Abstract
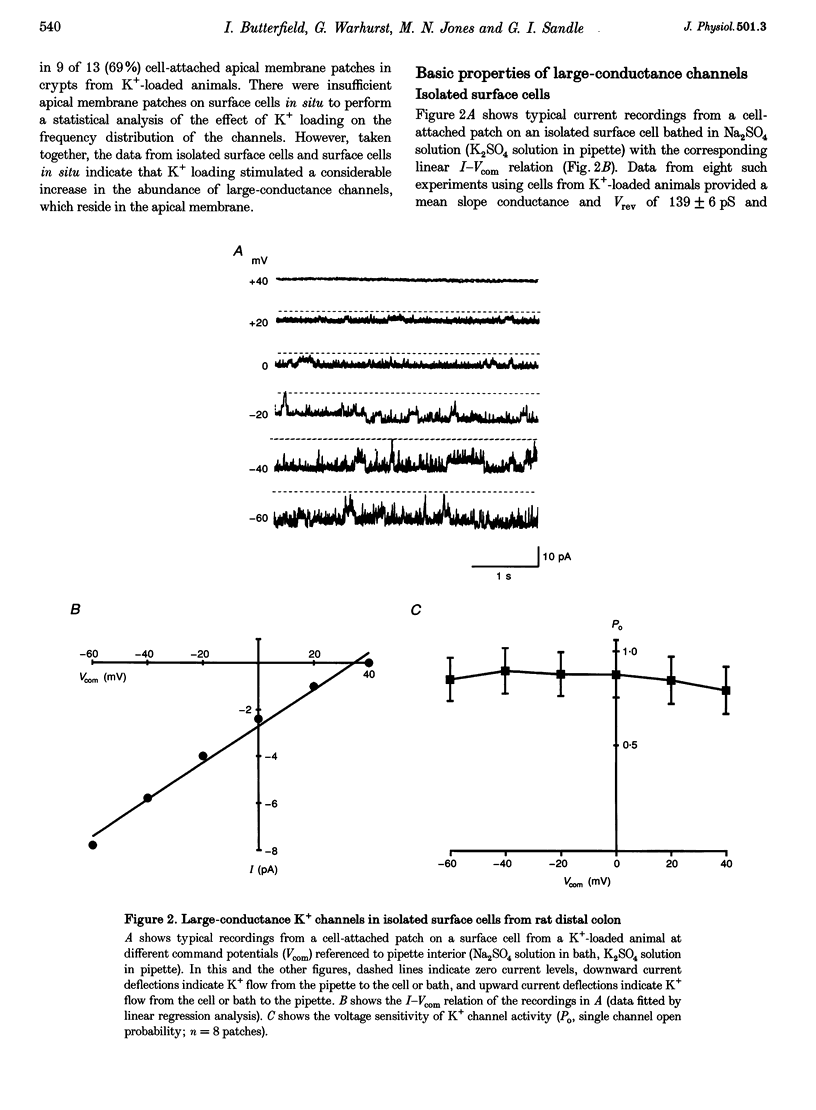
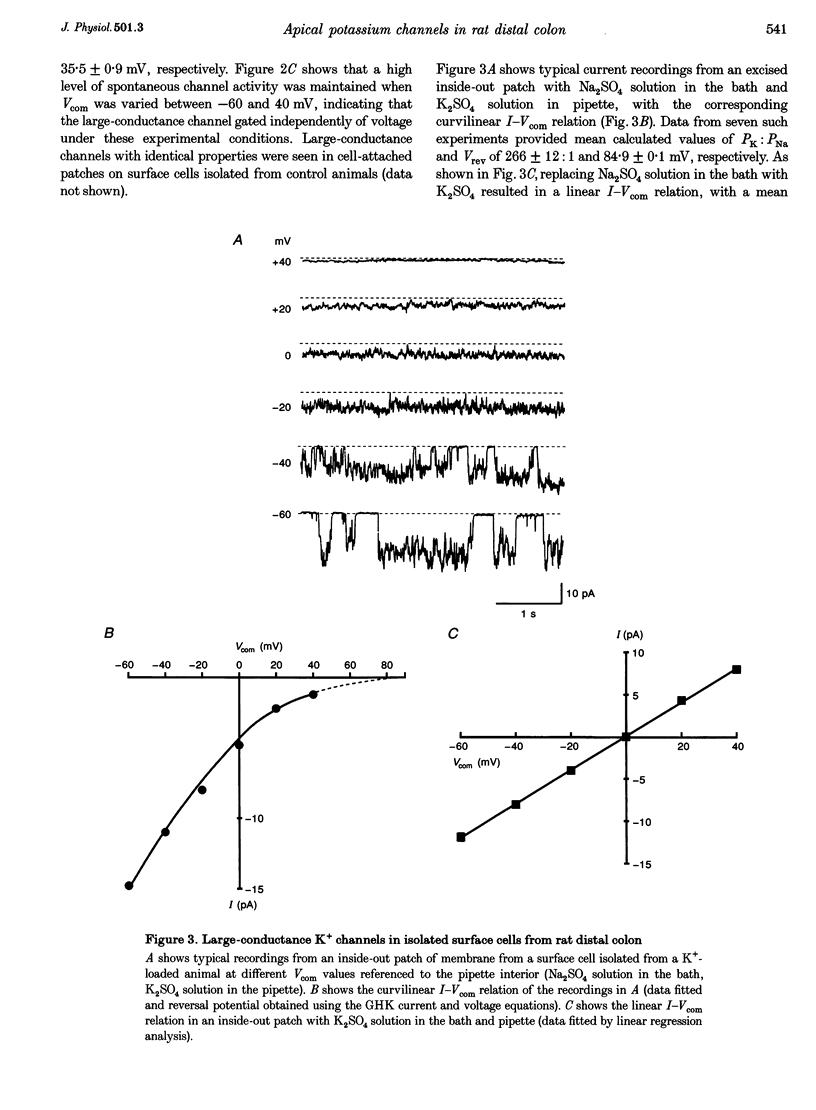
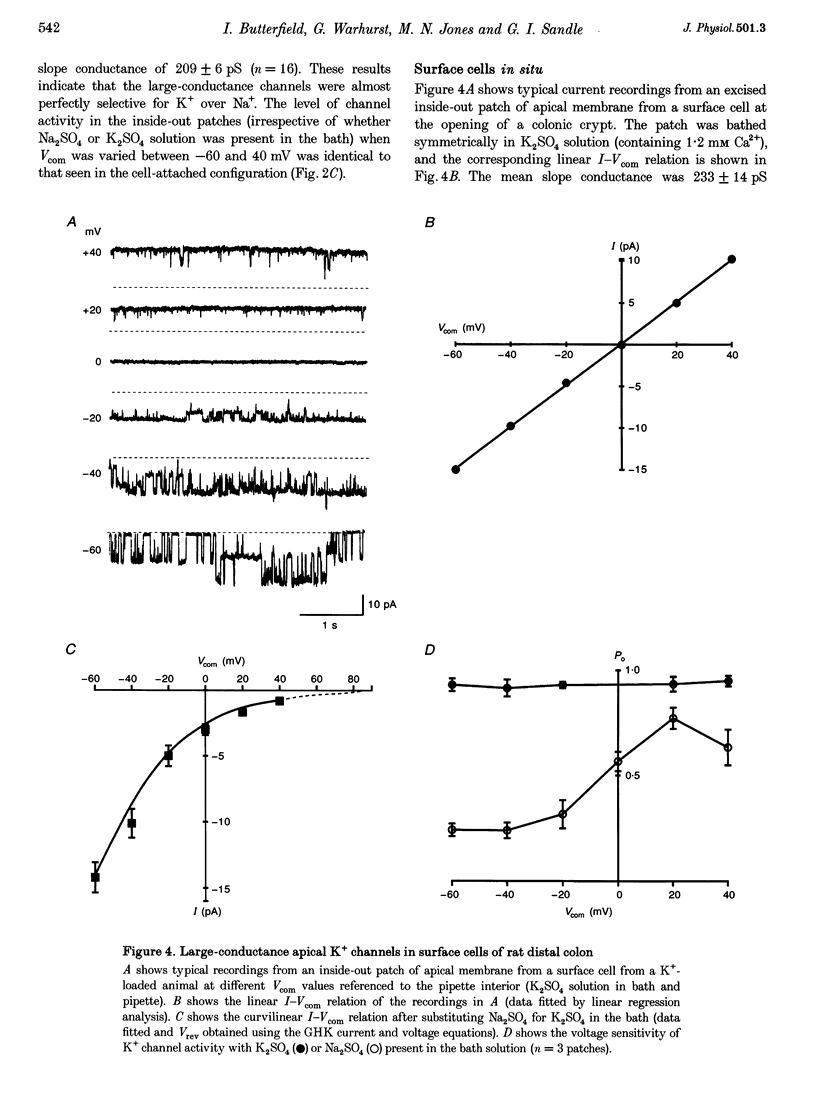
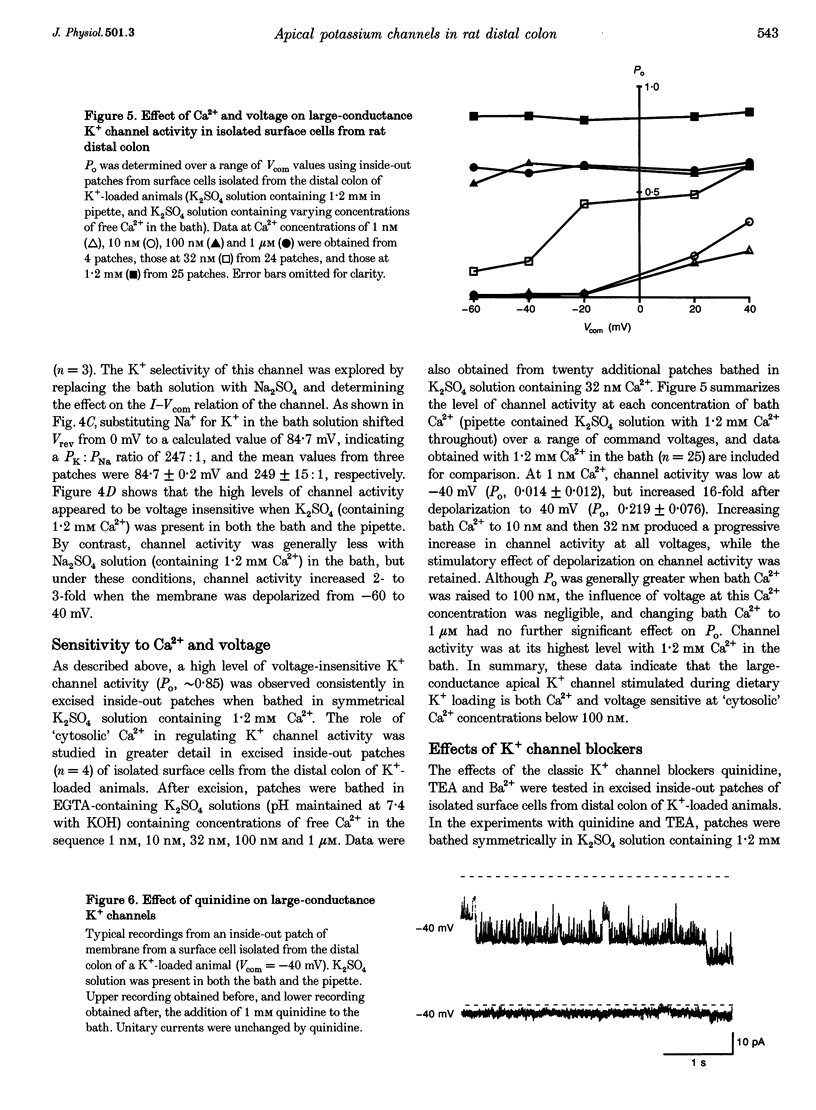
1. Chronic dietary K+ loading stimulates an active K+ secretory process in rat distal colon, which involves an increase in the macroscopic apical K+ conductance of surface epithelial cells. In the present study, the abundance and characteristics of K+ channels constituting this enhanced apical K+ conductance were evaluated using patch clamp recording techniques. 2. In isolated non-polarized surface cells, K+ channels were seen in 9 of 90 (10%) cell-attached patches in cells from control animals, and in 247 of 437 (57%) cell-attached patches in cells from K(+)-loaded animals, with a significant (P < 0.001) shift in distribution density. Similarly, recordings from cell-attached patches of the apical membrane of surface cells surrounding the openings of distal colonic crypts revealed identical K+ channels in 1 of 11 (9%) patches in control animals, and in 9 of 13 (69%) patches in K(+)-loaded animals. 3. In isolated surface cells and surface cells in situ, K+ channels had mean slope conductances of 209 +/- 6 and 233 +/- 14 pS, respectively, when inside-out patches were bathed symmetrically in K2SO4 solution. The channels were sensitive to 'cytosolic' Ca2+ concentration, were voltage sensitive at 'cytosolic' Ca2+ concentrations encountered in colonic epithelial cells, and were inhibited by 1 mM quinidine, 20 mM TEA or 5 mM Ba2+ ions. 4. The data show that dietary K+ loading increases the abundance of Ca(2+)- and voltage-sensitive large-conductance K+ channels in the apical membrane of surface cells in rat distal colon. These