Abstract
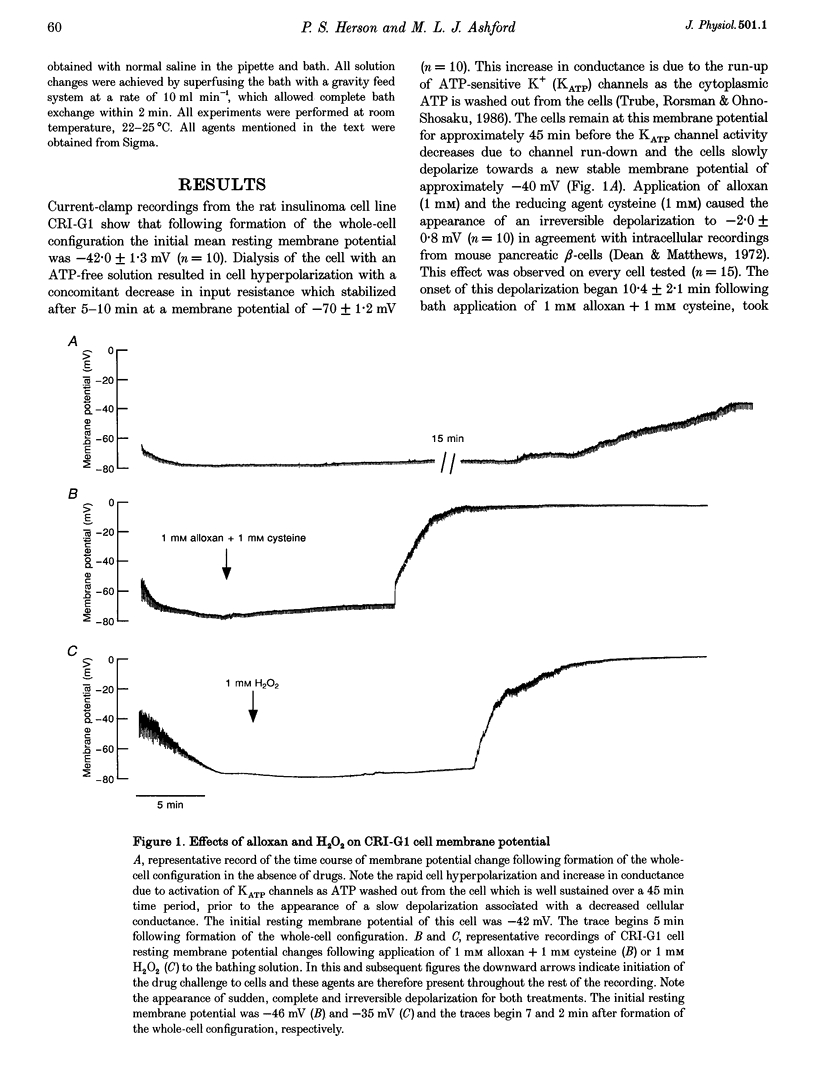
