Abstract
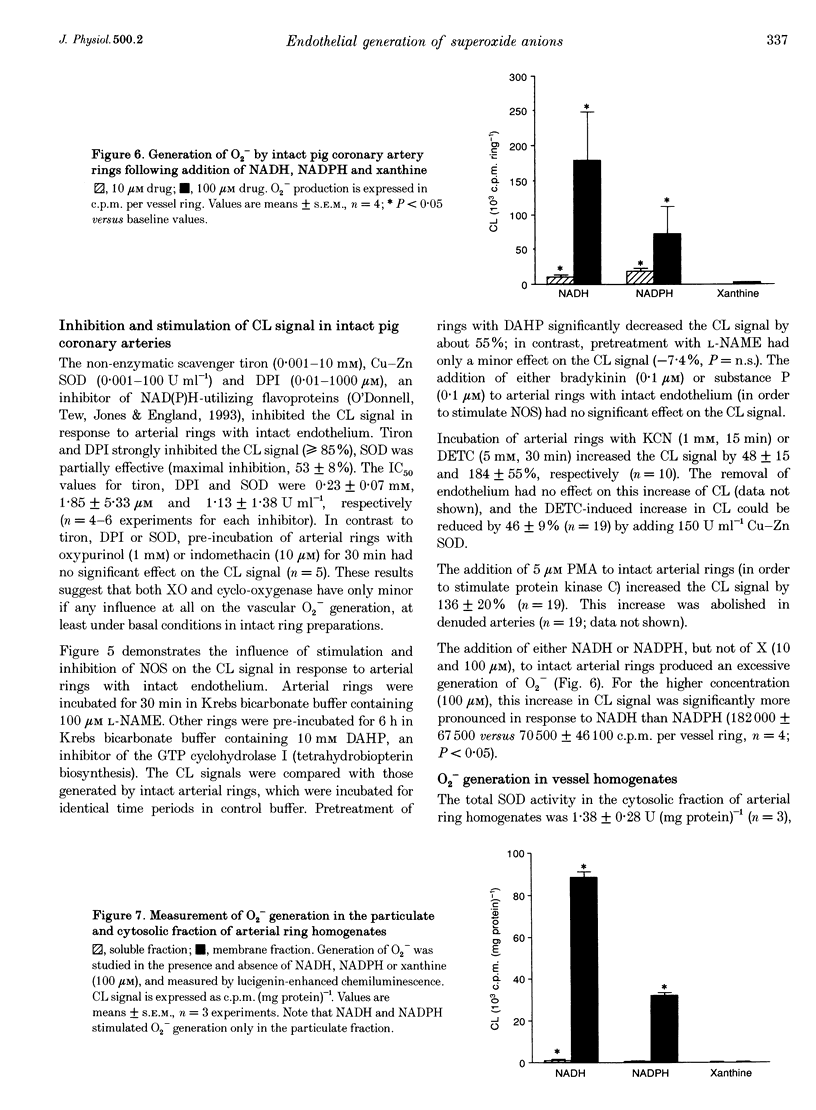
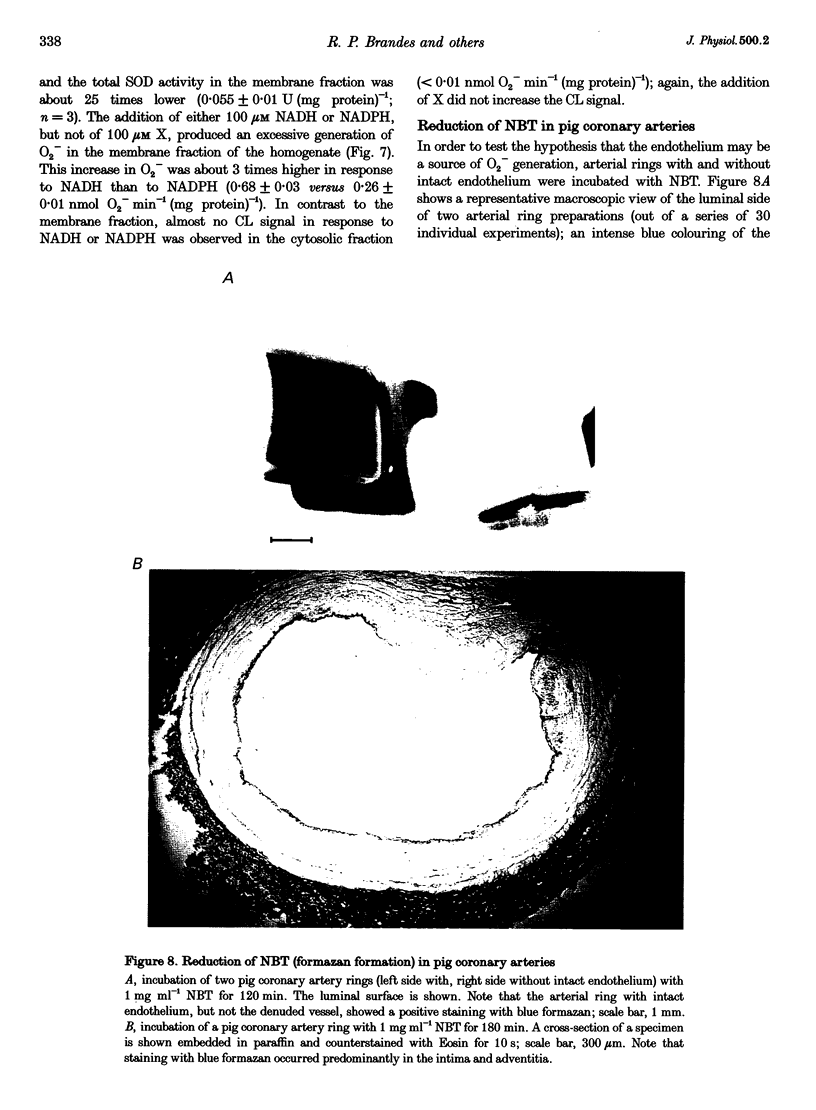
1. The generation of superoxide anions (O2-) by intact pig coronary artery rings was measured using a lucigenin-enhanced chemiluminescence technique and a histochemical technique with Nitroblue Tetrazolium (NBT) staining. 2. Isolated arteries with intact endothelium generated O2- at a rate of 9.0 +/- 0.8 pmol min-1 (mg dry weight)-1; this rate was diminished by about 24% when the endothelium was removed. The NBT staining of arterial ring preparations showed formazan precipitation mainly in the intima. Arterial rings were pretreated with diethylthiocarbamate in order to inhibit Cu-Zn superoxide dismutase (SOD) activity which increased the O2- generation by 184 +/- 55% (n = 10; P < 0.01). Stimulation of protein kinase C with phorbol 12-myristate 13-acetate (5 microM) enhanced endothelium-dependent O2- generation by 136 +/- 20% (n = 19; P < 0.01). Neither stimulation with bradykinin or substance P, nor inhibition with NG-nitro-L-arginine methyl ester of endothelial nitric oxide synthase had a significant effect on O2- generation. In contrast, the inhibition of flavoproteins with diphenyliodonium decreased concentration-dependent O2- generation (IC50, 1.85 +/- 5.33 microM). Inhibition of tetrahydrobiopterin synthesis with 2,4-diamino-6-hydroxy-pyrimidine resulted in a reduced generation of O2- by about 55%. 3. The addition of 100 microM NADH and 100 microM NADPH resulted in an excessive generation of O2- at a rate of 0.68 +/- 0.03 and 0.26 +/- 0.01 nmol O2- min-1 (mg protein)-1, respectively, in the membrane fraction, but not in the cytosolic fraction, of homogenates obtained from arteries. 4. The results suggest that intact coronary arteries do generate O2- under basal conditions and that the endothelial layer significantly contributes to this phenomenon. This generation of O2- is greatly influenced by intrinsic SOD activity. It is suggested that basal vascular O2- generation is mainly due to membrane-bound NAD(P)H oxidase activity and/or tetrahydrobiopterin-dependent processes.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman J. S., Minor R. L., Jr, White C. W., Repine J. E., Rosen G. M., Freeman B. A. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J Biol Chem. 1988 May 15;263(14):6884–6892. [PubMed] [Google Scholar]
