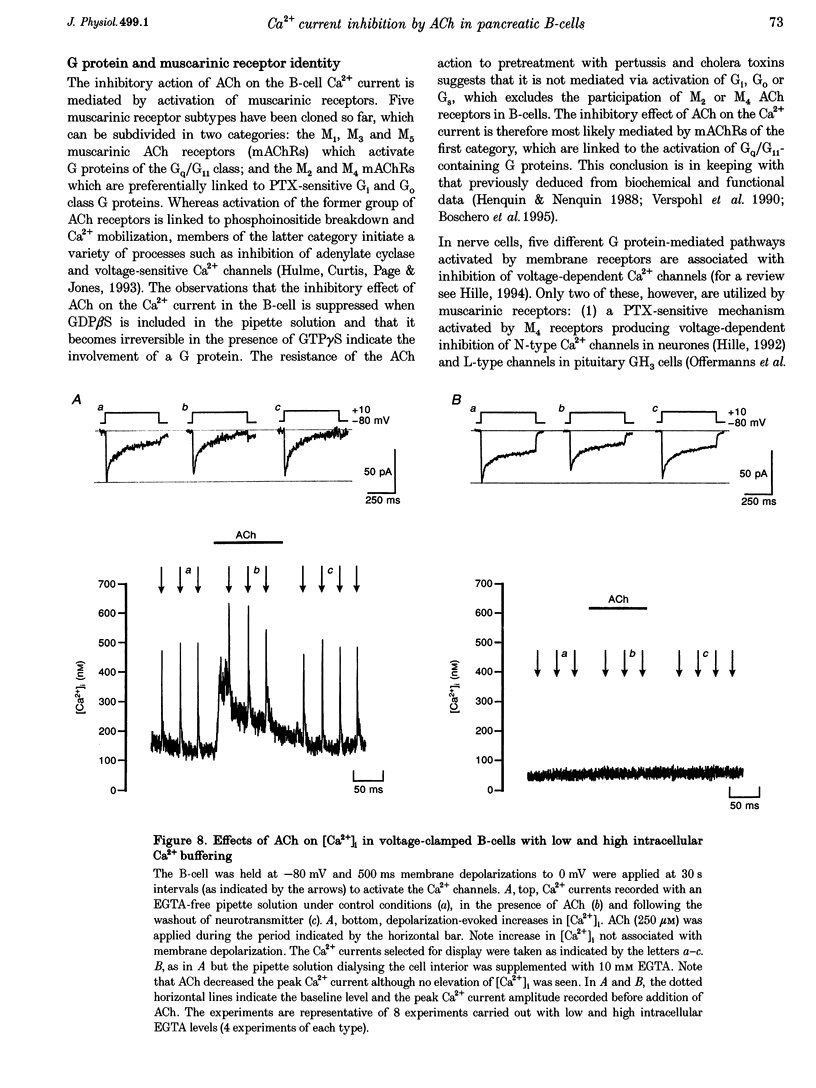
1. The effect of acetylcholine (ACh) on voltage-dependent Ca2+ currents in mouse pancreatic B-cells was studied using the whole-cell configuration of the patch-clamp technique. 2. ACh (0.25-250 microM) reversibly and dose-dependently inhibited the Ca2+ current elicited by depolarizations from -80 mV to +10 mV. Maximal inhibition was observed at concentrations > 25 microM where it amounted to approximately 35%. The effect was voltage independent and prevented by atropine (10 microM) suggesting that it was mediated by muscarinic receptors. 3. The inhibitory action of ACh on the Ca2+ current was abolished when the cytoplasmic solution contained GDP beta S (2 mM) and became irreversible when the non-hydrolysable GTP analogue GTP gamma S (10 microM) was included in the pipette. This indicates the participation of G proteins in the inhibitory effect of ACh but pretreatment of the cells with either pertussis or cholera toxin failed to prevent the effect of ACh on the Ca2+ current. 4. ACh remained equally effective as an inhibitor of the whole-cell Ca2+ current in the presence of the L-type Ca2+ channel agonist (-)-Bay K 8644 and after partial inhibition of the current by nifedipine. Addition of omega-agatoxin IVA, omega-conotoxin GVIA or omega-conotoxin MVIIC neither affected the peak Ca2+ current amplitude nor the extent of inhibition produced by ACh. These pharmacological properties indicate that ACh acts by inhibiting L-type Ca2+ channels. 5. The inhibitory action of ACh on the B-cell Ca2+ current was not secondary to elevation of [Ca2+]i and ACh remained equally effective as an inhibitor when Ba2+ was used as the charge carrier, when [Ca2+]i was buffered to low concentrations using EGTA and under experimental conditions preventing the mobilization of Ca2+ from intracellular stores. 6. These results suggest that ACh reduces the whole-cell Ca2+ current in the B-cell through a G protein-regulated, voltage- and Ca(2+)-independent inhibition of L-type Ca2+ channels.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahrén B., Taborsky G. J., Jr, Porte D., Jr Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone secretion. Diabetologia. 1986 Dec;29(12):827–836. doi: 10.1007/BF00870137. [DOI] [PubMed] [Google Scholar]
- Ammälä C., Berggren P. O., Bokvist K., Rorsman P. Inhibition of L-type calcium channels by internal GTP [gamma S] in mouse pancreatic beta cells. Pflugers Arch. 1992 Jan;420(1):72–77. doi: 10.1007/BF00378643. [DOI] [PubMed] [Google Scholar]
- Ammälä C., Eliasson L., Bokvist K., Berggren P. O., Honkanen R. E., Sjöholm A., Rorsman P. Activation of protein kinases and inhibition of protein phosphatases play a central role in the regulation of exocytosis in mouse pancreatic beta cells. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4343–4347. doi: 10.1073/pnas.91.10.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Berggren P. O., Arkhammar P., Nilsson T. Activation of protein kinase C assists insulin producing cells in recovery from raised cytoplasmic Ca2+ by stimulating Ca2+ efflux. Biochem Biophys Res Commun. 1989 Nov 30;165(1):416–421. doi: 10.1016/0006-291x(89)91086-3. [DOI] [PubMed] [Google Scholar]
