Abstract
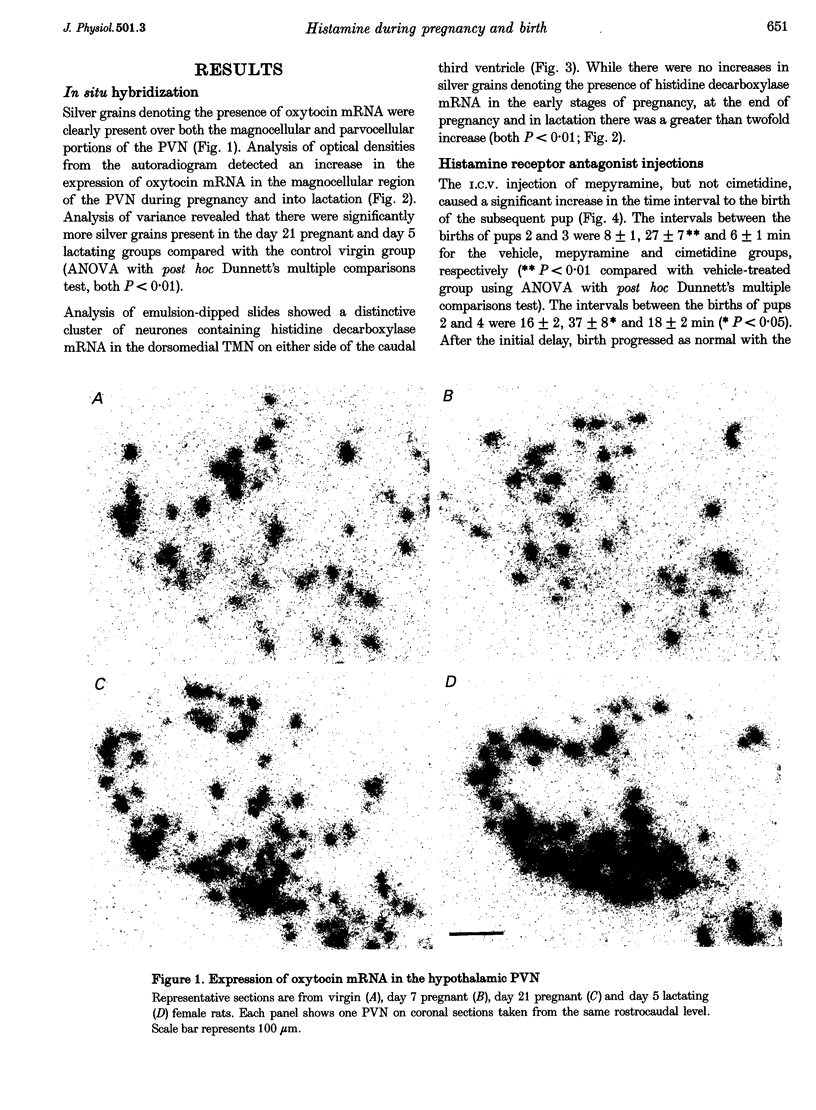
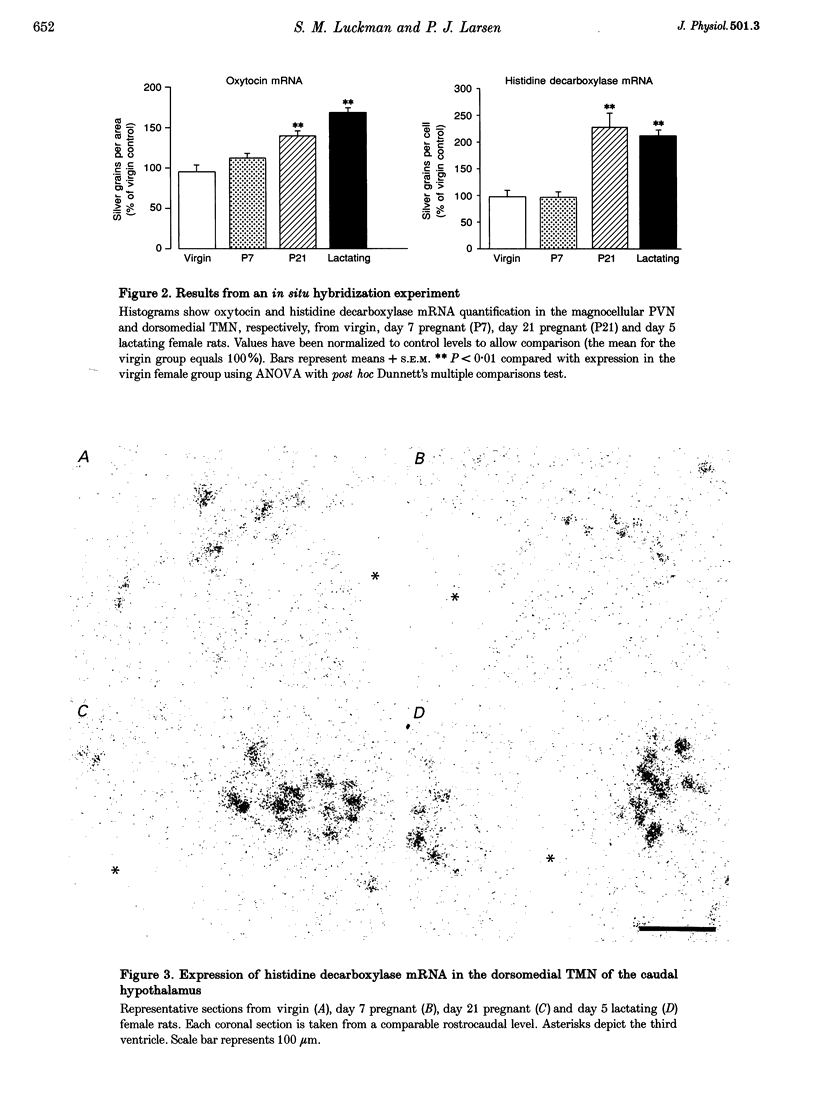
1. Previous studies have shown that histaminergic neurones of the tuberomammillary nucleus project directly to hypothalamic magnocellular nuclei and that intracerebroventricular administration of histamine increases the synthetic activity of magnocellular oxytocin neurones. 2. Histaminergic neurones of the dorsomedial tuberomammillary nucleus that project to the magnocellular region of the paraventricular nucleus are activated during late pregnancy and lactation, as measured by an increase in mRNA for the synthetic enzyme histidine decarboxylase. 3. There is a concomitant increase in oxytocin mRNA in magnocellular neurones of the paraventricular nucleus. This increase in mRNA contributes to an accumulation of oxytocin before birth and to continued oxytocin synthesis during lactation. 4. Intracerebroventricular administration of mepyramine, a specific antagonist of the H1 histamine receptor, causes a delay in the birth of subsequent pups if given to the mother during parturition. Vehicle or the H2 receptor antagonist cimetidine has no effect. Thus, histamine acts centrally, via H1 receptors, during parturition and may have an excitatory effect on oxytocin release. 5. These results suggest that afferent histaminergic neurones show increased activity during pregnancy and may be responsible for the increase of synthesis in magnocellular oxytocin neurones at this time. If, as previously reported, these histamine neurones can reduce the electrical activity of oxytocin neurones via H2 receptors, then they may have a dual effect, increasing the synthesis of oxytocin while inhibiting its premature release. At term, any inhibitory effects of histamine are overcome to allow oxytocin secretion.