- Brandes R. P., Dwenger A., Mügge A. The basal and stimulated release of the endothelium-derived relaxing factor from isolated pig coronary arteries does not interfere with the vascular release of superoxide. Naunyn Schmiedebergs Arch Pharmacol. 1994 Feb;349(2):183–187. doi: 10.1007/BF00169835. [DOI] [PubMed] [Google Scholar]
- Corbisier P., Houbion A., Remacle J. A new technique for highly sensitive detection of superoxide dismutase activity by chemiluminescence. Anal Biochem. 1987 Jul;164(1):240–247. doi: 10.1016/0003-2697(87)90392-7. [DOI] [PubMed] [Google Scholar]
- Cosentino F., Katusić Z. S. Tetrahydrobiopterin and dysfunction of endothelial nitric oxide synthase in coronary arteries. Circulation. 1995 Jan 1;91(1):139–144. doi: 10.1161/01.cir.91.1.139. [DOI] [PubMed] [Google Scholar]
- Devlin R. G., Lin C. S., Perper R. J., Dougherty H. Evaluation of free radical scavengers in studies of lymphocyte-mediated cytolysis. Immunopharmacology. 1981 Jun;3(2):147–159. doi: 10.1016/0162-3109(81)90016-3. [DOI] [PubMed] [Google Scholar]
- Fielden E. M., Roberts P. B., Bray R. C., Lowe D. J., Mautner G. N., Rotilio G., Calabrese L. Mechanism of action of superoxide dismutase from pulse radiolysis and electron paramagnetic resonance. Evidence that only half the active sites function in catalysis. Biochem J. 1974 Apr;139(1):49–60. doi: 10.1042/bj1390049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui T., Lassègue B., Kai H., Alexander R. W., Griendling K. K. Cytochrome b-558 alpha-subunit cloning and expression in rat aortic smooth muscle cells. Biochim Biophys Acta. 1995 Oct 10;1231(3):215–219. doi: 10.1016/0005-2728(95)00098-4. [DOI] [PubMed] [Google Scholar]
- Hattori Y., Kawasaki H., Abe K., Kanno M. Superoxide dismutase recovers altered endothelium-dependent relaxation in diabetic rat aorta. Am J Physiol. 1991 Oct;261(4 Pt 2):H1086–H1094. doi: 10.1152/ajpheart.1991.261.4.H1086. [DOI] [PubMed] [Google Scholar]
- Heim K. F., Thomas G., Ramwell P. W. Superoxide production in the isolated rabbit aorta and the effect of alloxan, indomethacin and nitrovasodilators. J Pharmacol Exp Ther. 1991 Feb;256(2):537–541. [PubMed] [Google Scholar]
- Holland J. A., Pritchard K. A., Pappolla M. A., Wolin M. S., Rogers N. J., Stemerman M. B. Bradykinin induces superoxide anion release from human endothelial cells. J Cell Physiol. 1990 Apr;143(1):21–25. doi: 10.1002/jcp.1041430104. [DOI] [PubMed] [Google Scholar]
