Abstract
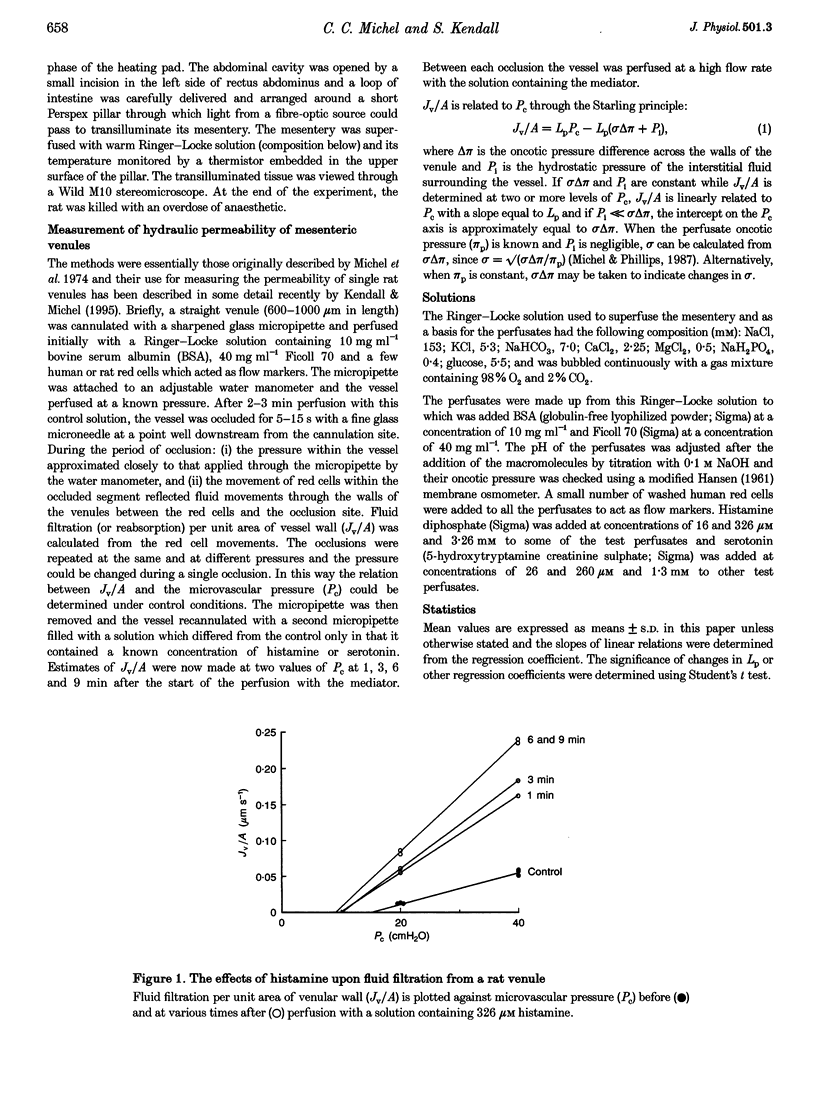
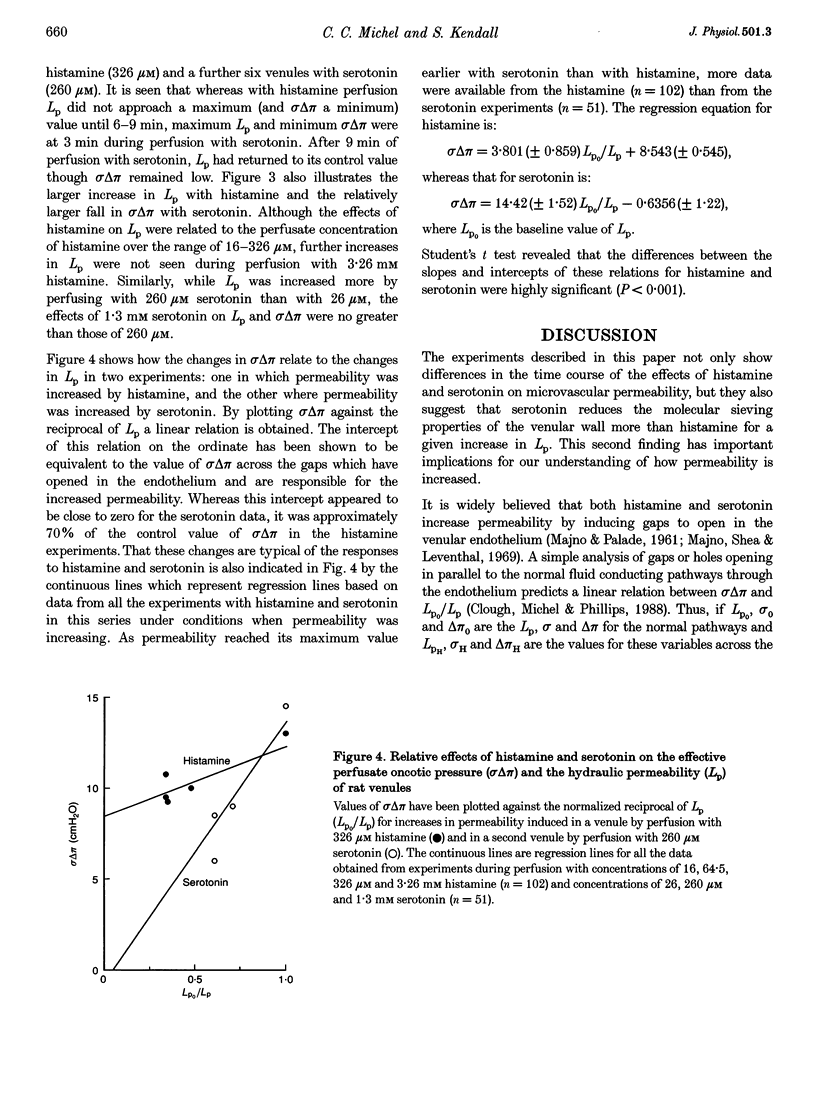
1. We have investigated simultaneous changes in the hydraulic permeability (Lp) and the retention of perfusate macromolecules in single mesenteric venules of anaesthetized rats during perfusion with either histamine or serotonin. 2. The venules were microperfused in situ. Retention of macromolecules was assessed from the effective oncotic pressure (omega delta pi) exerted by the perfusate across the vessel walls. Lp and omega delta pi were estimated by the red cell microperfusion technique. 3. Perfusion with histamine (at concentrations between 16 microM and 3.26 mM) and serotonin (at concentrations between 26 microM and 1.3 mM) transiently increased Lp and reduced omega delta pi. Maximal changes were seen at 6-9 min with histamine and at 3 min with serotonin. 4. Maximal increases in Lp were greater with histamine (approximately 3-fold) than with serotonin (1.5- to 2-fold). Serotonin, however, decreased omega delta pi from a baseline of 14-15 cmH2O to one of 6-7 cmH2O whereas the fall of omega delta pi with histamine was only from 14-15 cmH2O to 10-11 cmH2O. 5. The data are consistent with the hypothesis that serotonin increases permeability by inducing openings in the venular endothelium which do not retain macromolecules. If histamine also increases permeability by gap formation, these gaps are able to retain macromolecules to a significant extent.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clough G., Michel C. C., Phillips M. E. Inflammatory changes in permeability and ultrastructure of single vessels in the frog mesenteric microcirculation. J Physiol. 1988 Jan;395:99–114. doi: 10.1113/jphysiol.1988.sp016910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana J. N., Long S. C., Yao H. Effect of histamine on equivalent pore radius in capillaries of isolated dog hindlimb. Microvasc Res. 1972 Oct;4(4):413–437. doi: 10.1016/0026-2862(72)90074-x. [DOI] [PubMed] [Google Scholar]
- Feng D., Nagy J. A., Hipp J., Dvorak H. F., Dvorak A. M. Vesiculo-vacuolar organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine, and serotonin. J Exp Med. 1996 May 1;183(5):1981–1986. doi: 10.1084/jem.183.5.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J., Galey F., Wayland H. Action of histamine on the mesenteric microvasculature. Microvasc Res. 1980 Jan;19(1):108–126. doi: 10.1016/0026-2862(80)90087-4. [DOI] [PubMed] [Google Scholar]