Full text
PDF

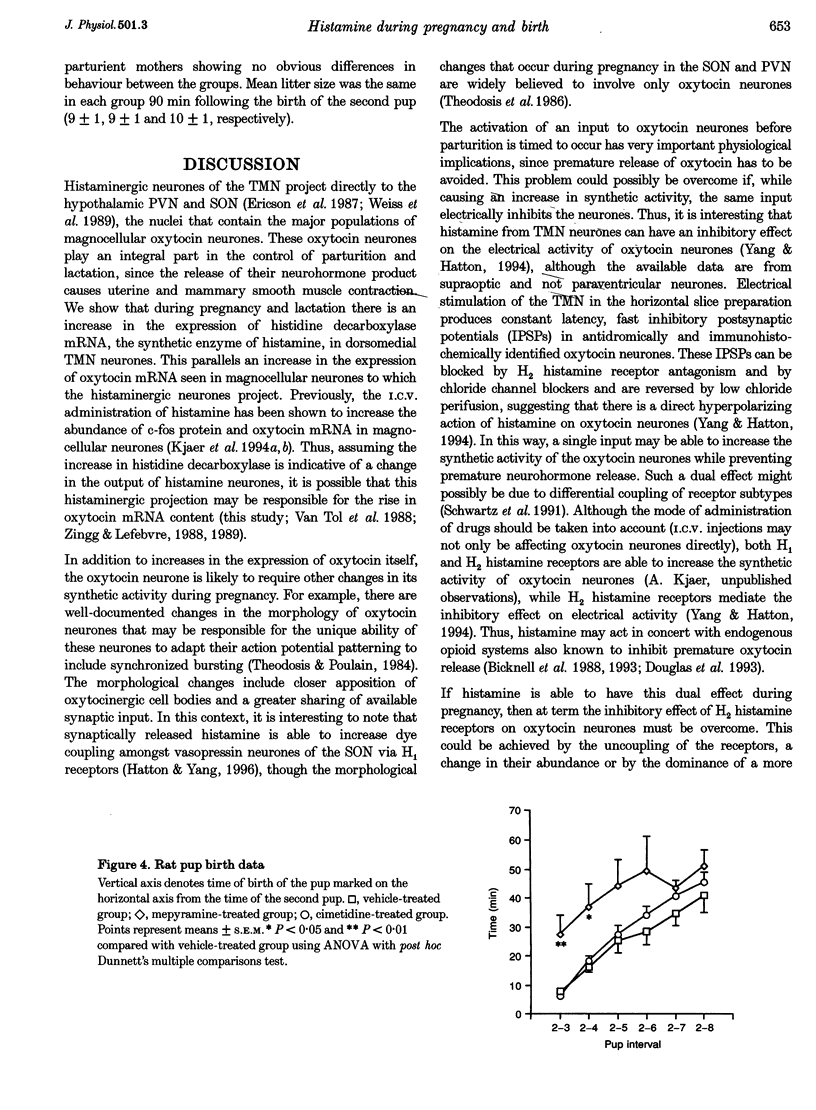


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong W. E., Sladek C. D. Evidence for excitatory actions of histamine on supraoptic neurons in vitro: mediation by an H1-type receptor. Neuroscience. 1985 Oct;16(2):307–322. doi: 10.1016/0306-4522(85)90004-1. [DOI] [PubMed] [Google Scholar]
- Bethea C. L., Kohama S. G., Widmann A. A. Search for progestin receptors (PR) in prolactin-releasing peptidergic neurons: oxytocin neurons lack PR, but respond to gonadal steroids in monkeys. Endocrinology. 1994 Feb;134(2):945–953. doi: 10.1210/endo.134.2.8299589. [DOI] [PubMed] [Google Scholar]
- Bicknell R. J., Leng G., Russell J. A., Dyer R. G., Mansfield S., Zhao B. G. Hypothalamic opioid mechanisms controlling oxytocin neurones during parturition. Brain Res Bull. 1988 Jun;20(6):743–749. doi: 10.1016/0361-9230(88)90086-x. [DOI] [PubMed] [Google Scholar]
- Douglas A. J., Dye S., Leng G., Russell J. A., Bicknell R. J. Endogenous opioid regulation of oxytocin secretion through pregnancy in the rat. J Neuroendocrinol. 1993 Jun;5(3):307–314. doi: 10.1111/j.1365-2826.1993.tb00487.x. [DOI] [PubMed] [Google Scholar]
- Ericson H., Watanabe T., Köhler C. Morphological analysis of the tuberomammillary nucleus in the rat brain: delineation of subgroups with antibody against L-histidine decarboxylase as a marker. J Comp Neurol. 1987 Sep 1;263(1):1–24. doi: 10.1002/cne.902630102. [DOI] [PubMed] [Google Scholar]
- Fox S. R., Harlan R. E., Shivers B. D., Pfaff D. W. Chemical characterization of neuroendocrine targets for progesterone in the female rat brain and pituitary. Neuroendocrinology. 1990 Mar;51(3):276–283. doi: 10.1159/000125350. [DOI] [PubMed] [Google Scholar]
- Hatton G. I., Yang Q. Z. Synaptically released histamine increases dye coupling among vasopressinergic neurons of the supraoptic nucleus: mediation by H1 receptors and cyclic nucleotides. J Neurosci. 1996 Jan;16(1):123–129. doi: 10.1523/JNEUROSCI.16-01-00123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivell R., Richter D. Structure and comparison of the oxytocin and vasopressin genes from rat. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2006–2010. doi: 10.1073/pnas.81.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph D. R., Sullivan P. M., Wang Y. M., Kozak C., Fenstermacher D. A., Behrendsen M. E., Zahnow C. A. Characterization and expression of the complementary DNA encoding rat histidine decarboxylase. Proc Natl Acad Sci U S A. 1990 Jan;87(2):733–737. doi: 10.1073/pnas.87.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer A., Knigge U., Warberg J. Involvement of oxytocin in histamine- and stress-induced ACTH and prolactin secretion. Neuroendocrinology. 1995 Jun;61(6):704–713. doi: 10.1159/000126898. [DOI] [PubMed] [Google Scholar]
