Abstract
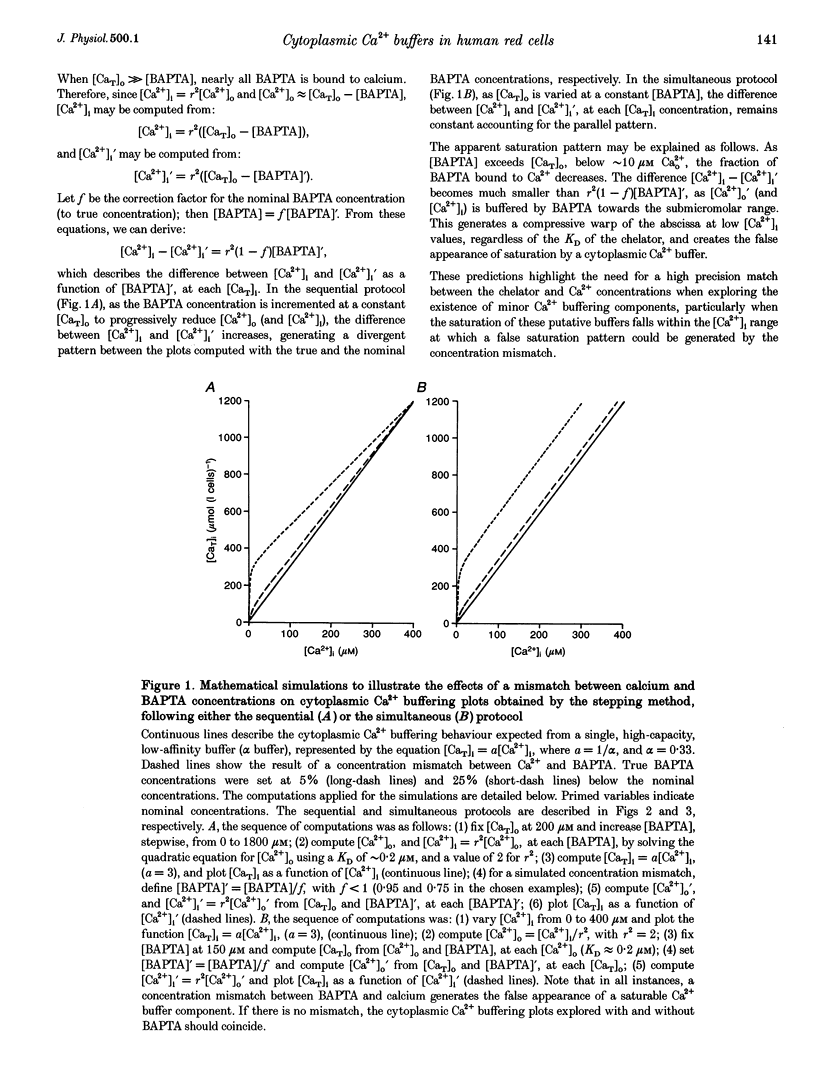
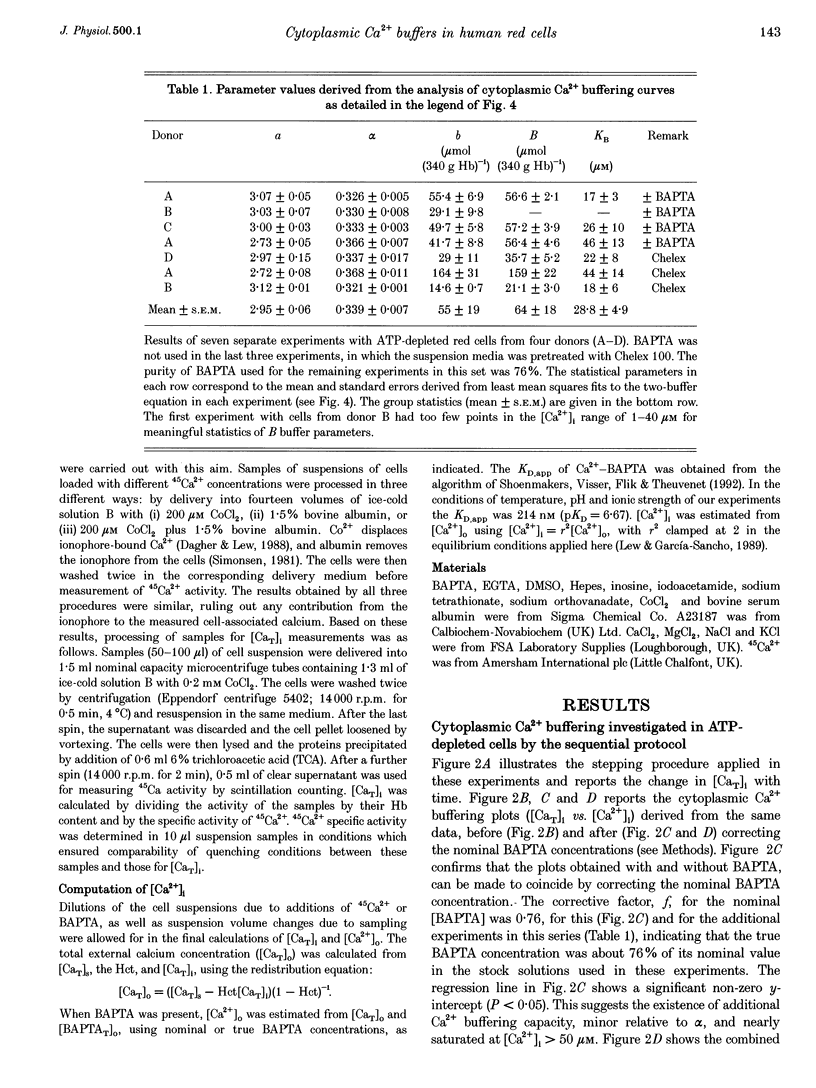
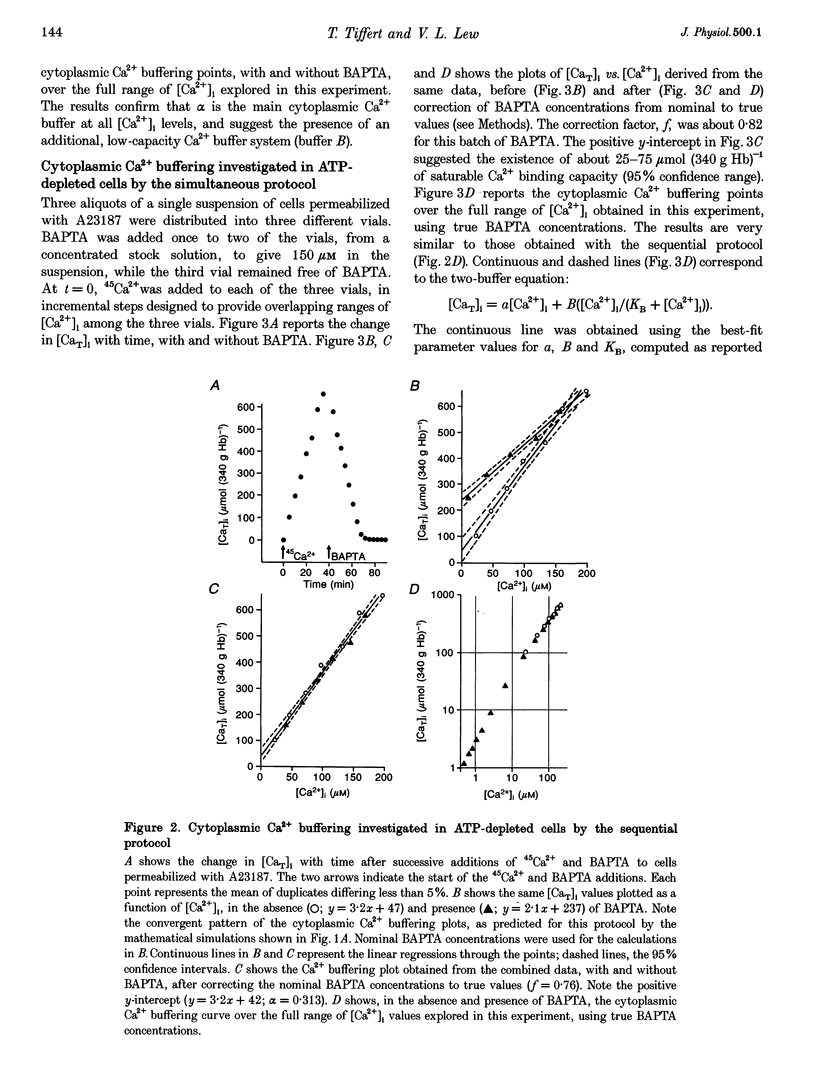
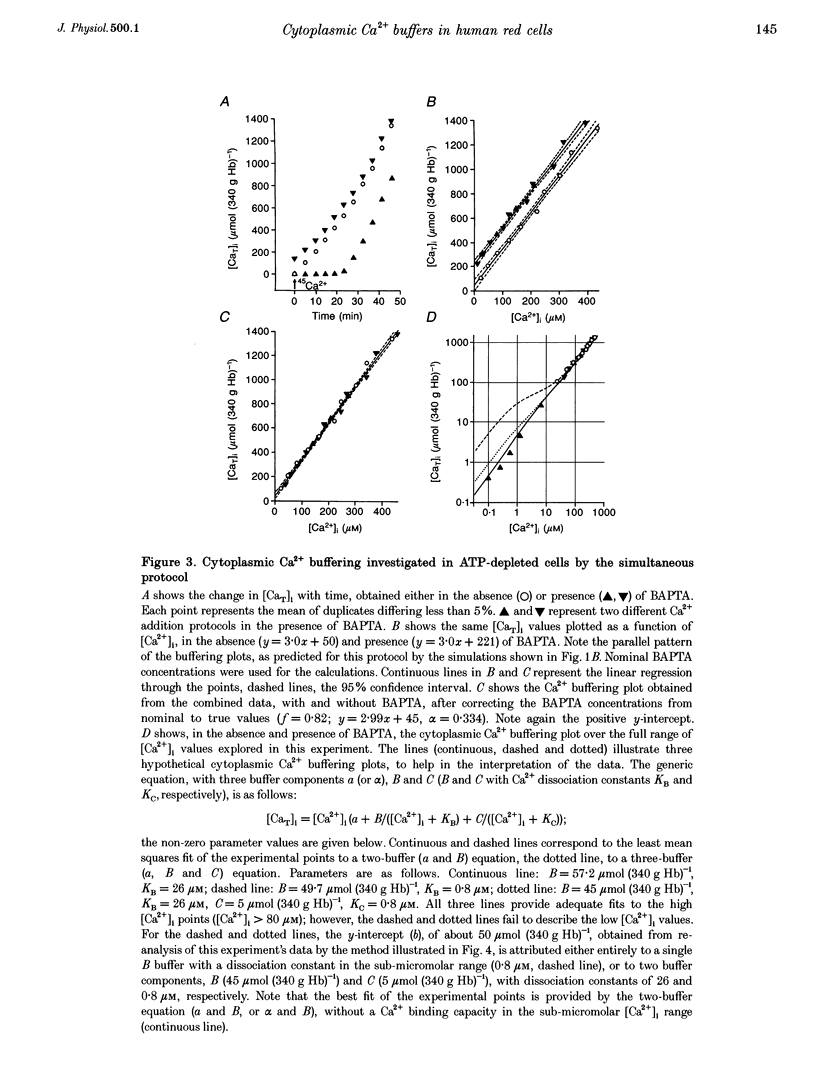
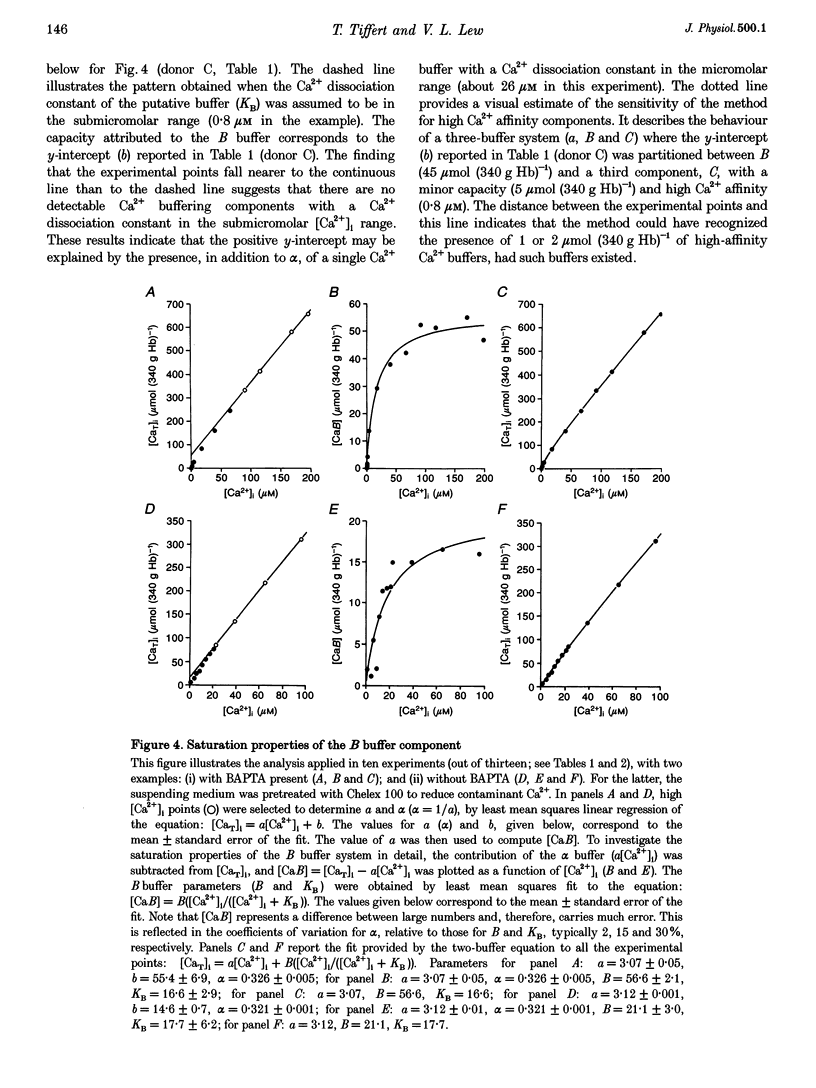
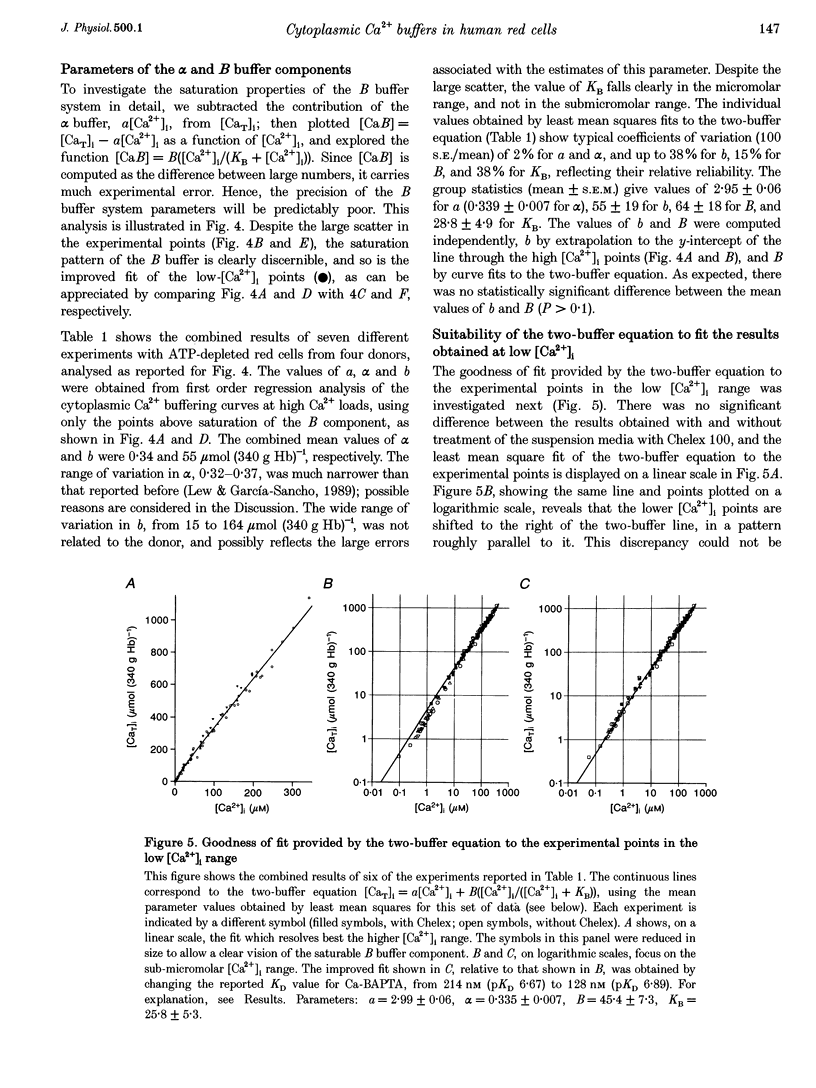
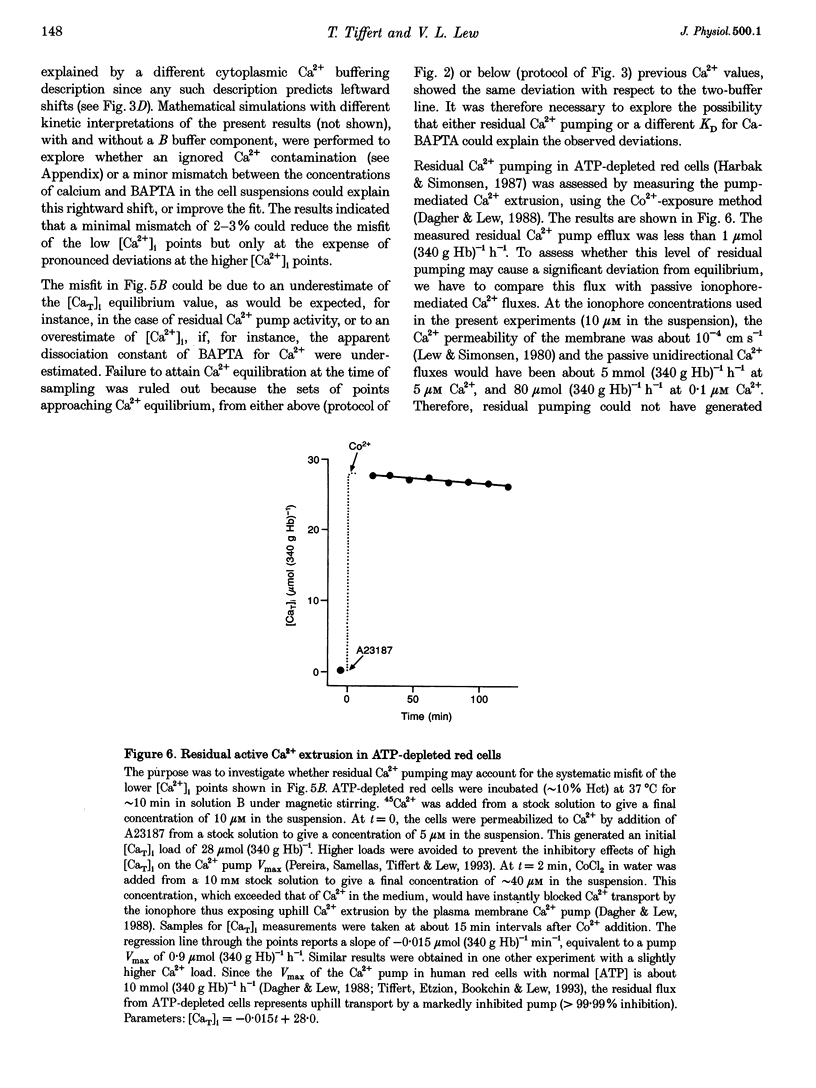
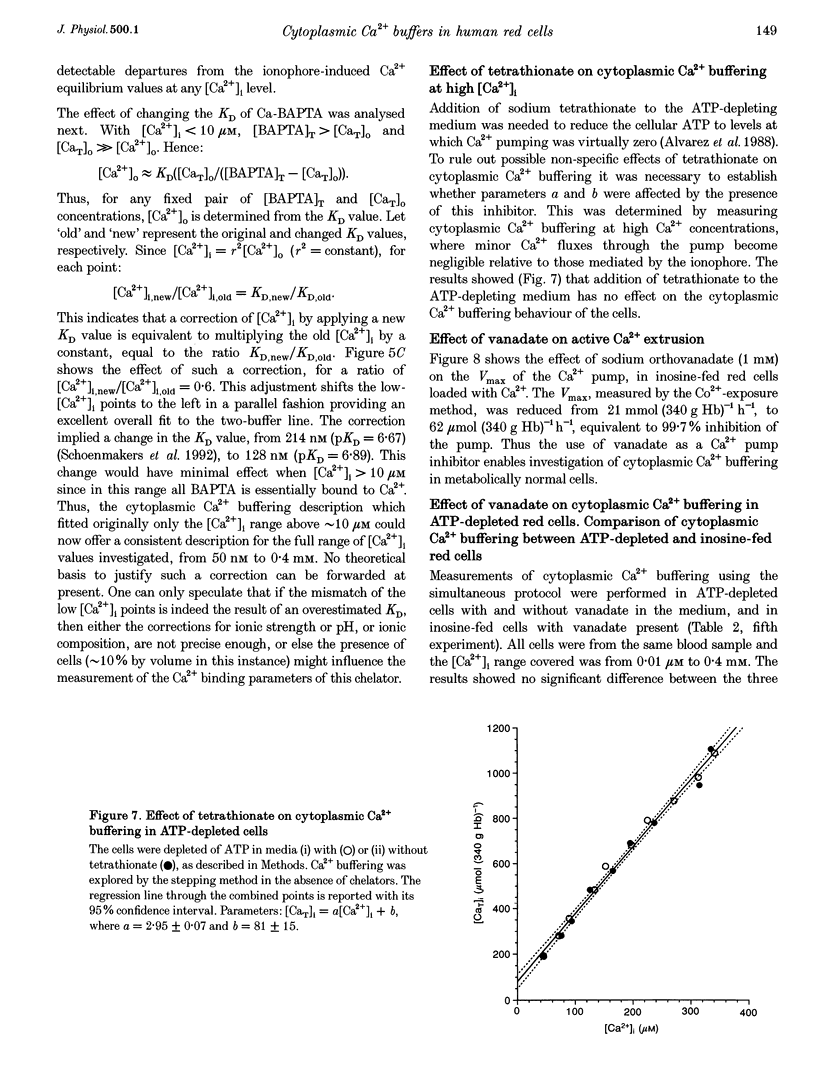
1. Precise knowledge of the cytoplasmic Ca2+ buffering behaviour in intact human red cells is essential for the characterization of their [Ca2+]i-dependent functions. This was investigated by using a refined method and experimental protocols which allowed continuity in the estimates of [Ca2+]i, from nanomolar to millimolar concentrations, in the presence and absence of external Ca2+ chelators. 2. The study was carried out in human red cells whose plasma membrane Ca2+ pump was inhibited either by depleting the cells of ATP or by adding vanadate to the cell suspension. Cytoplasmic Ca2+ buffering was analysed from plots of total cell calcium content vs. ionized cytoplasmic Ca2+ concentration ([CaT]i vs. [Ca2+]i) obtained from measurements of the equilibrium distribution of 45Ca2+ at different external Ca2+ concentrations ([Ca2+]o), in conditions known to clamp cell volume and pH. The equilibrium distribution of 45Ca2+ was induced by the divalent cation ionophore A23187. 