- Bernheim L., Mathie A., Hille B. Characterization of muscarinic receptor subtypes inhibiting Ca2+ current and M current in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9544–9548. doi: 10.1073/pnas.89.20.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L., Abramowitz J., Brown A. M. Receptor-effector coupling by G proteins. Biochim Biophys Acta. 1990 May 7;1031(2):163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Bokvist K., Eliasson L., Ammälä C., Renström E., Rorsman P. Co-localization of L-type Ca2+ channels and insulin-containing secretory granules and its significance for the initiation of exocytosis in mouse pancreatic B-cells. EMBO J. 1995 Jan 3;14(1):50–57. doi: 10.1002/j.1460-2075.1995.tb06974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschero A. C., Szpak-Glasman M., Carneiro E. M., Bordin S., Paul I., Rojas E., Atwater I. Oxotremorine-m potentiation of glucose-induced insulin release from rat islets involves M3 muscarinic receptors. Am J Physiol. 1995 Feb;268(2 Pt 1):E336–E342. doi: 10.1152/ajpendo.1995.268.2.E336. [DOI] [PubMed] [Google Scholar]
- Brown S., Miller W. G. Determination of magnesium in serum by the technicon SMAC with a calmagite method with blank correction. Clin Chem. 1990 Nov;36(11):1990–1991. [PubMed] [Google Scholar]
- Dolphin A. C., McGuirk S. M., Scott R. H. An investigation into the mechanisms of inhibition of calcium channel currents in cultured sensory neurones of the rat by guanine nucleotide analogues and (-)-baclofen. Br J Pharmacol. 1989 May;97(1):263–273. doi: 10.1111/j.1476-5381.1989.tb11950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C. Regulation of calcium channel activity by GTP binding proteins and second messengers. Biochim Biophys Acta. 1991 Jan 10;1091(1):68–80. doi: 10.1016/0167-4889(91)90224-l. [DOI] [PubMed] [Google Scholar]
- Gagerman E., Idahl L. A., Meissner H. P., Täljedal I. B. Insulin release, cGMP, cAMP, and membrane potential in acetylcholine-stimulated islets. Am J Physiol. 1978 Nov;235(5):E493–E500. doi: 10.1152/ajpendo.1978.235.5.E493. [DOI] [PubMed] [Google Scholar]
- Gao Z. Y., Gilon P., Henquin J. C. The role of protein kinase-C in signal transduction through vasopressin and acetylcholine receptors in pancreatic B-cells from normal mouse. Endocrinology. 1994 Jul;135(1):191–199. doi: 10.1210/endo.135.1.8013353. [DOI] [PubMed] [Google Scholar]