- Kjaer A., Larsen P. J., Knigge U., Møller M., Warberg J. Histamine stimulates c-fos expression in hypothalamic vasopressin-, oxytocin-, and corticotropin-releasing hormone-containing neurons. Endocrinology. 1994 Jan;134(1):482–491. doi: 10.1210/endo.134.1.8275963. [DOI] [PubMed] [Google Scholar]
- Kjaer A., Larsen P. J., Knigge U., Warberg J. Histaminergic activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 1994 Sep;135(3):1171–1177. doi: 10.1210/endo.135.3.8070360. [DOI] [PubMed] [Google Scholar]
- Li Z., Hatton G. I. Histamine-induced prolonged depolarization in rat supraoptic neurons: G-protein-mediated, Ca(2+)-independent suppression of K+ leakage conductance. Neuroscience. 1996 Jan;70(1):145–158. doi: 10.1016/0306-4522(95)00373-q. [DOI] [PubMed] [Google Scholar]
- Luckman S. M., Antonijevic I., Leng G., Dye S., Douglas A. J., Russell J. A., Bicknell R. J. The maintenance of normal parturition in the rat requires neurohypophysial oxytocin. J Neuroendocrinol. 1993 Feb;5(1):7–12. doi: 10.1111/j.1365-2826.1993.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Rhodes C. H., Morrell J. I., Pfaff D. W. Distribution of estrogen-concentrating, neurophysin-containing magnocellular neurons in the rat hypothalamus as demonstrated by a technique combining steroid autoradiography and immunohistology in the same tissue. Neuroendocrinology. 1981 Jul;33(1):18–23. doi: 10.1159/000123195. [DOI] [PubMed] [Google Scholar]
- Sar M. Distribution of progestin-concentrating cells in rat brain: colocalization of [3H]ORG.2058, a synthetic progestin, and antibodies to tyrosine hydroxylase in hypothalamus by combined autoradiography and immunocytochemistry. Endocrinology. 1988 Aug;123(2):1110–1118. doi: 10.1210/endo-123-2-1110. [DOI] [PubMed] [Google Scholar]
- Schagen F. H., Knigge U., Kjaer A., Larsen P. J., Warberg J. Involvement of histamine in suckling-induced release of oxytocin, prolactin and adrenocorticotropin in lactating rats. Neuroendocrinology. 1996 Jun;63(6):550–558. doi: 10.1159/000127084. [DOI] [PubMed] [Google Scholar]
- Schwartz J. C., Arrang J. M., Garbarg M., Pollard H., Ruat M. Histaminergic transmission in the mammalian brain. Physiol Rev. 1991 Jan;71(1):1–51. doi: 10.1152/physrev.1991.71.1.1. [DOI] [PubMed] [Google Scholar]
