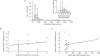Abstract
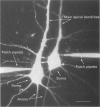
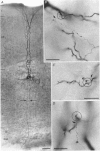
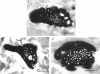
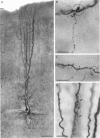
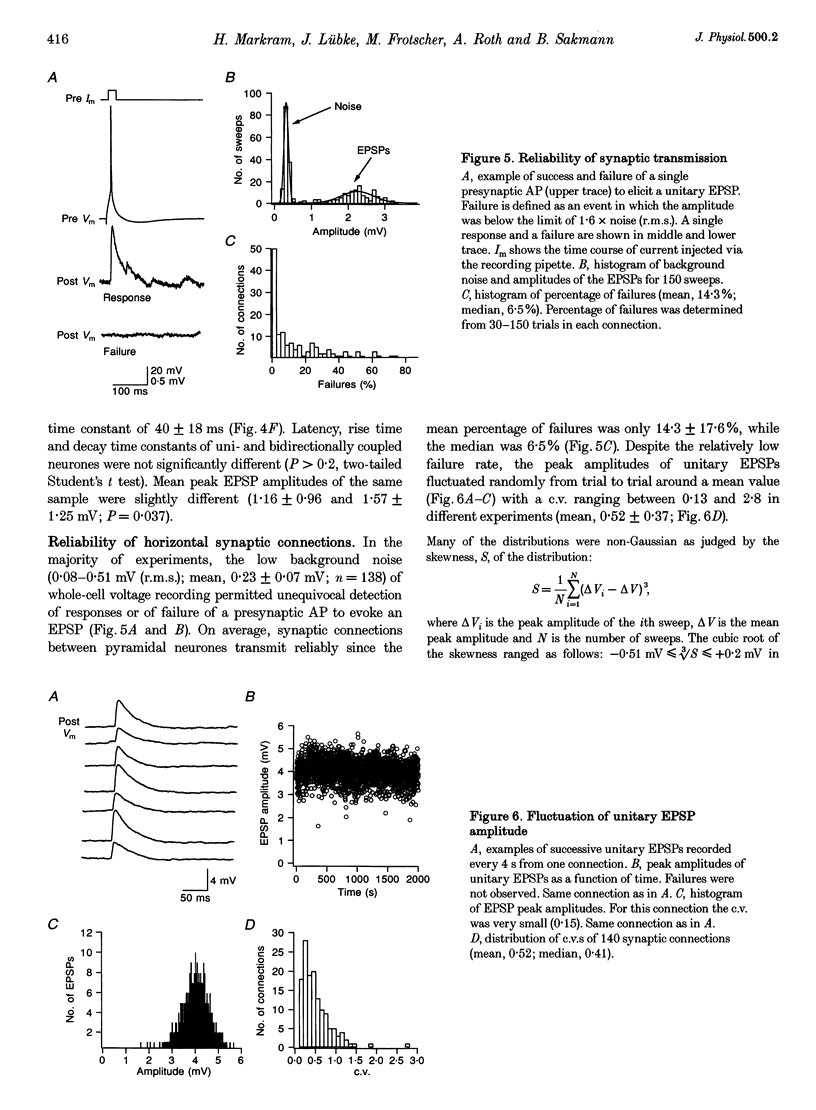
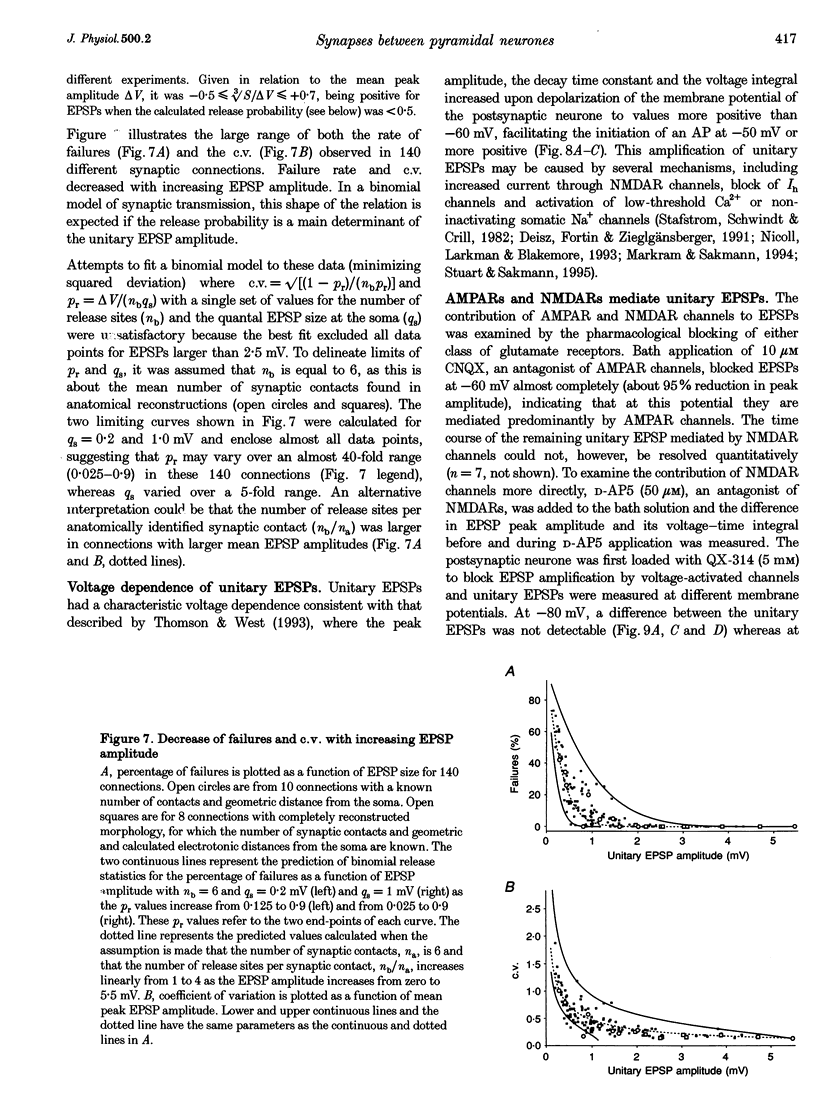
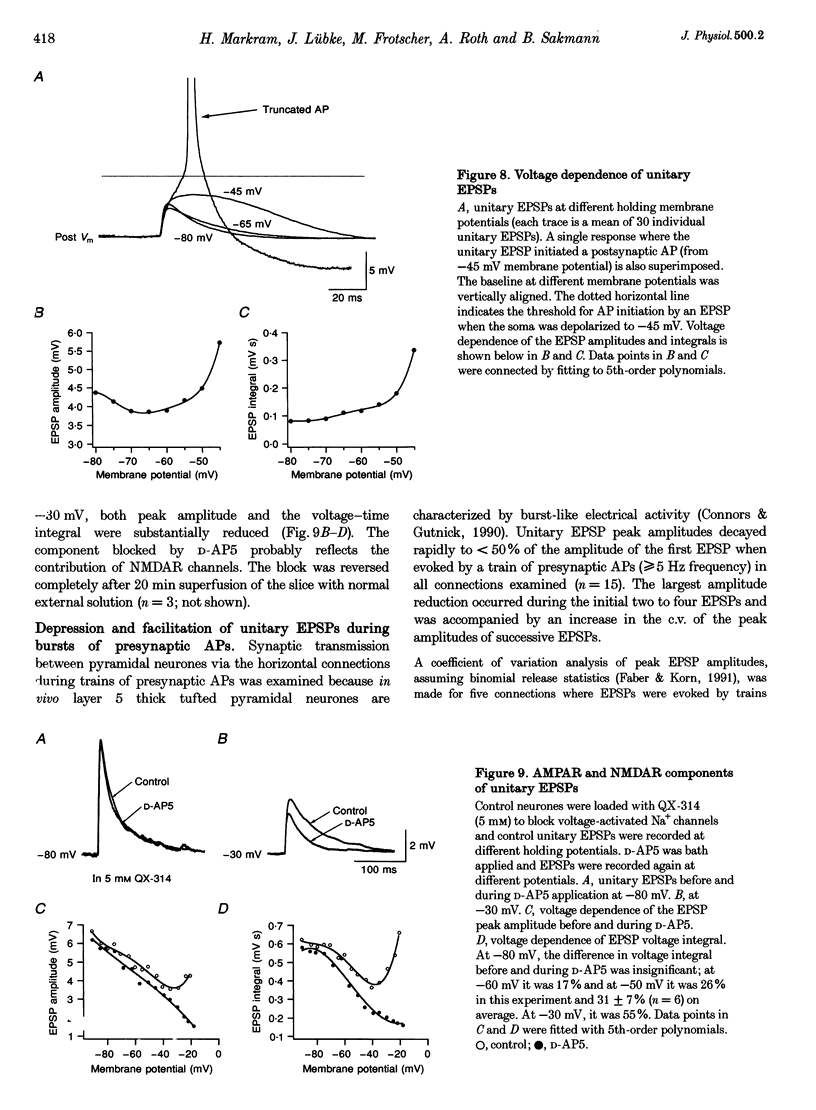
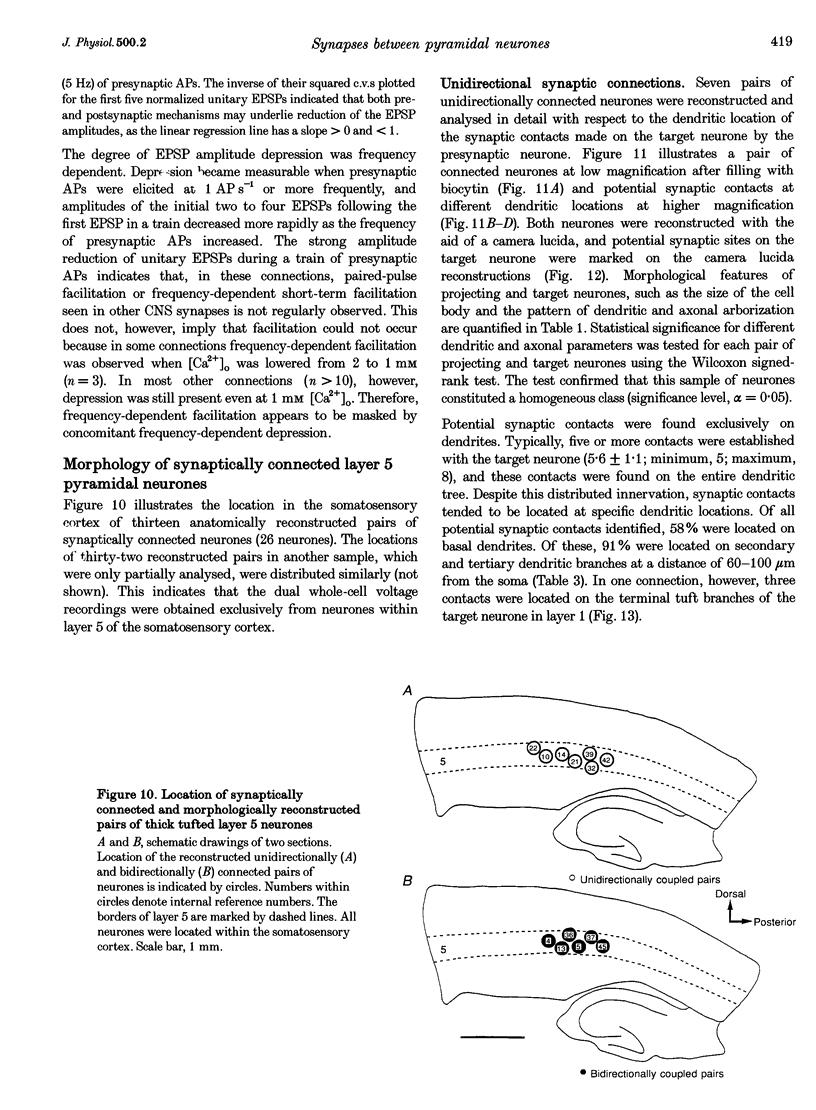
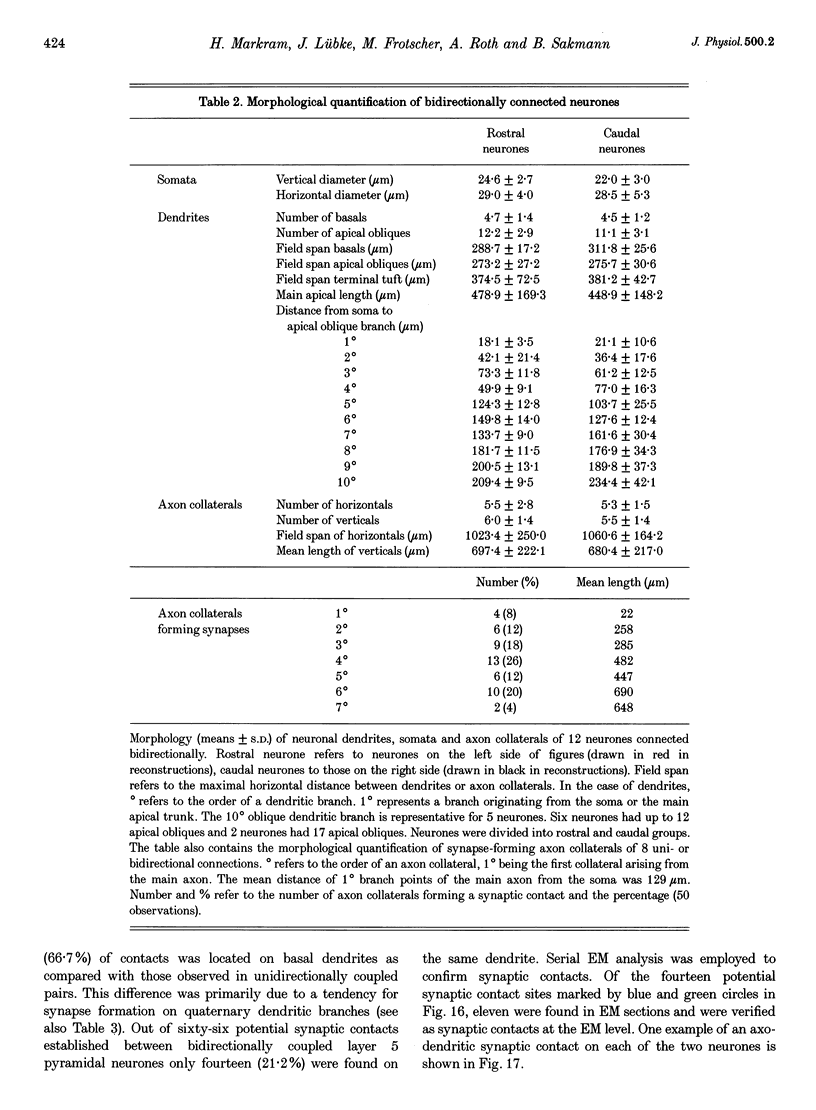
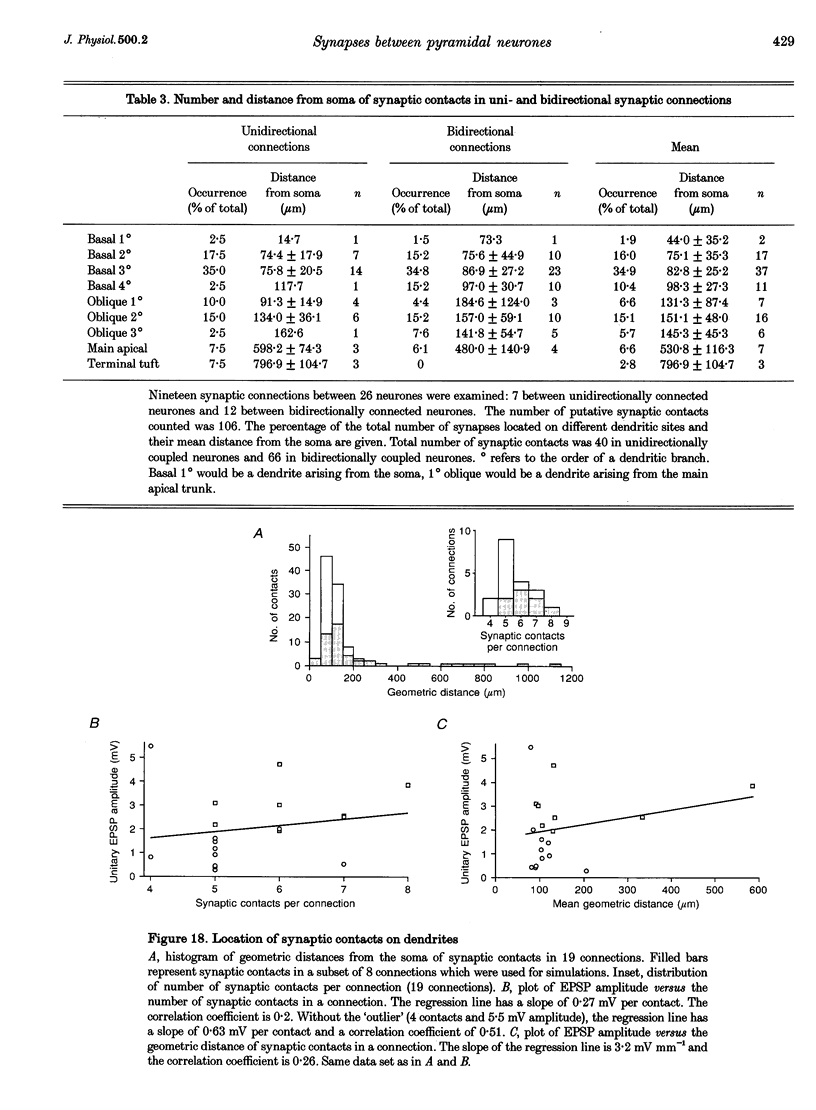
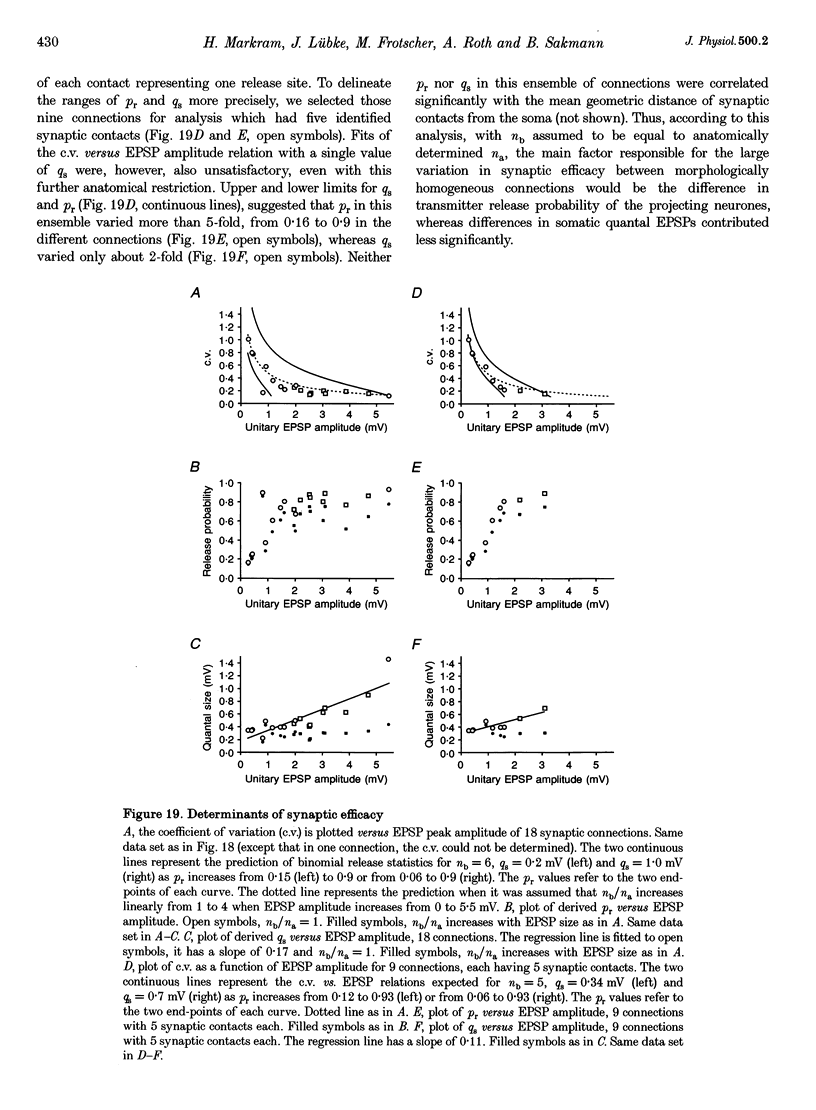
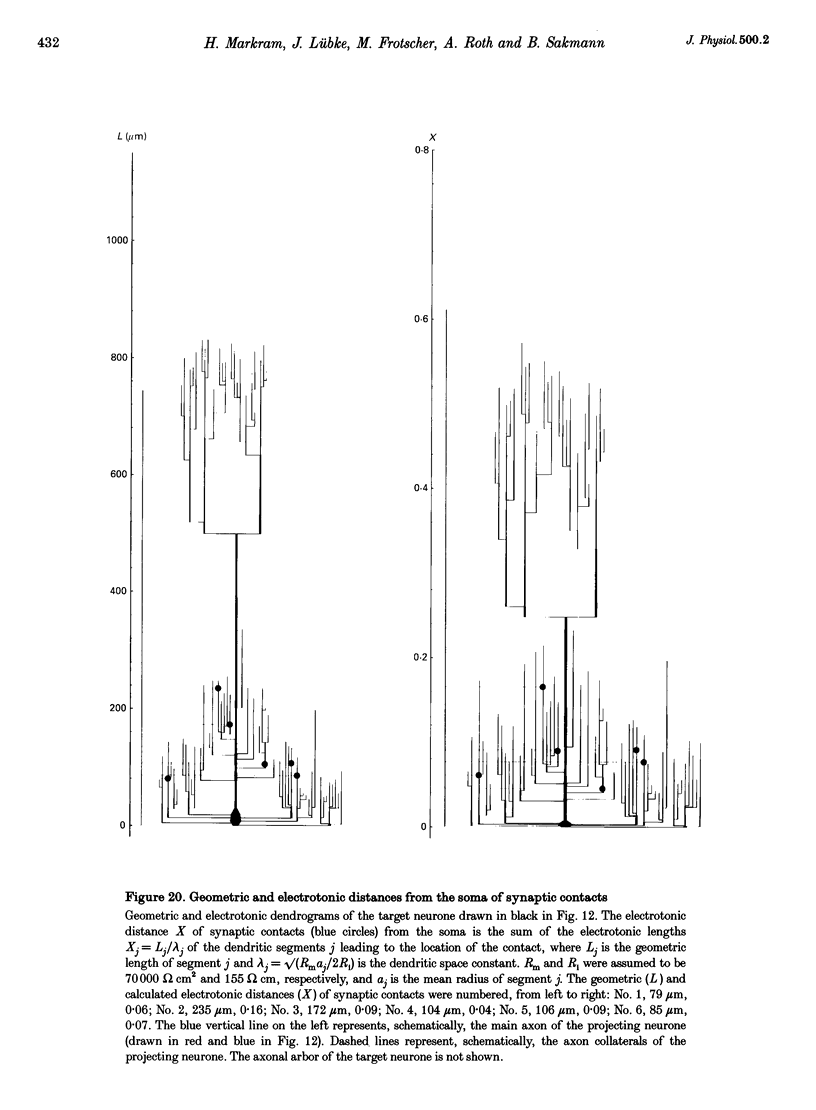
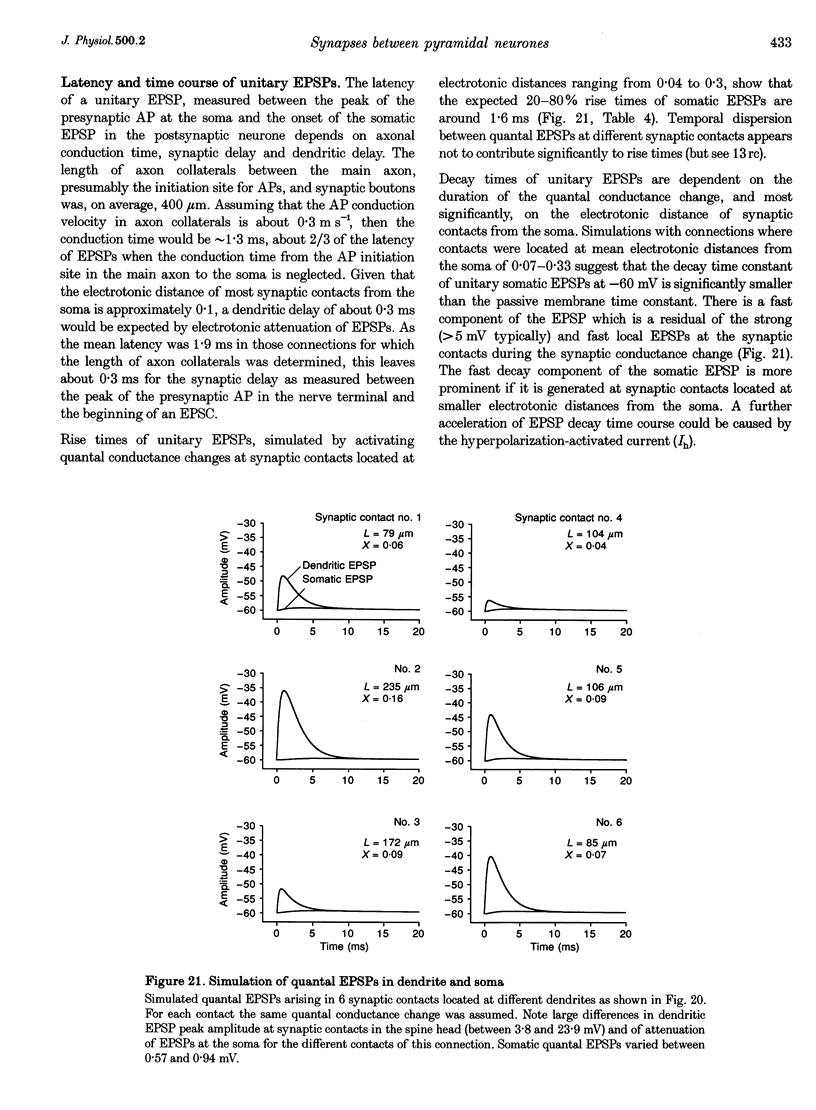
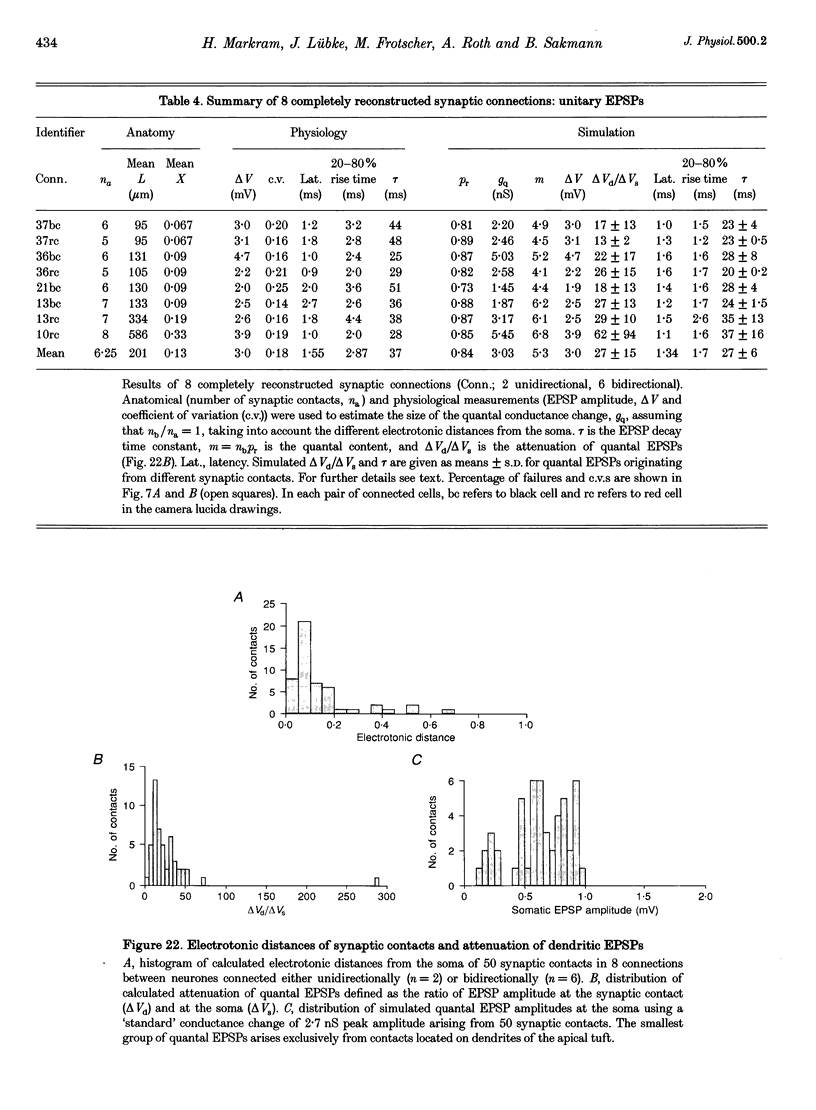
1. Dual voltage recordings were made from pairs of adjacent, synaptically connected thick tufted layer 5 pyramidal neurones in brain slices of young rat (14-16 days) somatosensory cortex to examine the physiological properties