channels constitute the enhanced macroscopic apical K+ conductance previously identified in these cells, and are likely to play a critical role in the active K+ secretory process that typifies this model of colonic K+ adaptation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. The mechanism of action of Ba2+ and TEA on single Ca2+-activated K+ -channels in arterial and intestinal smooth muscle cell membranes. Pflugers Arch. 1985 Feb;403(2):120–127. doi: 10.1007/BF00584088. [DOI] [PubMed] [Google Scholar]
- Burckhardt B. C., Gögelein H. Small and maxi K+ channels in the basolateral membrane of isolated crypts from rat distal colon: single-channel and slow whole-cell recordings. Pflugers Arch. 1992 Jan;420(1):54–60. doi: 10.1007/BF00378641. [DOI] [PubMed] [Google Scholar]
- Del Castillo J. R., Rajendran V. M., Binder H. J. Apical membrane localization of ouabain-sensitive K(+)-activated ATPase activities in rat distal colon. Am J Physiol. 1991 Dec;261(6 Pt 1):G1005–G1011. doi: 10.1152/ajpgi.1991.261.6.G1005. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Pandol S. J. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest. 1986 Feb;77(2):348–354. doi: 10.1172/JCI112311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener M., Rummel W., Mestres P., Lindemann B. Single chloride channels in colon mucosa and isolated colonic enterocytes of the rat. J Membr Biol. 1989 Apr;108(1):21–30. doi: 10.1007/BF01870422. [DOI] [PubMed] [Google Scholar]