3. The results showed the following. (i) The known red cell Ca2+ buffer represented by alpha, with a large capacity and low Ca2+ affinity, was the main cytoplasmic Ca2+ binding agent. (ii) The value of alpha was remarkably constant; the means for each of four donors ranged from 0.33 to 0.35, with a combined value of all independent measurements of 0.34 +/- 0.01 (mean +/- S.E.M., n = 16). This contrasts with the variability previously reported. (iii) There was an additional Ca2+ buffering complex with a low capacity (approximately 80 micromol (340 g Hb)(-1)) and intermediate Ca2+ affinity (apparent dissociation constant, K(D,app) approximately 4-50 microM) whose possible identity is discussed. (iv) The cell content of putative Ca2+ buffers with submicromolar Ca2+ dissociation constants was below the detection limit of the methods used here (less than 2 micromol (340 g Hb)(-1)). 4. Vanadate (1 mM) inhibited the Vmax of the Ca2+ pump in inosine-fed cells by 99.7%. The cytoplasmic Ca2+ buffering behaviour in these cells was similar to that found in ATP-depleted cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez J., García-Sancho J., Herreros B. All or none cell responses of Ca2+-dependent K channels elicited by calcium or lead in human red cells can be explained by heterogeneity of agonist distribution. J Membr Biol. 1988 Sep;104(2):129–138. doi: 10.1007/BF01870925. [DOI] [PubMed] [Google Scholar]