of unitary EPSPs. Pre- and postsynaptic neurones were filled with biocytin and examined in the light and electron microscope to quantify the morphology of axonal and dendritic arbors and the number and location of synaptic contacts on the target neurone. 2. In 138 synaptic connections between pairs of pyramidal neurones 96 (70%) were unidirectional and 42 (30%) were bidirectional. The probability of finding a synaptic connection in dual recordings was 0.1. Unitary EPSPs evoked by a single presynaptic action potential (AP) had a mean peak amplitude ranging from 0.15 to 5.5 mV in different connections with a mean of 1.3 +/- 1.1 mV, a latency of 1.7 +/- 0.9 ms, a 20-80% rise time of 2.9 +/- 2.3 ms and a decay time constant of 40 +/- 18 ms at 32-24 degrees C and -60 +/- 2 mV membrane potential. 3. Peak amplitudes of unitary EPSPs fluctuated randomly from trial to trial. The coefficient of variation (c.v.) of the unitary EPSP amplitudes ranged from 0.13 to 2.8 in different synaptic connections (mean, 0.52; median, 0.41). The percentage of failures of single APs to evoke a unitary EPSP ranged from 0 to 73% (mean, 14%; median, 7%). Both c.v. and percentage of failures decreased with increasing mean EPSP amplitude. 4. Postsynaptic glutamate receptors which mediate unitary EPSPs at -60 mV were predominantly of the L-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor type. Receptors of the N-methyl-D-aspartate (NMDA) type contributed only a small fraction (< 20%) to the voltage-time integral of the unitary EPSP at -60 mV, but their contribution increased at more positive membrane potentials. 