- Edmonds C. J. Amiloride sensitivity of the transepithelial electrical potential and of sodium and potassium transport in rat distal colon in vivo. J Physiol. 1981;313:547–559. doi: 10.1113/jphysiol.1981.sp013681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Foster E. S., Jones W. J., Hayslett J. P., Binder H. J. Role of aldosterone and dietary potassium in potassium adaptation in the distal colon of the rat. Gastroenterology. 1985 Jan;88(1 Pt 1):41–46. doi: 10.1016/s0016-5085(85)80130-x. [DOI] [PubMed] [Google Scholar]
- Foster E. S., Sandle G. I., Hayslett J. P., Binder H. J. Cyclic adenosine monophosphate stimulates active potassium secretion in the rat colon. Gastroenterology. 1983 Feb;84(2):324–330. [PubMed] [Google Scholar]
- Furuya K., Enomoto K., Furuya S., Yamagishi S., Edwards C., Oka T. Single calcium-activated potassium channel in cultured mammary epithelial cells. Pflugers Arch. 1989 Jun;414(2):118–124. doi: 10.1007/BF00580952. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Shapiro A. B., Rao M. C., Layden T. J. In vivo evidence of altered chloride but not potassium secretion in cystic fibrosis rectal mucosa. Gastroenterology. 1991 Oct;101(4):1012–1019. doi: 10.1016/0016-5085(91)90728-4. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K., Pavenstädt H., Greger R. Characterization of potassium channels in respiratory cells. II. Inhibitors and regulation. Pflugers Arch. 1989 Jul;414(3):297–303. doi: 10.1007/BF00584630. [DOI] [PubMed] [Google Scholar]
- Lomax R. B., Warhurst G., Sandle G. I. Characteristics of two basolateral potassium channel populations in human colonic crypts. Gut. 1996 Feb;38(2):243–247. doi: 10.1136/gut.38.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo D. D., Kaunitz J. D. Ca2+ and cAMP activate K+ channels in the basolateral membrane of crypt cells isolated from rabbit distal colon. J Membr Biol. 1989 Aug;110(1):19–28. doi: 10.1007/BF01870989. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Matsunaga H., Hoshi T. Ca2+- and voltage activated K+ channel in apical cell membrane of gallbladder epithelium from Triturus. Pflugers Arch. 1986 Jun;406(6):563–567. doi: 10.1007/BF00584021. [DOI] [PubMed] [Google Scholar]
