Abstract
1. The action of the two diastereometric phosphorothioate derivatives of cAMP, Rp-cAMPs and Sp-cAMPs, was investigated on hyperpolarization-activated 'pacemaker' current (i(f)) recorded in inside-out macropatches from rabbit sino-atrial (SA) node myocytes. 2. When superfused on the intracellular side of f-channels at the concentration of 10 microM, both cAMP derivatives accelerated i(f) activation; their action was moderately less pronounced than that due to the same concentration of cAMP. 3. The measurement of the i(f) conductance-voltage relation by voltage ramp protocols indicated that both cAMP analogues shift the activation curve of i(f) to more positive voltages with no change in maximal (fully activated) conductance. 4. Dose-response relationships of the shift of the i(f) activation curve showed that both Rp-cAMPs and Sp-cAMPs act as agonists in the cAMP-dependent direct f-channel activation. Fitting data to the Hill equation resulted in maximal shifts of 9.6 and 9.5 mV, apparent dissociation constants of 0.82 and 5.4 microM, and Hill coefficients of 0.82 and 1.12 for Sp-cAMPs and Rp-cAMPs, respectively. 5. The activating action of Rp-cAMPs, a known antagonist of cAMP in the activation of cAMP-dependent protein kinase, confirms previously established evidence that f-channel activation does not involve phosphorylation. These results also suggest that the cAMP binding site of f-channels may be structurally similar to the cyclic nucleotide binding site of olfactory receptor channels.
Full text
PDF

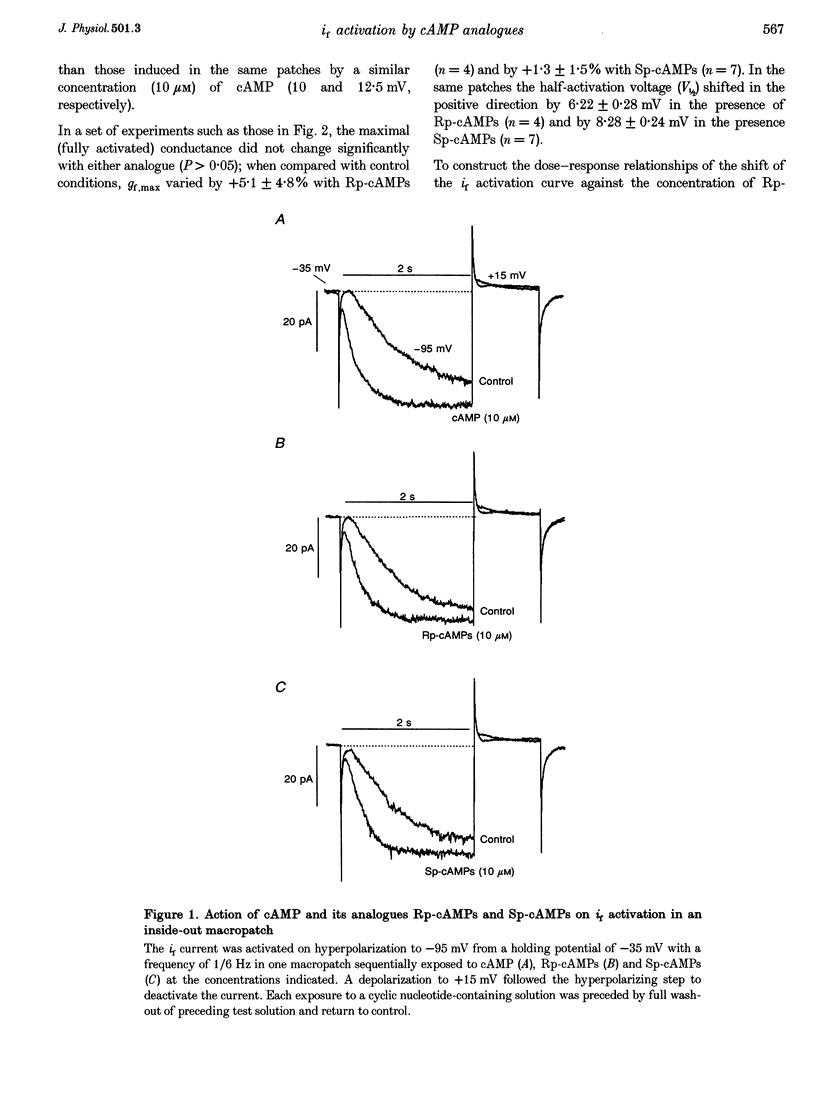
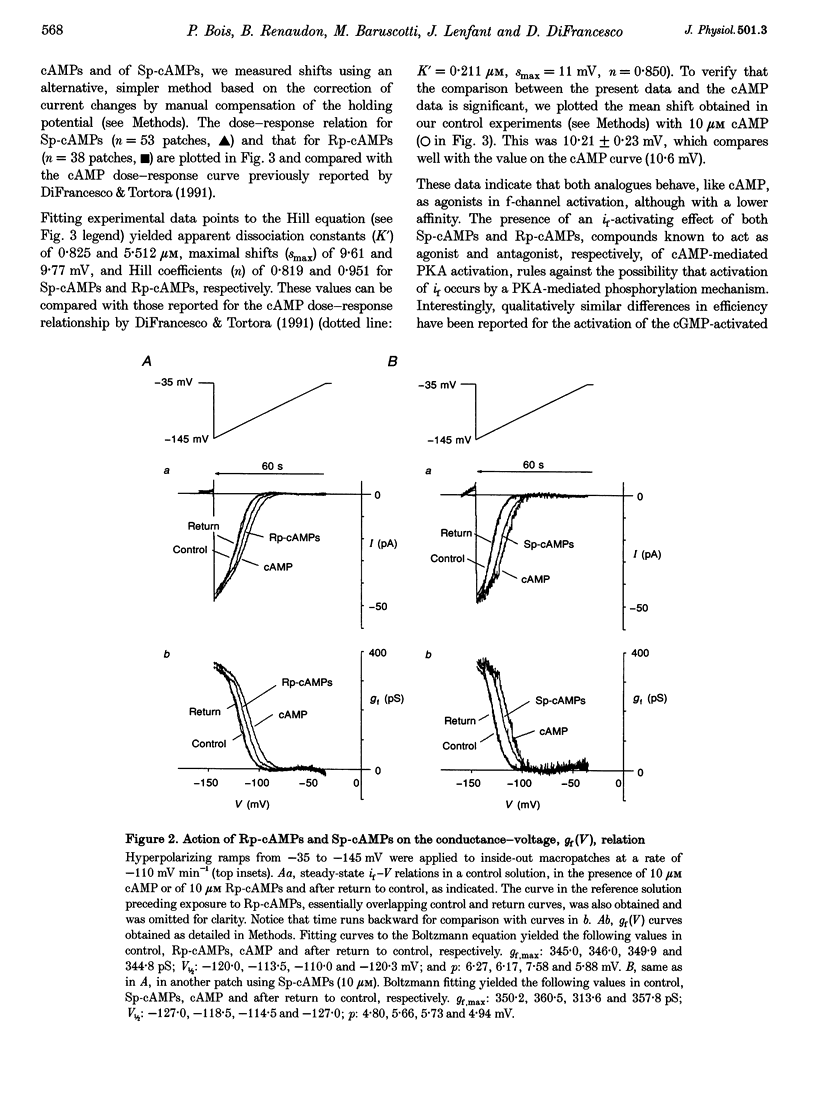
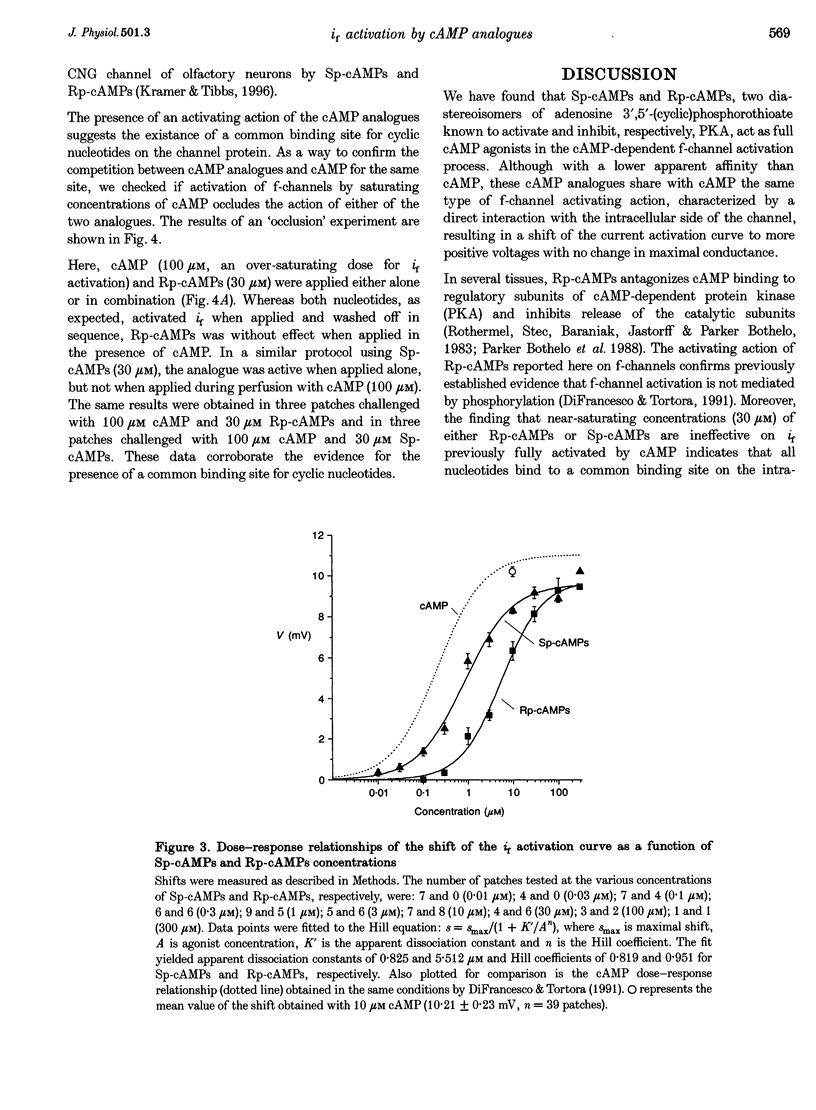
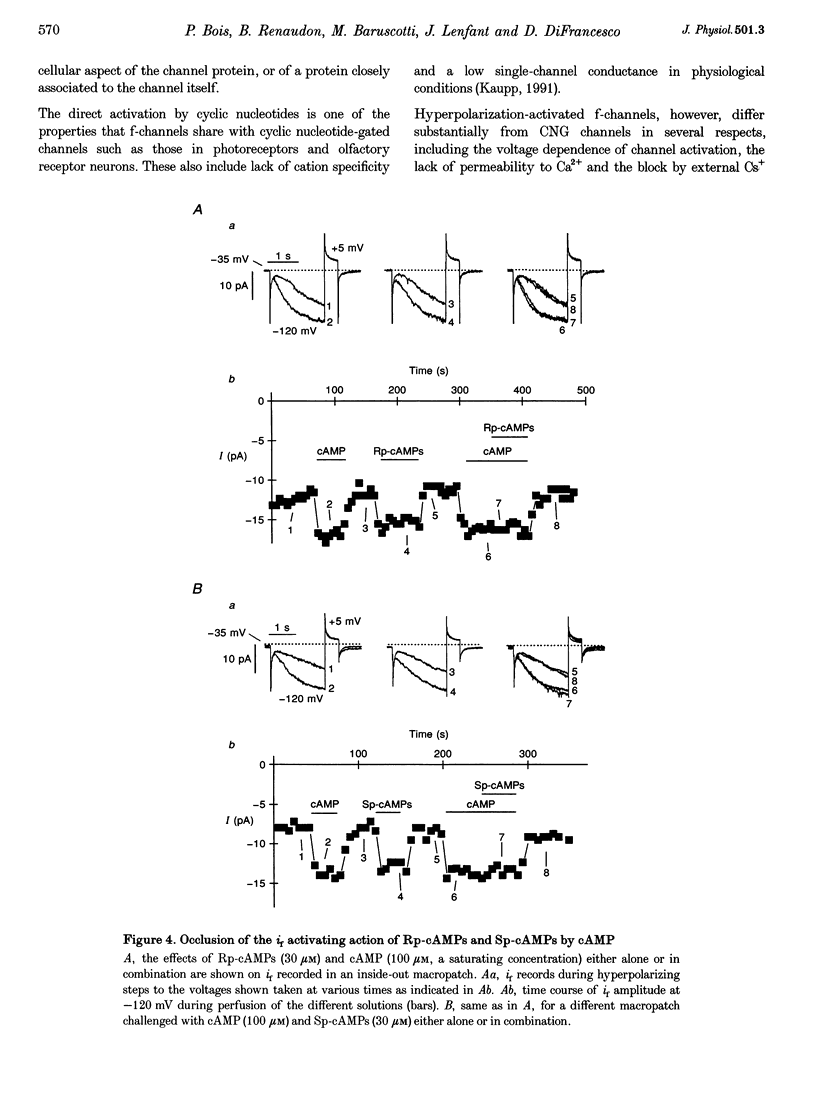

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accili E. A., DiFrancesco D. Inhibition of the hyperpolarization-activated current (if) of rabbit SA node myocytes by niflumic acid. Pflugers Arch. 1996 Mar;431(5):757–762. doi: 10.1007/BF02253840. [DOI] [PubMed] [Google Scholar]
- Botelho L. H., Rothermel J. D., Coombs R. V., Jastorff B. cAMP analog antagonists of cAMP action. Methods Enzymol. 1988;159:159–172. doi: 10.1016/0076-6879(88)59017-1. [DOI] [PubMed] [Google Scholar]
- Chen T. Y., Peng Y. W., Dhallan R. S., Ahamed B., Reed R. R., Yau K. W. A new subunit of the cyclic nucleotide-gated cation channel in retinal rods. Nature. 1993 Apr 22;362(6422):764–767. doi: 10.1038/362764a0. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Mazzanti M., Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986 Aug;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Mangoni M. Modulation of single hyperpolarization-activated channels (i(f)) by cAMP in the rabbit sino-atrial node. J Physiol. 1994 Feb 1;474(3):473–482. doi: 10.1113/jphysiol.1994.sp020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Noble D. A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Philos Trans R Soc Lond B Biol Sci. 1985 Jan 10;307(1133):353–398. doi: 10.1098/rstb.1985.0001. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991 May 9;351(6322):145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- Goulding E. H., Ngai J., Kramer R. H., Colicos S., Axel R., Siegelbaum S. A., Chess A. Molecular cloning and single-channel properties of the cyclic nucleotide-gated channel from catfish olfactory neurons. Neuron. 1992 Jan;8(1):45–58. doi: 10.1016/0896-6273(92)90107-o. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B., Niidome T., Tanabe T., Terada S., Bönigk W., Stühmer W., Cook N. J., Kangawa K., Matsuo H., Hirose T. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature. 1989 Dec 14;342(6251):762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B. The cyclic nucleotide-gated channels of vertebrate photoreceptors and olfactory epithelium. Trends Neurosci. 1991 Apr;14(4):150–157. doi: 10.1016/0166-2236(91)90087-b. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Tibbs G. R. Antagonists of cyclic nucleotide-gated channels and molecular mapping of their site of action. J Neurosci. 1996 Feb 15;16(4):1285–1293. doi: 10.1523/JNEUROSCI.16-04-01285.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel J. D., Stec W. J., Baraniak J., Jastorff B., Botelho L. H. Inhibition of glycogenolysis in isolated rat hepatocytes by the Rp diastereomer of adenosine cyclic 3',5'-phosphorothioate. J Biol Chem. 1983 Oct 25;258(20):12125–12128. [PubMed] [Google Scholar]
- Yatani A., Brown A. M. Regulation of cardiac pacemaker current If in excised membranes from sinoatrial node cells. Am J Physiol. 1990 Jun;258(6 Pt 2):H1947–H1951. doi: 10.1152/ajpheart.1990.258.6.H1947. [DOI] [PubMed] [Google Scholar]
- Zagotta W. N., Siegelbaum S. A. Structure and function of cyclic nucleotide-gated channels. Annu Rev Neurosci. 1996;19:235–263. doi: 10.1146/annurev.ne.19.030196.001315. [DOI] [PubMed] [Google Scholar]