- Babu Y. S., Bugg C. E., Cook W. J. Structure of calmodulin refined at 2.2 A resolution. J Mol Biol. 1988 Nov 5;204(1):191–204. doi: 10.1016/0022-2836(88)90608-0. [DOI] [PubMed] [Google Scholar]
- Bookchin R. M., Lew V. L. Progressive inhibition of the Ca pump and Ca:Ca exchange in sickle red cells. Nature. 1980 Apr 10;284(5756):561–563. doi: 10.1038/284561a0. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya R., Meador W. E., Means A. R., Quiocho F. A. Calmodulin structure refined at 1.7 A resolution. J Mol Biol. 1992 Dec 20;228(4):1177–1192. doi: 10.1016/0022-2836(92)90324-d. [DOI] [PubMed] [Google Scholar]
- Dagher G., Lew V. L. Maximal calcium extrusion capacity and stoichiometry of the human red cell calcium pump. J Physiol. 1988 Dec;407:569–586. doi: 10.1113/jphysiol.1988.sp017432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers R. J. Notification of final adoption of an international method and standard solution for hemoglobinometry specifications for preparation of standard solution. Am J Clin Pathol. 1967 Feb;47(2):212–214. doi: 10.1093/ajcp/47.2.212. [DOI] [PubMed] [Google Scholar]