5. Branching patterns of dendrites and axon collaterals of forty-five synaptically connected neurones, when examined in the light microscope, indicated that the axonal and dendritic anatomy of both projecting and target neurones and of uni- and bidirectionally connected neurones was uniform. 6. The number of potential synaptic contacts formed by a presynaptic neurone on a target neurone varied between four and eight (mean, 5.5 +/- 1.1 contacts; n = 19 connections). Synaptic contacts were preferentially located on basal dendrites (63%, 82 +/- 35 microns from the soma, n = 67) and apical oblique dendrites (27%, 145 +/- 59 microns, n = 29), and 35% of all contacts were located on tertiary basal dendritic branches. The mean geometric distances (from the soma) of the contacts of a connection varied between 80 and 585 microns (mean, 147 microns; median, 105 microns). The correlation between EPSP amplitude and the number of morphologically determined synaptic contacts or the mean geometric distances from the soma was only weak (correlation coefficients were 0.2 and 0.26, respectively). 7. Compartmental models constructed from camera lucida drawings of eight target neurones showed that synaptic contacts were located at mean electrotonic distances between 0.07 and 0.33 from the soma (mean, 0.13). Simulations of unitary EPSPs, assuming quantal conductance changes with fast rise time and short duration, indicated that amplitudes of quantal EPSPs at the soma were attenuated, on average, to < 10% of dendritic EPSPs and varied in amplitude up to 10-fold depending on the dendritic location of synaptic contacts. The inferred quantal peak conductance increase varied between 1.5 and 5.5 nS (mean, 3 nS). 