- Kendall S., Michel C. C. The measurement of permeability in single rat venules using the red cell microperfusion technique. Exp Physiol. 1995 May;80(3):359–372. doi: 10.1113/expphysiol.1995.sp003853. [DOI] [PubMed] [Google Scholar]
- Koller M. E., Woie K., Reed R. K. Increased negativity of interstitial fluid pressure in rat trachea after mast cell degranulation. J Appl Physiol (1985) 1993 May;74(5):2135–2139. doi: 10.1152/jappl.1993.74.5.2135. [DOI] [PubMed] [Google Scholar]
- Korthuis R. J., Wang C. Y., Scott J. B. Transient effects of histamine on microvascular fluid movement. Microvasc Res. 1982 May;23(3):316–328. doi: 10.1016/s0026-2862(82)80004-6. [DOI] [PubMed] [Google Scholar]
- Lund T., Wiig H., Reed R. K. Acute postburn edema: role of strongly negative interstitial fluid pressure. Am J Physiol. 1988 Nov;255(5 Pt 2):H1069–H1074. doi: 10.1152/ajpheart.1988.255.5.H1069. [DOI] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G., Shea S. M., Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol. 1969 Sep;42(3):647–672. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C. C., Mason J. C., Curry F. E., Tooke J. E., Hunter P. J. A development of the Landis technique for measuring the filtration coefficient of individual capillaries in the frog mesentery. Q J Exp Physiol Cogn Med Sci. 1974 Oct;59(4):283–309. doi: 10.1113/expphysiol.1974.sp002275. [DOI] [PubMed] [Google Scholar]
- Michel C. C., Phillips M. E. Steady-state fluid filtration at different capillary pressures in perfused frog mesenteric capillaries. J Physiol. 1987 Jul;388:421–435. doi: 10.1113/jphysiol.1987.sp016622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal C. R., Michel C. C. Transcellular gaps in microvascular walls of frog and rat when permeability is increased by perfusion with the ionophore A23187. J Physiol. 1995 Oct 15;488(Pt 2):427–437. doi: 10.1113/jphysiol.1995.sp020977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R. K., Rodt S. A. Increased negativity of interstitial fluid pressure during the onset stage of inflammatory edema in rat skin. Am J Physiol. 1991 Jun;260(6 Pt 2):H1985–H1991. doi: 10.1152/ajpheart.1991.260.6.H1985. [DOI] [PubMed] [Google Scholar]
- Renkin E. M., Carter R. D., Joyner W. L. Mechanism of the sustained action of histamine and bradykinin on transport of large molecules across capillary walls in the dog paw. Microvasc Res. 1974 Jan;7(1):49–60. doi: 10.1016/0026-2862(74)90036-3. [DOI] [PubMed] [Google Scholar]