- Engelmann B., Duhm J. Intracellular calcium content of human erythrocytes: relation to sodium transport systems. J Membr Biol. 1987;98(1):79–87. doi: 10.1007/BF01871047. [DOI] [PubMed] [Google Scholar]
- Ferreira H. G., Lew V. L. Use of ionophore A23187 to measure cytoplasmic Ca buffering and activation of the Ca pump by internal Ca. Nature. 1976 Jan 1;259(5538):47–49. doi: 10.1038/259047a0. [DOI] [PubMed] [Google Scholar]
- GARDOS G. The function of calcium in the potassium permeability of human erythrocytes. Biochim Biophys Acta. 1958 Dec;30(3):653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- Glaser T., Schwarz-Benmeir N., Barnoy S., Barak S., Eshhar Z., Kosower N. S. Calpain (Ca(2+)-dependent thiol protease) in erythrocytes of young and old individuals. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):7879–7883. doi: 10.1073/pnas.91.17.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. G., Long C. The calcium content of human erythrocytes. J Physiol. 1968 Dec;199(2):367–381. doi: 10.1113/jphysiol.1968.sp008658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. M., Bers D. M. The effect of temperature and ionic strength on the apparent Ca-affinity of EGTA and the analogous Ca-chelators BAPTA and dibromo-BAPTA. Biochim Biophys Acta. 1987 Aug 13;925(2):133–143. doi: 10.1016/0304-4165(87)90102-4. