Abstract
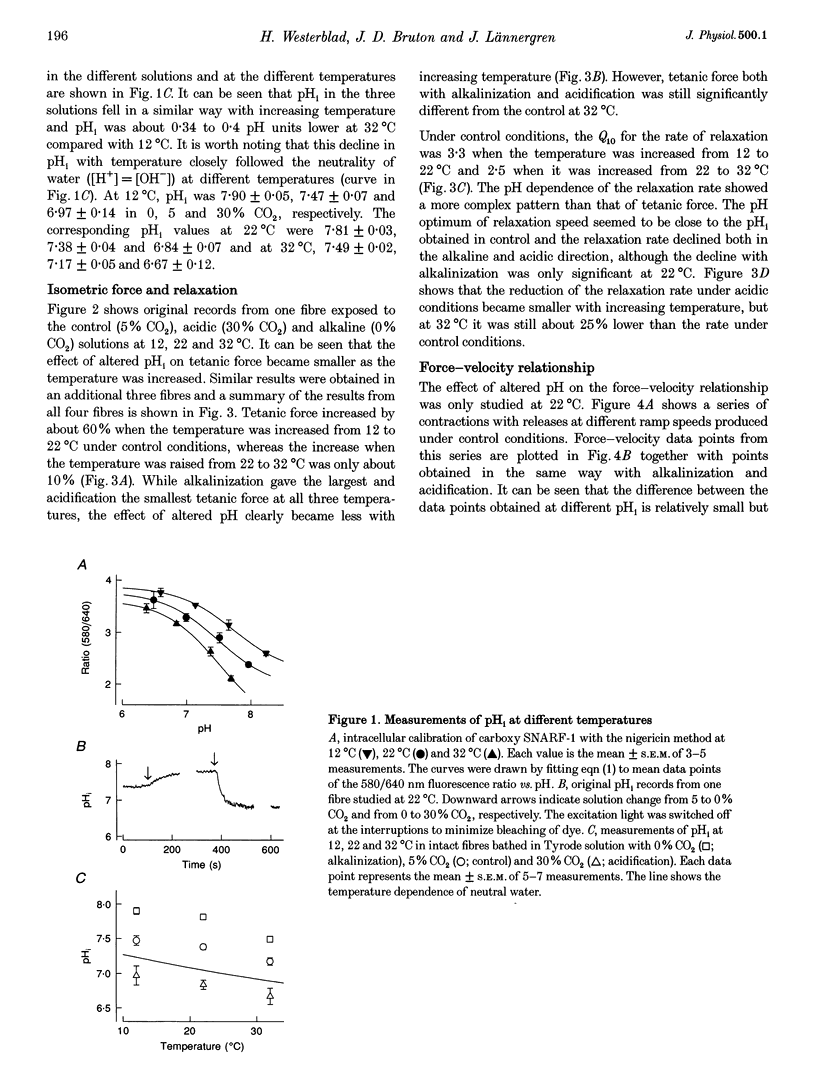
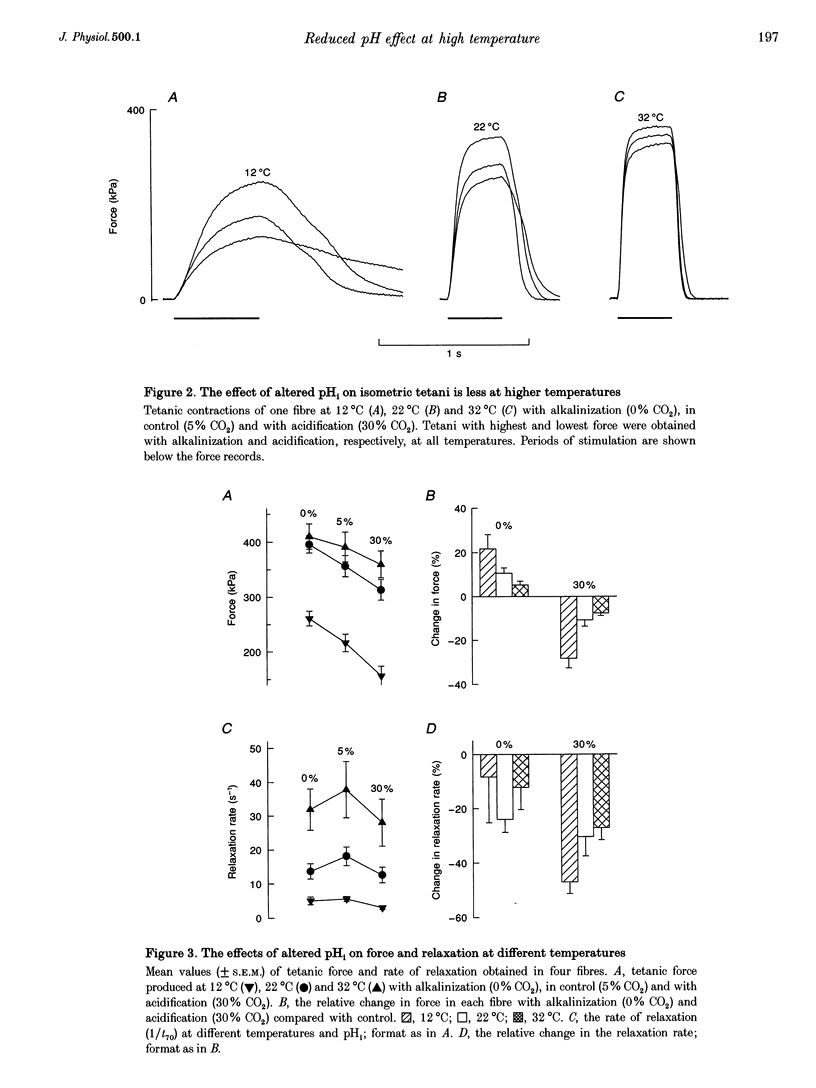
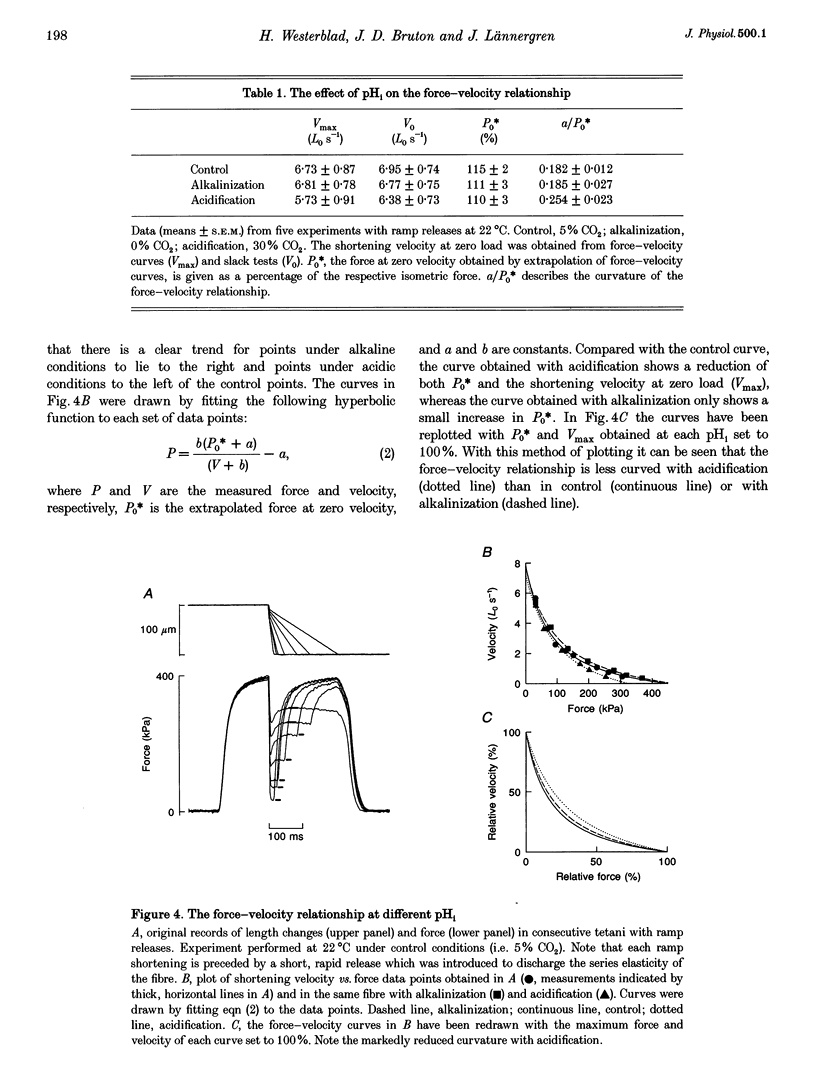
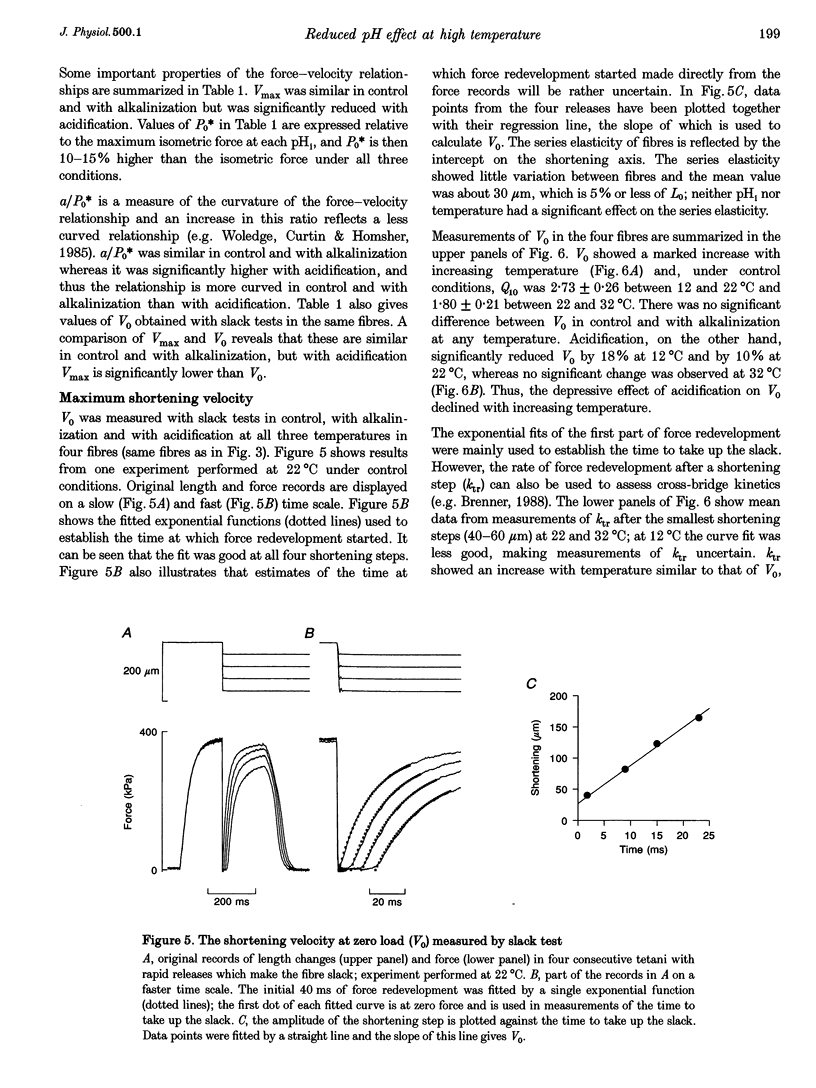
1. The effect of altered intracellular pH (pHi) on isometric contractions and shortening velocity at 12, 22 and 32 degrees C was studied in intact, single fibres of mouse skeletal muscle. Changes in pHi were obtained by exposing fibres to solutions with different CO2 concentrations. 2. Under control conditions (5% CO2), pHi (measured with carboxy SNARF-1) was about 0.3 pH units more alkaline than neutral water at each temperature. An acidification of about 0.5 pH units was produced by 30% CO2 and an alkalinization of similar size by 0% CO2. 3. In acidified fibres tetanic force was reduced by 28% at 12 degrees C but only by 10% at 32 degrees C. The force increase with alkalinization showed a similar reduction with increasing temperature. Acidification caused a marked slowing of relaxation and this slowing became less with increasing temperature. 4. Acidification reduced the maximum shortening velocity (V0) by almost 20% at 12 degrees C, but had no significant effect at 32 degrees C. Alkalinization had no significant effect on V0 at any temperature. 5. In conclusion, the effect of pHi on contraction of mammalian muscle declines markedly with increasing temperature. Thus, the direct inhibition of force production by acidification is not a major factor in muscle fatigue at physiological temperatures.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. R., Fisher M. J., Meyer R. A. Hypercapnic acidosis and increased H2PO4- concentration do not decrease force in cat skeletal muscle. Am J Physiol. 1991 Apr;260(4 Pt 1):C805–C812. doi: 10.1152/ajpcell.1991.260.4.C805. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Lännergren J., Westerblad H. Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Exp Physiol. 1995 Jul;80(4):497–527. doi: 10.1113/expphysiol.1995.sp003864. [DOI] [PubMed] [Google Scholar]