8. The combined physiological and morphological measurements in conjunction with EPSP simulations indicated that the 20-fold range in efficacy of the synaptic connections between thick tufted pyramidal neurones, which have their synaptic contacts preferentially located on basal and apical oblique dendrites, was due to differences in transmitter release probability of the projecting neurones and, to a lesser extent, to differenc
Full text
PDF



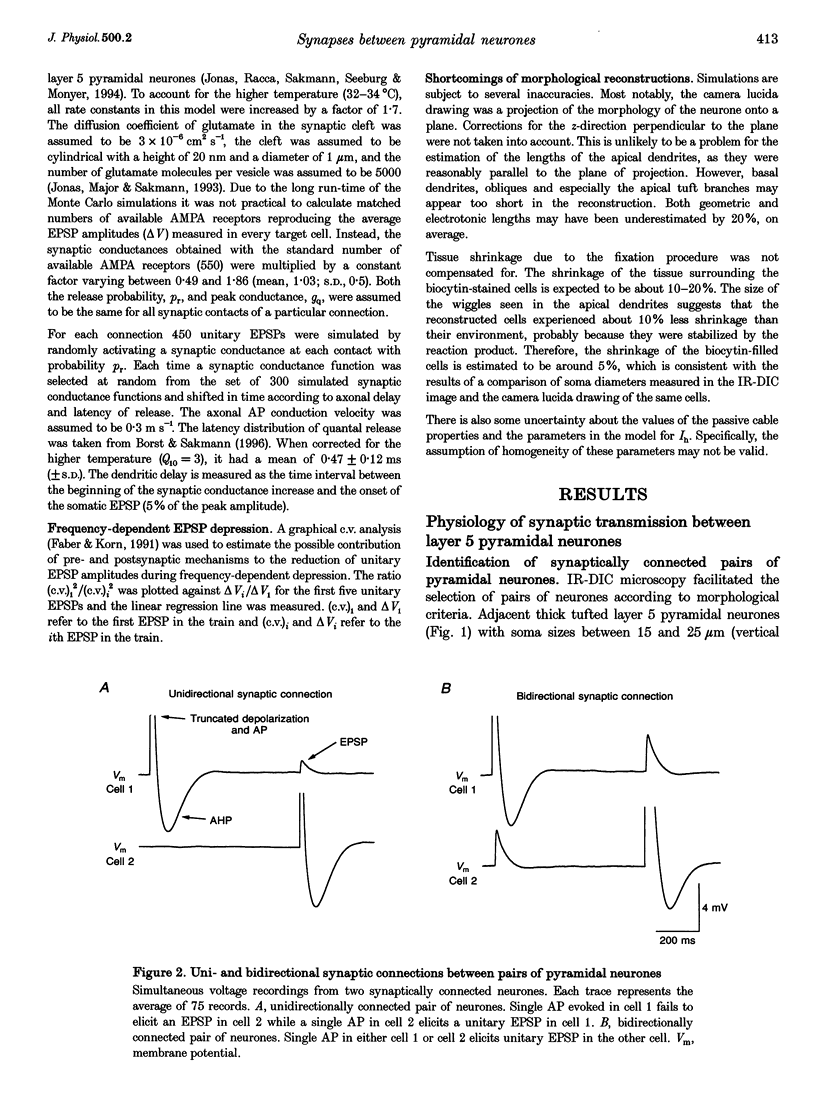








Images in this article
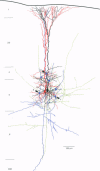
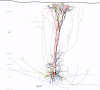
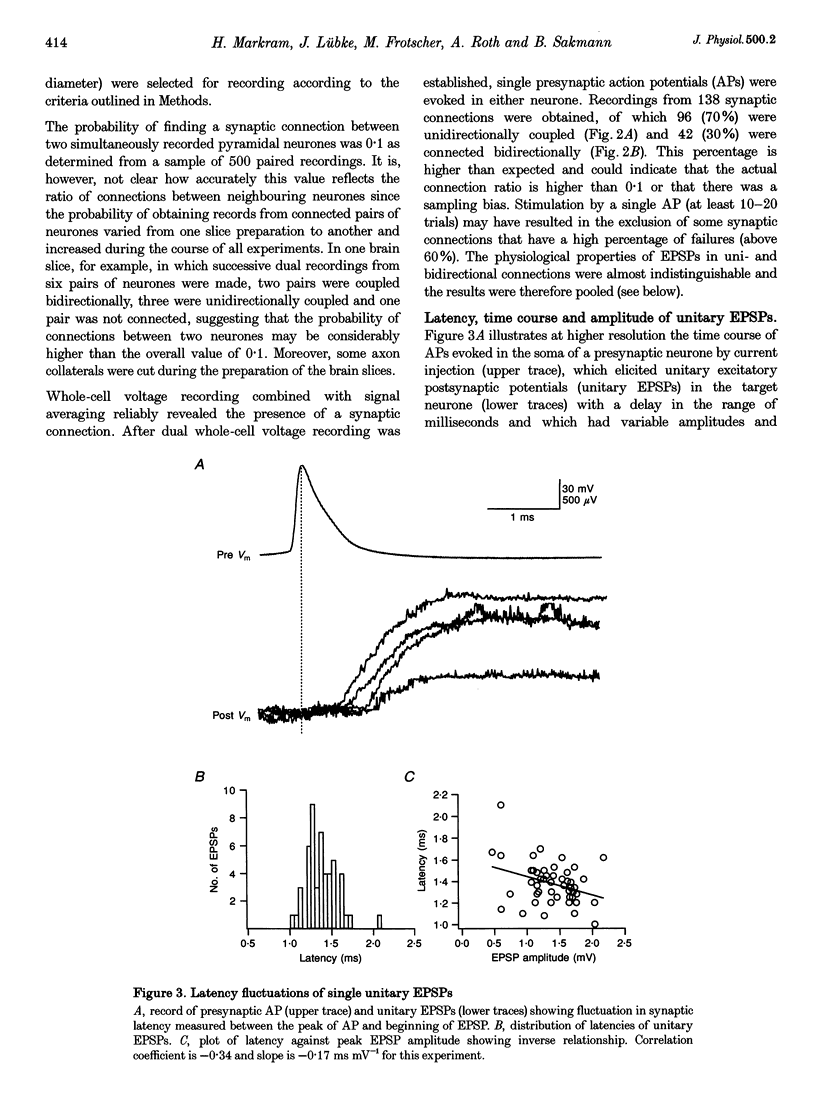
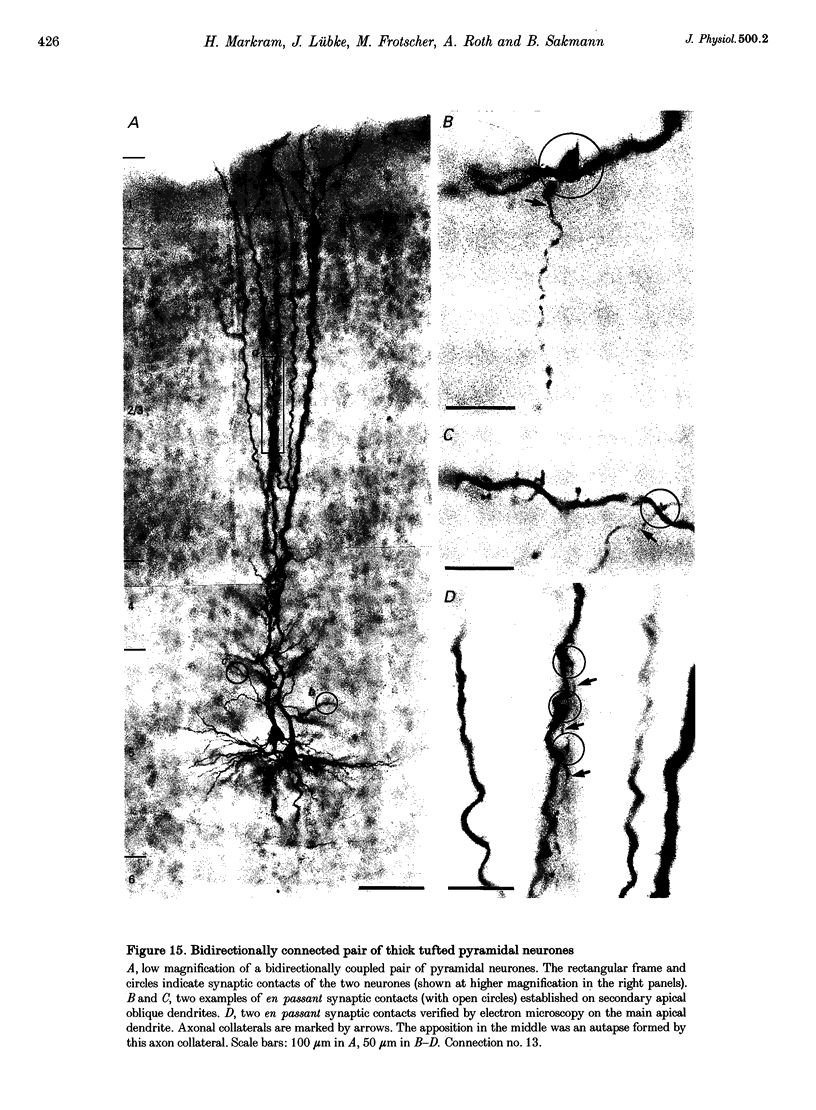
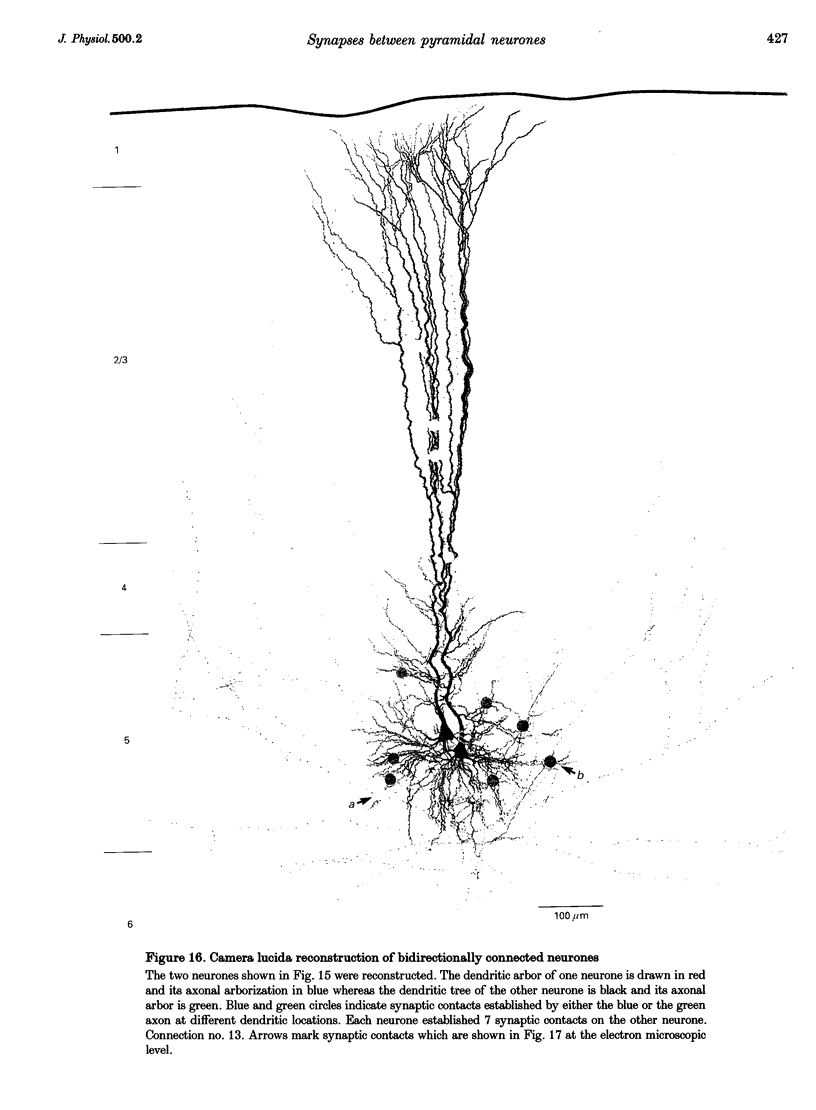
Selected References
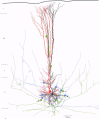
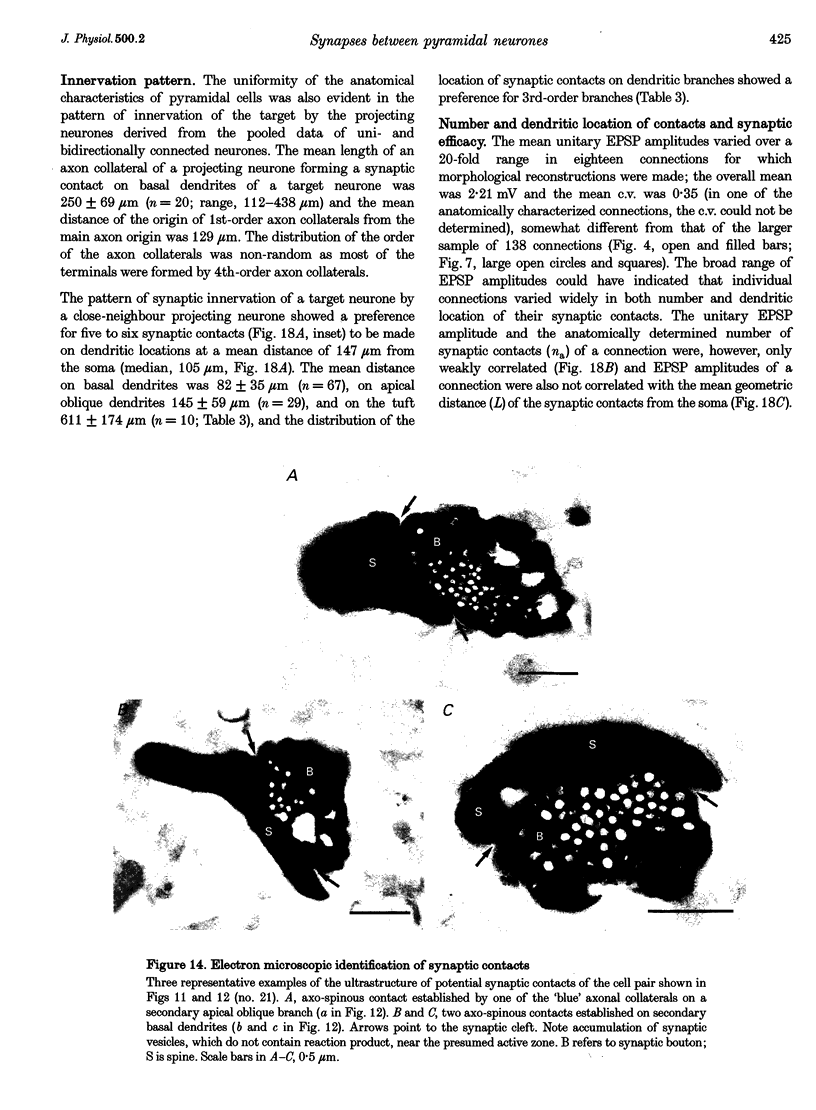
These references are in PubMed. This may not be the complete list of references from this article.