- Hope B. T., Michael G. J., Knigge K. M., Vincent S. R. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huie R. E., Padmaja S. The reaction of no with superoxide. Free Radic Res Commun. 1993;18(4):195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- Kanner J., Harel S., Granit R. Nitric oxide as an antioxidant. Arch Biochem Biophys. 1991 Aug 15;289(1):130–136. doi: 10.1016/0003-9861(91)90452-o. [DOI] [PubMed] [Google Scholar]
- Kaufman S. New tetrahydrobiopterin-dependent systems. Annu Rev Nutr. 1993;13:261–286. doi: 10.1146/annurev.nu.13.070193.001401. [DOI] [PubMed] [Google Scholar]
- Klatt P., Schmidt K., Uray G., Mayer B. Multiple catalytic functions of brain nitric oxide synthase. Biochemical characterization, cofactor-requirement, and the role of N omega-hydroxy-L-arginine as an intermediate. J Biol Chem. 1993 Jul 15;268(20):14781–14787. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurindo F. R., Pedro M. de A., Barbeiro H. V., Pileggi F., Carvalho M. H., Augusto O., da Luz P. L. Vascular free radical release. Ex vivo and in vivo evidence for a flow-dependent endothelial mechanism. Circ Res. 1994 Apr;74(4):700–709. doi: 10.1161/01.res.74.4.700. [DOI] [PubMed] [Google Scholar]
- Marletta M. A. Nitric oxide synthase structure and mechanism. J Biol Chem. 1993 Jun 15;268(17):12231–12234. [PubMed] [Google Scholar]
- Matsubara T., Ziff M. Increased superoxide anion release from human endothelial cells in response to cytokines. J Immunol. 1986 Nov 15;137(10):3295–3298. [PubMed] [Google Scholar]
- Matsubara T., Ziff M. Superoxide anion release by human endothelial cells: synergism between a phorbol ester and a calcium ionophore. J Cell Physiol. 1986 May;127(2):207–210. doi: 10.1002/jcp.1041270203. [DOI] [PubMed] [Google Scholar]
- Mayer B., Klatt P., Werner E. R., Schmidt K. Kinetics and mechanism of tetrahydrobiopterin-induced oxidation of nitric oxide. J Biol Chem. 1995 Jan 13;270(2):655–659. doi: 10.1074/jbc.270.2.655. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968 Nov 10;243(21):5753–5760. [PubMed] [Google Scholar]
- Mohazzab K. M., Kaminski P. M., Wolin M. S. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am J Physiol. 1994 Jun;266(6 Pt 2):H2568–H2572. doi: 10.1152/ajpheart.1994.266.6.H2568. [DOI] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Mügge A., Brandes R. P., Böger R. H., Dwenger A., Bode-Böger S., Kienke S., Frölich J. C., Lichtlen P. R. Vascular release of superoxide radicals is enhanced in hypercholesterolemic rabbits. J Cardiovasc Pharmacol. 1994 Dec;24(6):994–998. doi: 10.1097/00005344-199424060-00019. [DOI] [PubMed] [Google Scholar]