- McNicholas C. M., Fraser G., Sandle G. I. Properties and regulation of basolateral K+ channels in rat duodenal crypts. J Physiol. 1994 Jun 15;477(Pt 3):381–392. doi: 10.1113/jphysiol.1994.sp020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Lee J. A. A large conductance, voltage- and calcium-activated K+ channel in the basolateral membrane of rat enterocytes. FEBS Lett. 1986 Sep 29;206(1):87–92. doi: 10.1016/0014-5793(86)81346-1. [DOI] [PubMed] [Google Scholar]
- Muto S., Sansom S., Giebisch G. Effects of a high potassium diet on electrical properties of cortical collecting ducts from adrenalectomized rabbits. J Clin Invest. 1988 Feb;81(2):376–380. doi: 10.1172/JCI113329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberleithner H., Kersting U., Hunter M. Cytoplasmic pH determines K+ conductance in fused renal epithelial cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8345–8349. doi: 10.1073/pnas.85.21.8345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzel D., Ganz M. B., Nestler E. J., Lewis J. J., Goldenring J., Akcicek F., Hayslett J. P. Correlates of aldosterone-induced increases in Cai2+ and Isc suggest that Cai2+ is the second messenger for stimulation of apical membrane conductance. J Clin Invest. 1992 Jan;89(1):150–156. doi: 10.1172/JCI115555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandle G. I., Foster E. S., Lewis S. A., Binder H. J., Hayslett J. P. The electrical basis for enhanced potassium secretion in rat distal colon during dietary potassium loading. Pflugers Arch. 1985 Apr;403(4):433–439. doi: 10.1007/BF00589258. [DOI] [PubMed] [Google Scholar]