- Garcia M. C., Hermans M. P., Henquin J. C. Glucose-, calcium- and concentration-dependence of acetylcholine stimulation of insulin release and ionic fluxes in mouse islets. Biochem J. 1988 Aug 15;254(1):211–218. doi: 10.1042/bj2540211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilon P., Nenquin M., Henquin J. C. Muscarinic stimulation exerts both stimulatory and inhibitory effects on the concentration of cytoplasmic Ca2+ in the electrically excitable pancreatic B-cell. Biochem J. 1995 Oct 1;311(Pt 1):259–267. doi: 10.1042/bj3110259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C., Garcia M. C., Bozem M., Hermans M. P., Nenquin M. Muscarinic control of pancreatic B cell function involves sodium-dependent depolarization and calcium influx. Endocrinology. 1988 May;122(5):2134–2142. doi: 10.1210/endo-122-5-2134. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Nenquin M. The muscarinic receptor subtype in mouse pancreatic B-cells. FEBS Lett. 1988 Aug 15;236(1):89–92. doi: 10.1016/0014-5793(88)80290-4. [DOI] [PubMed] [Google Scholar]
- Hille B. G protein-coupled mechanisms and nervous signaling. Neuron. 1992 Aug;9(2):187–195. doi: 10.1016/0896-6273(92)90158-a. [DOI] [PubMed] [Google Scholar]
- Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994 Dec;17(12):531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Homaidan F. R., Sharp G. W., Nowak L. M. Galanin inhibits a dihydropyridine-sensitive Ca2+ current in the RINm5f cell line. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8744–8748. doi: 10.1073/pnas.88.19.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins W. F., Satin L. S., Cook D. L. Inactivation kinetics and pharmacology distinguish two calcium currents in mouse pancreatic B-cells. J Membr Biol. 1991 Feb;119(3):229–239. doi: 10.1007/BF01868728. [DOI] [PubMed] [Google Scholar]
- Hsu W. H., Xiang H. D., Rajan A. S., Kunze D. L., Boyd A. E., 3rd Somatostatin inhibits insulin secretion by a G-protein-mediated decrease in Ca2+ entry through voltage-dependent Ca2+ channels in the beta cell. J Biol Chem. 1991 Jan 15;266(2):837–843. [PubMed] [Google Scholar]
- Hulme E. C., Curtis C. A., Page K. M., Jones P. G. Agonist activation of muscarinic acetylcholine receptors. Cell Signal. 1993 Nov;5(6):687–694. doi: 10.1016/0898-6568(93)90030-p. [DOI] [PubMed] [Google Scholar]
- Jones S. V. Muscarinic receptor subtypes: modulation of ion channels. Life Sci. 1993;52(5-6):457–464. doi: 10.1016/0024-3205(93)90302-j. [DOI] [PubMed] [Google Scholar]
- Keahey H. H., Boyd A. E., 3rd, Kunze D. L. Catecholamine modulation of calcium currents in clonal pancreatic beta-cells. Am J Physiol. 1989 Dec;257(6 Pt 1):C1171–C1176. doi: 10.1152/ajpcell.1989.257.6.C1171. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Kaczmarek L. K., Levitan E. S. Neuropeptide inhibition of voltage-gated calcium channels mediated by mobilization of intracellular calcium. Neuron. 1991 Apr;6(4):557–563. doi: 10.1016/0896-6273(91)90058-8. [DOI] [PubMed] [Google Scholar]
- Magnelli V., Pollo A., Sher E., Carbone E. Block of non-L-, non-N-type Ca2+ channels in rat insulinoma RINm5F cells by omega-agatoxin IVA and omega-conotoxin MVIIC. Pflugers Arch. 1995 Apr;429(6):762–771. doi: 10.1007/BF00374799. [DOI] [PubMed] [Google Scholar]