- Mügge A., Elwell J. H., Peterson T. E., Harrison D. G. Release of intact endothelium-derived relaxing factor depends on endothelial superoxide dismutase activity. Am J Physiol. 1991 Feb;260(2 Pt 1):C219–C225. doi: 10.1152/ajpcell.1991.260.2.C219. [DOI] [PubMed] [Google Scholar]
- Nakazono K., Watanabe N., Matsuno K., Sasaki J., Sato T., Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10045–10048. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell B. V., Tew D. G., Jones O. T., England P. J. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J. 1993 Feb 15;290(Pt 1):41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara Y., Peterson T. E., Harrison D. G. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993 Jun;91(6):2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano P. J., Tornheim K., Cohen R. A. Superoxide anion production by rabbit thoracic aorta: effect of endothelium-derived nitric oxide. Am J Physiol. 1993 Aug;265(2 Pt 2):H707–H712. doi: 10.1152/ajpheart.1993.265.2.H707. [DOI] [PubMed] [Google Scholar]
- Paky A., Michael J. R., Burke-Wolin T. M., Wolin M. S., Gurtner G. H. Endogenous production of superoxide by rabbit lungs: effects of hypoxia or metabolic inhibitors. J Appl Physiol (1985) 1993 Jun;74(6):2868–2874. doi: 10.1152/jappl.1993.74.6.2868. [DOI] [PubMed] [Google Scholar]
- Pou S., Pou W. S., Bredt D. S., Snyder S. H., Rosen G. M. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992 Dec 5;267(34):24173–24176. [PubMed] [Google Scholar]
- Pritchard K. A., Jr, Groszek L., Smalley D. M., Sessa W. C., Wu M., Villalon P., Wolin M. S., Stemerman M. B. Native low-density lipoprotein increases endothelial cell nitric oxide synthase generation of superoxide anion. Circ Res. 1995 Sep;77(3):510–518. doi: 10.1161/01.res.77.3.510. [DOI] [PubMed] [Google Scholar]
- Rosen G. M., Freeman B. A. Detection of superoxide generated by endothelial cells. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7269–7273. doi: 10.1073/pnas.81.23.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecher U. P. Role of superoxide in endothelial-cell modification of low-density lipoproteins. Biochim Biophys Acta. 1988 Mar 4;959(1):20–30. doi: 10.1016/0005-2760(88)90145-2. [DOI] [PubMed] [Google Scholar]
- White C. R., Brock T. A., Chang L. Y., Crapo J., Briscoe P., Ku D., Bradley W. A., Gianturco S. H., Gore J., Freeman B. A. Superoxide and peroxynitrite in atherosclerosis. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1044–1048. doi: 10.1073/pnas.91.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]