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Bookchin R. M. Role of reticulocyte transport heterogeneity in the generation of mature sickle cells with different volumes. Biochem Soc Trans. 1992 Nov;20(4):797–800. doi: 10.1042/bst0200797. [DOI] [PubMed] [Google Scholar]
- Lew V. L., García-Sancho J. Measurement and control of intracellular calcium in intact red cells. Methods Enzymol. 1989;173:100–112. doi: 10.1016/s0076-6879(89)73008-1. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Hockaday A., Sepulveda M. I., Somlyo A. P., Somlyo A. V., Ortiz O. E., Bookchin R. M. Compartmentalization of sickle-cell calcium in endocytic inside-out vesicles. Nature. 1985 Jun 13;315(6020):586–589. doi: 10.1038/315586a0. [DOI] [PubMed] [Google Scholar]
- Lew V. L. On the ATP dependence of the Ca 2+ -induced increase in K + permeability observed in human red cells. Biochim Biophys Acta. 1971 Jun 1;233(3):827–830. doi: 10.1016/0005-2736(71)90185-4. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Tsien R. Y., Miner C., Bookchin R. M. Physiological [Ca2+]i level and pump-leak turnover in intact red cells measured using an incorporated Ca chelator. Nature. 1982 Jul 29;298(5873):478–481. doi: 10.1038/298478a0. [DOI] [PubMed] [Google Scholar]
- Linse S., Helmersson A., Forsén S. Calcium binding to calmodulin and its globular domains. J Biol Chem. 1991 May 5;266(13):8050–8054. [PubMed] [Google Scholar]