- Baker A. J., Brandes R., Weiner M. W. Effects of intracellular acidosis on Ca2+ activation, contraction, and relaxation of frog skeletal muscle. Am J Physiol. 1995 Jan;268(1 Pt 1):C55–C63. doi: 10.1152/ajpcell.1995.268.1.C55. [DOI] [PubMed] [Google Scholar]
- Barclay C. J., Constable J. K., Gibbs C. L. Energetics of fast- and slow-twitch muscles of the mouse. J Physiol. 1993 Dec;472:61–80. doi: 10.1113/jphysiol.1993.sp019937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank P. S., Silverman H. S., Chung O. Y., Hogue B. A., Stern M. D., Hansford R. G., Lakatta E. G., Capogrossi M. C. Cytosolic pH measurements in single cardiac myocytes using carboxy-seminaphthorhodafluor-1. Am J Physiol. 1992 Jul;263(1 Pt 2):H276–H284. doi: 10.1152/ajpheart.1992.263.1.H276. [DOI] [PubMed] [Google Scholar]
- Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci U S A. 1988 May;85(9):3265–3269. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci U S A. 1986 May;83(10):3542–3546. doi: 10.1073/pnas.83.10.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Application of a new pH-sensitive fluoroprobe (carboxy-SNARF-1) for intracellular pH measurement in small, isolated cells. Pflugers Arch. 1990 Oct;417(2):234–239. doi: 10.1007/BF00370705. [DOI] [PubMed] [Google Scholar]
- Cady E. B., Jones D. A., Lynn J., Newham D. J. Changes in force and intracellular metabolites during fatigue of human skeletal muscle. J Physiol. 1989 Nov;418:311–325. doi: 10.1113/jphysiol.1989.sp017842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase P. B., Kushmerick M. J. Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophys J. 1988 Jun;53(6):935–946. doi: 10.1016/S0006-3495(88)83174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Franks K., Luciani G. B., Pate E. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J Physiol. 1988 Jan;395:77–97. doi: 10.1113/jphysiol.1988.sp016909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A., Edman K. A. Force-velocity relation for frog muscle fibres: effects of moderate fatigue and of intracellular acidification. J Physiol. 1994 Mar 15;475(3):483–494. doi: 10.1113/jphysiol.1994.sp020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson S. K., Hermansen L., Bolles L. Differential, direct effects of H+ on Ca2+ -activated force of skinned fibers from the soleus, cardiac and adductor magnus muscles of rabbits. Pflugers Arch. 1978 Aug 25;376(1):55–65. doi: 10.1007/BF00585248. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Mulieri L. A., Scubon-Mulieri B. Non-hyperbolic force-velocity relationship in single muscle fibres. Acta Physiol Scand. 1976 Oct;98(2):143–156. doi: 10.1111/j.1748-1716.1976.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M. A., Goldman Y. E., Simmons R. M. The dependence of force and shortening velocity on substrate concentration in skinned muscle fibres from Rana temporaria. J Physiol. 1984 May;350:519–543. doi: 10.1113/jphysiol.1984.sp015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts R. H. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994 Jan;74(1):49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Good N. E., Izawa S. Hydrogen ion buffers. Methods Enzymol. 1972;24:53–68. doi: 10.1016/0076-6879(72)24054-x. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Rapid 'give' and the tension 'shoulder' in the relaxation of frog muscle fibres. J Physiol. 1970 Sep;210(1):32P–33P. [PubMed] [Google Scholar]