- Sandle G. I., Gaiger E., Tapster S., Goodship T. H. Enhanced rectal potassium secretion in chronic renal insufficiency: evidence for large intestinal potassium adaptation in man. Clin Sci (Lond) 1986 Oct;71(4):393–401. doi: 10.1042/cs0710393. [DOI] [PubMed] [Google Scholar]
- Sandle G. I., McGlone F. Segmental variability of membrane conductances in rat and human colonic epithelia. Implications for Na, K and Cl transport. Pflugers Arch. 1987 Sep;410(1-2):173–180. doi: 10.1007/BF00581912. [DOI] [PubMed] [Google Scholar]
- Siemer C., Gögelein H. Activation of nonselective cation channels in the basolateral membrane of rat distal colon crypt cells by prostaglandin E2. Pflugers Arch. 1992 Mar;420(3-4):319–328. doi: 10.1007/BF00374465. [DOI] [PubMed] [Google Scholar]
- Stieger B., Marxer A., Hauri H. P. Isolation of brush-border membranes from rat and rabbit colonocytes: is alkaline phosphatase a marker enzyme? J Membr Biol. 1986;91(1):19–31. doi: 10.1007/BF01870211. [DOI] [PubMed] [Google Scholar]
- Sweiry J. H., Binder H. J. Characterization of aldosterone-induced potassium secretion in rat distal colon. J Clin Invest. 1989 Mar;83(3):844–851. doi: 10.1172/JCI113967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warhurst G., Turnberg L. A., Higgs N. B., Tonge A., Grundy J., Fogg K. E. Multiple G-protein-dependent pathways mediate the antisecretory effects of somatostatin and clonidine in the HT29-19A colonic cell line. J Clin Invest. 1993 Aug;92(2):603–611. doi: 10.1172/JCI116627. [DOI] [PMC free article] [PubMed] [Google Scholar]