- Lombardo C. R., Low P. S. Calmodulin modulates protein 4.1 binding to human erythrocyte membranes. Biochim Biophys Acta. 1994 Dec 30;1196(2):139–144. doi: 10.1016/0005-2736(94)00233-9. [DOI] [PubMed] [Google Scholar]
- Moore R. B., Mankad M. V., Shriver S. K., Mankad V. N., Plishker G. A. Reconstitution of Ca(2+)-dependent K+ transport in erythrocyte membrane vesicles requires a cytoplasmic protein. J Biol Chem. 1991 Oct 5;266(28):18964–18968. [PubMed] [Google Scholar]
- Moore R. B., Plishker G. A., Shriver S. K. Purification and measurement of calpromotin, the cytoplasmic protein which activates calcium-dependent potassium transport. Biochem Biophys Res Commun. 1990 Jan 15;166(1):146–153. doi: 10.1016/0006-291x(90)91923-g. [DOI] [PubMed] [Google Scholar]
- Olwin B. B., Edelman A. M., Krebs E. G., Storm D. R. Quantitation of energy coupling between Ca2+, calmodulin, skeletal muscle myosin light chain kinase, and kinase substrates. J Biol Chem. 1984 Sep 10;259(17):10949–10955. [PubMed] [Google Scholar]
- Pereira A. C., Samellas D., Tiffert T., Lew V. L. Inhibition of the calcium pump by high cytosolic Ca2+ in intact human red blood cells. J Physiol. 