- Bartol T. M., Jr, Land B. R., Salpeter E. E., Salpeter M. M. Monte Carlo simulation of miniature endplate current generation in the vertebrate neuromuscular junction. Biophys J. 1991 Jun;59(6):1290–1307. doi: 10.1016/S0006-3495(91)82344-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolshakov V. Y., Siegelbaum S. A. Regulation of hippocampal transmitter release during development and long-term potentiation. Science. 1995 Sep 22;269(5231):1730–1734. doi: 10.1126/science.7569903. [DOI] [PubMed] [Google Scholar]
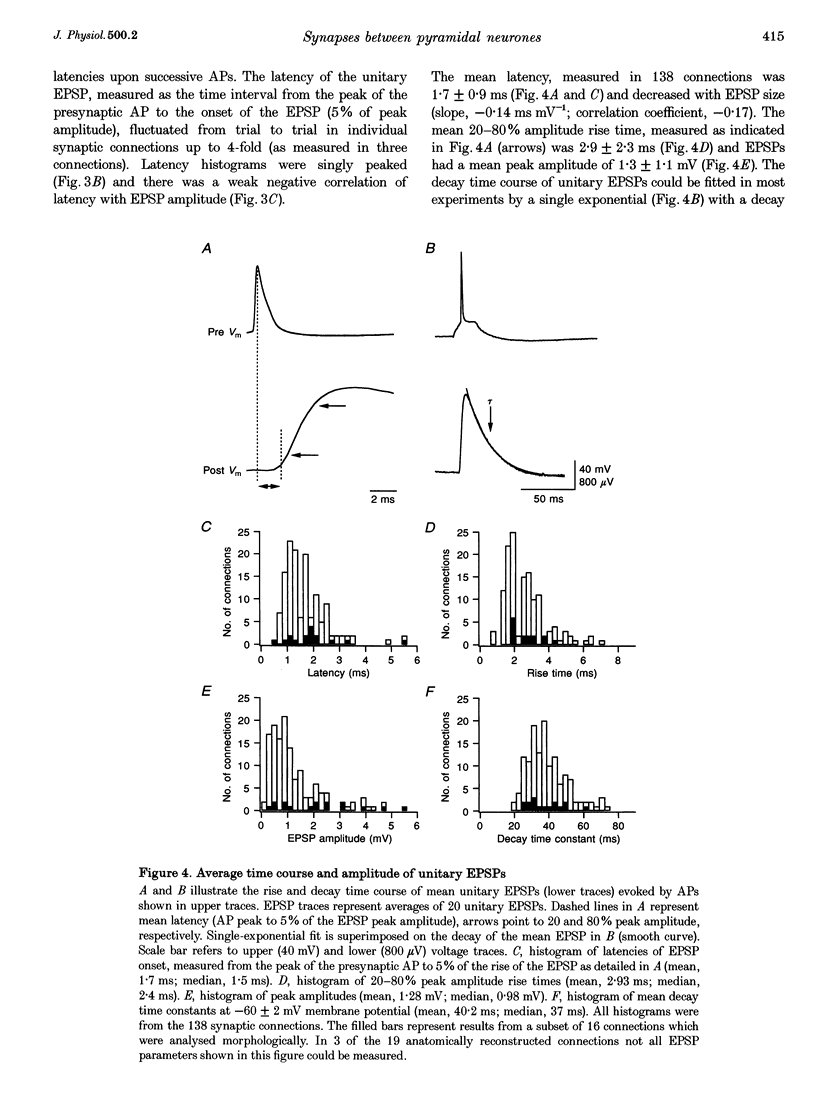
- Borst J. G., Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996 Oct 3;383(6599):431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- Burnashev N., Zhou Z., Neher E., Sakmann B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J Physiol. 1995 Jun 1;485(Pt 2):403–418. doi: 10.1113/jphysiol.1995.sp020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnac-Amitai Y., Luhmann H. J., Prince D. A. Burst generating and regular spiking layer 5 pyramidal neurons of rat neocortex have different morphological features. J Comp Neurol. 1990 Jun 22;296(4):598–613. doi: 10.1002/cne.902960407. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Gutnick M. J. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990 Mar;13(3):99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- DeFelipe J., Fariñas I. The pyramidal neuron of the cerebral cortex: morphological and chemical characteristics of the synaptic inputs. Prog Neurobiol. 1992 Dec;39(6):563–607. doi: 10.1016/0301-0082(92)90015-7. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Fortin G., Zieglgänsberger W. Voltage dependence of excitatory postsynaptic potentials of rat neocortical neurons. J Neurophysiol. 1991 Feb;65(2):371–382. doi: 10.1152/jn.1991.65.2.371. [DOI] [PubMed] [Google Scholar]
- Deuchars J., West D. C., Thomson A. M. Relationships between morphology and physiology of pyramid-pyramid single axon connections in rat neocortex in vitro. J Physiol. 1994 Aug 1;478(Pt 3):423–435. doi: 10.1113/jphysiol.1994.sp020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber D. S., Korn H. Applicability of the coefficient of variation method for analyzing synaptic plasticity. Biophys J. 1991 Nov;60(5):1288–1294. doi: 10.1016/S0006-3495(91)82162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M., Misgeld U., Nitsch C. Ultrastructure of mossy fiber endings in in vitro hippocampal slices. Exp Brain Res. 1981;41(3-4):247–255. doi: 10.1007/BF00238881. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature. 1979 Jul 12;280(5718):120–125. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
- Grinnell A. D., Trussell L. O. Synaptic strength as a function of motor unit size in the normal frog sartorius. J Physiol. 1983 May;338:221–241. doi: 10.1113/jphysiol.1983.sp014670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P., Major G., Sakmann B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J Physiol. 1993 Dec;472:615–663. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P., Racca C., Sakmann B., Seeburg P. H., Monyer H. Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron. 1994 Jun;12(6):1281–1289. doi: 10.1016/0896-6273(94)90444-8. [DOI] [PubMed] [Google Scholar]
- Kasper E. M., Larkman A. U., Lübke J., Blakemore C. Pyramidal neurons in layer 5 of the rat visual cortex. I. Correlation among cell morphology, intrinsic electrophysiological properties, and axon targets. J Comp Neurol. 1994 Jan 22;339(4):459–474. doi: 10.1002/cne.903390402. [DOI] [PubMed] [Google Scholar]
- Kasper E. M., Lübke J., Larkman A. U., Blakemore C. Pyramidal neurons in layer 5 of the rat visual cortex. III. Differential maturation of axon targeting, dendritic morphology, and electrophysiological properties. J Comp Neurol. 1994 Jan 22;339(4):495–518. doi: 10.1002/cne.903390404. [DOI] [PubMed] [Google Scholar]
- Korn H., Faber D. S., Triller A. Probabilistic determination of synaptic strength. J Neurophysiol. 1986 Feb;55(2):402–421. doi: 10.1152/jn.1986.55.2.402. [DOI] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman A., Mason A. Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. I. Establishment of cell classes. J Neurosci. 1990 May;10(5):1407–1414. doi: 10.1523/JNEUROSCI.10-05-01407.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübke J., Markram H., Frotscher M., Sakmann B. Frequency and dendritic distribution of autapses established by layer 5 pyramidal neurons in the developing rat neocortex: comparison with synaptic innervation of adjacent neurons of the same class. J Neurosci. 1996 May 15;16(10):3209–3218. doi: 10.1523/JNEUROSCI.16-10-03209.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H., Sakmann B. Calcium transients in dendrites of neocortical neurons evoked by single subthreshold excitatory postsynaptic potentials via low-voltage-activated calcium channels. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5207–5211. doi: 10.1073/pnas.91.11.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A., Larkman A. Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. II. Electrophysiology. J Neurosci. 1990 May;10(5):1415–1428. doi: 10.1523/JNEUROSCI.10-05-01415.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A., Nicoll A., Stratford K. Synaptic transmission between individual pyramidal neurons of the rat visual cortex in vitro. J Neurosci. 1991 Jan;11(1):72–84. doi: 10.1523/JNEUROSCI.11-01-00072.