- Julian F. J., Rome L. C., Stephenson D. G., Striz S. The maximum speed of shortening in living and skinned frog muscle fibres. J Physiol. 1986 Jan;370:181–199. doi: 10.1113/jphysiol.1986.sp015929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luff A. R. Dynamic properties of the inferior rectus, extensor digitorum longus, diaphragm and soleus muscles of the mouse. J Physiol. 1981;313:161–171. doi: 10.1113/jphysiol.1981.sp013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J., Lindblom P., Johansson B. Contractile properties of two varieties of twitch muscle fibres in Xenopus laevis. Acta Physiol Scand. 1982 Apr;114(4):523–535. doi: 10.1111/j.1748-1716.1982.tb07020.x. [DOI] [PubMed] [Google Scholar]
- Lännergren J. The force-velocity relation of isolated twitch and slow muscle fibres of Xenopus laevis. J Physiol. 1978 Oct;283:501–521. doi: 10.1113/jphysiol.1978.sp012516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J., Westerblad H. The temperature dependence of isometric contractions of single, intact fibres dissected from a mouse foot muscle. J Physiol. 1987 Sep;390:285–293. doi: 10.1113/jphysiol.1987.sp016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. pH modulation of the kinetics of a Ca2(+)-sensitive cross-bridge state transition in mammalian single skeletal muscle fibres. J Physiol. 1990 Sep;428:751–764. doi: 10.1113/jphysiol.1990.sp018239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. G., Boska M. D., Moussavi R. S., Carson P. J., Weiner M. W. 31P nuclear magnetic resonance studies of high energy phosphates and pH in human muscle fatigue. Comparison of aerobic and anaerobic exercise. J Clin Invest. 1988 Apr;81(4):1190–1196. doi: 10.1172/JCI113434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate E., Bhimani M., Franks-Skiba K., Cooke R. Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue. J Physiol. 