1993 Feb;461:63–73. doi: 10.1113/jphysiol.1993.sp019501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plishker G. A., Chevalier D., Seinsoth L., Moore R. B. Calcium-activated potassium transport and high molecular weight forms of calpromotin. J Biol Chem. 1992 Oct 25;267(30):21839–21843. [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Scharff O., Foder B. Delayed activation of calcium pump during transient increases in cellular Ca2+ concentration and K+ conductance in hyperpolarizing human red cells. Biochim Biophys Acta. 1986 Oct 23;861(3):471–479. doi: 10.1016/0005-2736(86)90456-6. [DOI] [PubMed] [Google Scholar]
- Scharff O., Foder B. Reversible shift between two states of Ca2+-ATPase in human erythrocytes mediated by Ca2+ and a membrane-bound activator. Biochim Biophys Acta. 1978 May 4;509(1):67–77. doi: 10.1016/0005-2736(78)90008-1. [DOI] [PubMed] [Google Scholar]
- Scharff O. Kinetics of calmodulin-dependent (Ca2+ + Mg2+)-ATPase in plasma membranes and solubilized membranes from erythrocytes. Arch Biochem Biophys. 1981 Jun;209(1):72–80. doi: 10.1016/0003-9861(81)90258-7. [DOI] [PubMed] [Google Scholar]
- Schoenmakers T. J., Visser G. J., Flik G., Theuvenet A. P. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques. 1992 Jun;12(6):870-4, 876-9. [PubMed] [Google Scholar]