- Mathie A., Bernheim L., Hille B. Inhibition of N- and L-type calcium channels by muscarinic receptor activation in rat sympathetic neurons. Neuron. 1992 May;8(5):907–914. doi: 10.1016/0896-6273(92)90205-r. [DOI] [PubMed] [Google Scholar]
- Offermanns S., Gollasch M., Hescheler J., Spicher K., Schmidt A., Schultz G., Rosenthal W. Inhibition of voltage-dependent Ca2+ currents and activation of pertussis toxin-sensitive G-proteins via muscarinic receptors in GH3 cells. Mol Endocrinol. 1991 Jul;5(7):995–1002. doi: 10.1210/mend-5-7-995. [DOI] [PubMed] [Google Scholar]
- Plant T. D. Properties and calcium-dependent inactivation of calcium currents in cultured mouse pancreatic B-cells. J Physiol. 1988 Oct;404:731–747. doi: 10.1113/jphysiol.1988.sp017316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Zawalich K. C., Ganesan S., Calle R., Zawalich W. S. Physiology and pathophysiology of insulin secretion. Diabetes Care. 1990 Jun;13(6):655–666. doi: 10.2337/diacare.13.6.655. [DOI] [PubMed] [Google Scholar]
- Renström E., Ding W. G., Bokvist K., Rorsman P. Neurotransmitter-induced inhibition of exocytosis in insulin-secreting beta cells by activation of calcineurin. Neuron. 1996 Sep;17(3):513–522. doi: 10.1016/s0896-6273(00)80183-x. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Ashcroft F. M., Trube G. Single Ca channel currents in mouse pancreatic B-cells. Pflugers Arch. 1988 Oct;412(6):597–603. doi: 10.1007/BF00583760. [DOI] [PubMed] [Google Scholar]
- Smith P. A., Aschroft F. M., Fewtrell C. M. Permeation and gating properties of the L-type calcium channel in mouse pancreatic beta cells. J Gen Physiol. 1993 May;101(5):767–797. doi: 10.1085/jgp.101.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Andrés J. V., Ripoll C., Soria B. Evidence that muscarinic potentiation of insulin release is initiated by an early transient calcium entry. FEBS Lett. 1988 Apr 11;231(1):143–147. doi: 10.1016/0014-5793(88)80719-1. [DOI] [PubMed] [Google Scholar]
- Velasco J. M., Petersen O. H. The effect of a cell-permeable diacylglycerol analogue on single Ca2+ (Ba2+) channel currents in the insulin-secreting cell line RINm5F. Q J Exp Physiol. 1989 May;74(3):367–370. doi: 10.1113/expphysiol.1989.sp003280. [DOI] [PubMed] [Google Scholar]
- Verspohl E. J., Tacke R., Mutschler E., Lambrecht G. Muscarinic receptor subtypes in rat pancreatic islets: binding and functional studies. Eur J Pharmacol. 1990 Mar 27;178(3):303–311. doi: 10.1016/0014-2999(90)90109-j. [DOI] [PubMed] [Google Scholar]
- Yada T., Hamakawa N., Yaekura K. Two distinct modes of Ca2+ signalling by ACh in rat pancreatic beta-cells: concentration, glucose dependence and Ca2+ origin. J Physiol. 1995 Oct 1;488(Pt 1):13–24. doi: 10.1113/jphysiol.1995.sp020942. [DOI] [PMC free article] [PubMed] [Google Scholar]