1995 Aug 1;486(Pt 3):689–694. doi: 10.1113/jphysiol.1995.sp020844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga K. W. The force-velocity relation of rat fast- and slow-twitch muscles examined at different temperatures. J Physiol. 1984 Jun;351:517–529. doi: 10.1113/jphysiol.1984.sp015260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud J. M., Allard Y., Mainwood G. W. Is the change in intracellular pH during fatigue large enough to be the main cause of fatigue? Can J Physiol Pharmacol. 1986 Jun;64(6):764–767. doi: 10.1139/y86-130. [DOI] [PubMed] [Google Scholar]
- Sahlin K., Ren J. M. Relationship of contraction capacity to metabolic changes during recovery from a fatiguing contraction. J Appl Physiol (1985) 1989 Aug;67(2):648–654. doi: 10.1152/jappl.1989.67.2.648. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Pettit A. I., Davies N. W., Stanfield P. R. Activation of ATP-dependent K+ currents in intact skeletal muscle fibres by reduced intracellular pH. Proc Biol Sci. 1992 Mar 23;247(1320):195–198. doi: 10.1098/rspb.1992.0028. [DOI] [PubMed] [Google Scholar]
- Stevens E. D., Godt R. E. Effects of temperature and concomitant change in pH on muscle. Am J Physiol. 1990 Aug;259(2 Pt 2):R204–R209. doi: 10.1152/ajpregu.1990.259.2.R204. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Trivedi B., Danforth W. H. Effect of pH on the kinetics of frog muscle phosphofructokinase. J Biol Chem. 1966 Sep 10;241(17):4110–4112. [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. Changes of intracellular pH due to repetitive stimulation of single fibres from mouse skeletal muscle. J Physiol. 1992 Apr;449:49–71. doi: 10.1113/jphysiol.1992.sp019074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. The influence of intracellular pH on contraction, relaxation and [Ca2+]i in intact single fibres from mouse muscle. J Physiol. 1993 Jul;466:611–628. [PMC free article] [PubMed] [Google Scholar]