- Schwarz-Benmeir N., Glaser T., Barnoy S., Kosower N. S. Calpastatin in erythrocytes of young and old individuals. Biochem J. 1994 Dec 1;304(Pt 2):365–370. doi: 10.1042/bj3040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons T. J. A method for estimating free Ca within human red blood cells, with an application to the study of their Ca-dependent K permeability. J Membr Biol. 1982;66(3):235–247. doi: 10.1007/BF01868498. [DOI] [PubMed] [Google Scholar]
- Tiffert T., Etzion Z., Bookchin R. M., Lew V. L. Effects of deoxygenation on active and passive Ca2+ transport and cytoplasmic Ca2+ buffering in normal human red cells. J Physiol. 1993 May;464:529–544. doi: 10.1113/jphysiol.1993.sp019649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffert T., Spivak J. L., Lew V. L. Magnitude of calcium influx required to induce dehydration of normal human red cells. Biochim Biophys Acta. 1988 Aug 18;943(2):157–165. doi: 10.1016/0005-2736(88)90547-0. [DOI] [PubMed] [Google Scholar]
- Varecka L., Carafoli E. Vanadate-induced movements of Ca2+ and K+ in human red blood cells. J Biol Chem. 1982 Jul 10;257(13):7414–7421. [PubMed] [Google Scholar]
- Williamson P., Puchulu E., Penniston J. T., Westerman M. P., Schlegel R. A. Ca2+ accumulation and loss by aberrant endocytic vesicles in sickle erythrocytes. J Cell Physiol. 1992 Jul;152(1):1–9. doi: 10.1002/jcp.1041520102. [DOI] [PubMed] [Google Scholar]