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971 Jan;34(1):171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Weiner R. Analysis of pairs of individual Ia-E.P.S.P.S in single motoneurones. J Physiol. 1976 Feb;255(1):81–104. doi: 10.1113/jphysiol.1976.sp011271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R. Variation in strength of inhibitory synapses in the CA3 region of guinea-pig hippocampus in vitro. J Physiol. 1990 Dec;431:659–676. doi: 10.1113/jphysiol.1990.sp018353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H., Sprengel R., Schoepfer R., Herb A., Higuchi M., Lomeli H., Burnashev N., Sakmann B., Seeburg P. H. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992 May 22;256(5060):1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Nicoll A., Larkman A., Blakemore C. Modulation of EPSP shape and efficacy by intrinsic membrane conductances in rat neocortical pyramidal neurons in vitro. J Physiol. 1993 Aug;468:693–710. doi: 10.1113/jphysiol.1993.sp019795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A., Kaiserman-Abramof I. R. The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am J Anat. 1970 Apr;127(4):321–355. doi: 10.1002/aja.1001270402. [DOI] [PubMed] [Google Scholar]
- Raastad M., Lipowski R. Diversity of postsynaptic amplitude and failure probability of unitary excitatory synapses between CA3 and CA1 cells in the rat hippocampus. Eur J Neurosci. 1996 Jun;8(6):1265–1274. doi: 10.1111/j.1460-9568.1996.tb01295.x. [DOI] [PubMed] [Google Scholar]
- Silva L. R., Amitai Y., Connors B. W. Intrinsic oscillations of neocortex generated by layer 5 pyramidal neurons. Science. 1991 Jan 25;251(4992):432–435. doi: 10.1126/science.1824881. [DOI] [PubMed] [Google Scholar]
- Silver R. A., Cull-Candy S. G., Takahashi T. Non-NMDA glutamate receptor occupancy and open probability at a rat cerebellar synapse with single and multiple release sites. J Physiol. 1996 Jul 1;494(Pt 1):231–250. doi: 10.1113/jphysiol.1996.sp021487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorra K. E., Harris K. M. Occurrence and three-dimensional structure of multiple synapses between individual radiatum axons and their target pyramidal cells in hippocampal area CA1. J Neurosci. 1993 Sep;13(9):3736–3748. doi: 10.1523/JNEUROSCI.13-09-03736.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain W. J., Schwindt P. C., Crill W. E. Anomalous rectification in neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1987 May;57(5):1555–1576. doi: 10.1152/jn.1987.57.5.1555. [DOI] [PubMed] [Google Scholar]
- Spruston N., Johnston D. Perforated patch-clamp analysis of the passive membrane properties of three classes of hippocampal neurons. J Neurophysiol. 1992 Mar;67(3):508–529. doi: 10.1152/jn.1992.67.3.508. [DOI] [PubMed] [Google Scholar]
- Stafstrom C. E., Schwindt P. C., Crill W. E. Negative slope conductance due to a persistent subthreshold sodium current in cat neocortical neurons in vitro. Brain Res. 1982 Mar 18;236(1):221–226. doi: 10.1016/0006-8993(82)90050-6. [DOI] [PubMed] [Google Scholar]
- Stratford K. J., Tarczy-Hornoch K., Martin K. A., Bannister N. J., Jack J. J. Excitatory synaptic inputs to spiny stellate cells in cat visual cortex. Nature. 1996 Jul 18;382(6588):258–261. doi: 10.1038/382258a0. [DOI] [PubMed] [Google Scholar]
- Stricker C., Field A. C., Redman S. J. Statistical analysis of amplitude fluctuations in EPSCs evoked in rat CA1 pyramidal neurones in vitro. J Physiol. 1996 Jan 15;490(Pt 2):419–441. doi: 10.1113/jphysiol.1996.sp021155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G. J., Dodt H. U., Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 1993 Jun;423(5-6):511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Stuart G., Sakmann B. Amplification of EPSPs by axosomatic sodium channels in neocortical pyramidal neurons. Neuron. 1995 Nov;15(5):1065–1076. doi: 10.1016/0896-6273(95)90095-0. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., Deuchars J. Temporal and spatial properties of local circuits in neocortex. Trends Neurosci. 1994 Mar;17(3):119–126. doi: 10.1016/0166-2236(94)90121-x. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., Deuchars J., West D. C. Large, deep layer pyramid-pyramid single axon EPSPs in slices of rat motor cortex display paired pulse and frequency-dependent depression, mediated presynaptically and self-facilitation, mediated postsynaptically. J Neurophysiol. 1993 Dec;70(6):2354–2369. doi: 10.1152/jn.1993.70.6.2354. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., West D. C. Fluctuations in pyramid-pyramid excitatory postsynaptic potentials modified by presynaptic firing pattern and postsynaptic membrane potential using paired intracellular recordings in rat neocortex. Neuroscience. 1993 May;54(2):329–346. doi: 10.1016/0306-4522(93)90256-f. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Grinnell A. D. The regulation of synaptic strength within motor units of the frog cutaneous pectoris muscle. J Neurosci. 1985 Jan;5(1):243–254. doi: 10.1523/JNEUROSCI.05-01-00243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde F. Intrinsic neocortical organization: some comparative aspects. Neuroscience. 1986 May;18(1):1–23. doi: 10.1016/0306-4522(86)90174-0. [DOI] [PubMed] [Google Scholar]
- Wang Z., McCormick D. A. Control of firing mode of corticotectal and corticopontine layer V burst-generating neurons by norepinephrine, acetylcholine, and 1S,3R-ACPD. J Neurosci. 1993 May;13(5):2199–2216. doi: 10.1523/JNEUROSCI.13-05-02199.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]