- Smith B. N., Armstrong W. E. Histamine enhances the depolarizing afterpotential of immunohistochemically identified vasopressin neurons in the rat supraoptic nucleus via H1-receptor activation. Neuroscience. 1993 Apr;53(3):855–864. doi: 10.1016/0306-4522(93)90630-x. [DOI] [PubMed] [Google Scholar]
- Theodosis D. T., Chapman D. B., Montagnese C., Poulain D. A., Morris J. F. Structural plasticity in the hypothalamic supraoptic nucleus at lactation affects oxytocin-, but not vasopressin-secreting neurones. Neuroscience. 1986 Mar;17(3):661–678. doi: 10.1016/0306-4522(86)90038-2. [DOI] [PubMed] [Google Scholar]
- Theodosis D. T., Poulain D. A. Evidence for structural plasticity in the supraoptic nucleus of the rat hypothalamus in relation to gestation and lactation. Neuroscience. 1984 Jan;11(1):183–193. doi: 10.1016/0306-4522(84)90222-7. [DOI] [PubMed] [Google Scholar]
- Van Tol H. H., Bolwerk E. L., Liu B., Burbach J. P. Oxytocin and vasopressin gene expression in the hypothalamo-neurohypophyseal system of the rat during the estrous cycle, pregnancy, and lactation. Endocrinology. 1988 Mar;122(3):945–951. doi: 10.1210/endo-122-3-945. [DOI] [PubMed] [Google Scholar]
- Wada H., Inagaki N., Yamatodani A., Watanabe T. Is the histaminergic neuron system a regulatory center for whole-brain activity? Trends Neurosci. 1991 Sep;14(9):415–418. doi: 10.1016/0166-2236(91)90034-r. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Taguchi Y., Shiosaka S., Tanaka J., Kubota H., Terano Y., Tohyama M., Wada H. Distribution of the histaminergic neuron system in the central nervous system of rats; a fluorescent immunohistochemical analysis with histidine decarboxylase as a marker. Brain Res. 1984 Mar 12;295(1):13–25. doi: 10.1016/0006-8993(84)90811-4. [DOI] [PubMed] [Google Scholar]
- Weiss M. L., Yang Q. Z., Hatton G. I. Magnocellular tuberomammillary nucleus input to the supraoptic nucleus in the rat: anatomical and in vitro electrophysiological investigations. Neuroscience. 1989;31(2):299–311. doi: 10.1016/0306-4522(89)90375-8. [DOI] [PubMed] [Google Scholar]
- Yang Q. Z., Hatton G. I. Histamine mediates fast synaptic inhibition of rat supraoptic oxytocin neurons via chloride conductance activation. Neuroscience. 1994 Aug;61(4):955–964. doi: 10.1016/0306-4522(94)90415-4. [DOI] [PubMed] [Google Scholar]
- Zingg H. H., Lefebvre D. L. Oxytocin and vasopressin gene expression during gestation and lactation. Brain Res. 1988 Aug;464(1):1–6. doi: 10.1016/0169-328x(88)90011-3. [DOI] [PubMed] [Google Scholar]
- Zingg H. H., Lefebvre D. L. Oxytocin mRNA: increase of polyadenylate tail size during pregnancy and lactation. Mol Cell Endocrinol. 1989 Aug;65(1-2):59–62. doi: 10.1016/0303-7207(89)90165-2. [DOI] [PubMed] [Google Scholar]