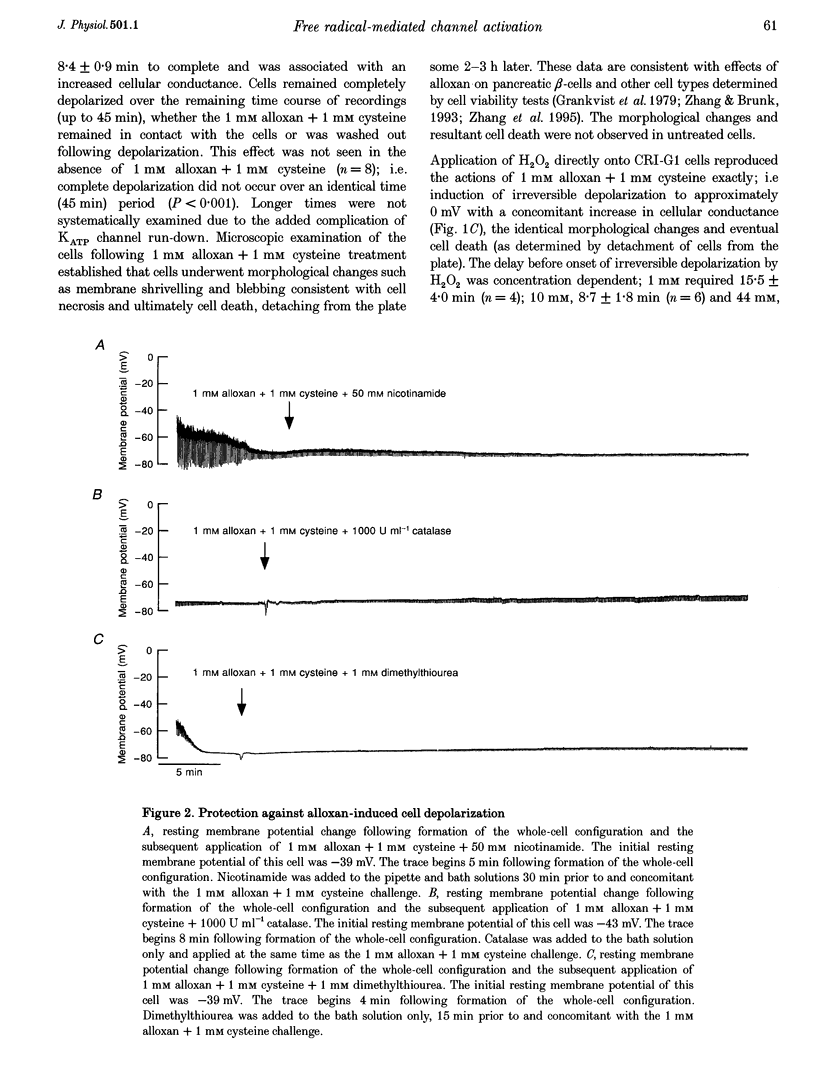
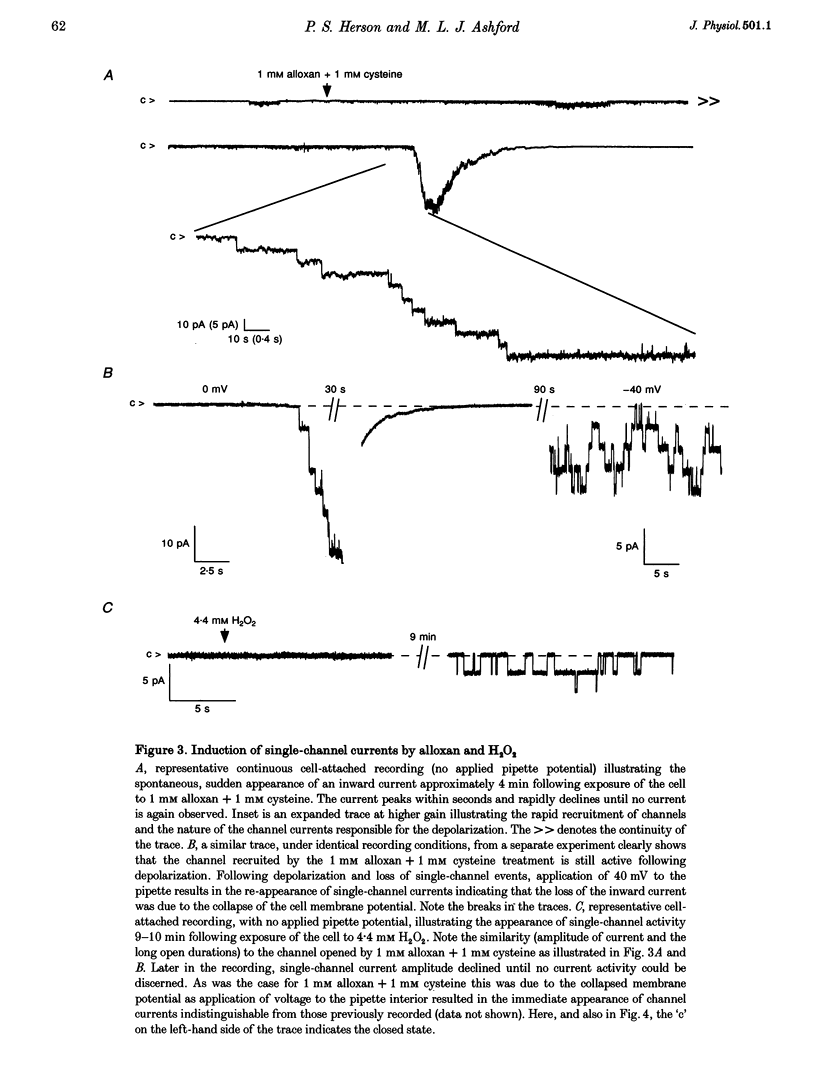
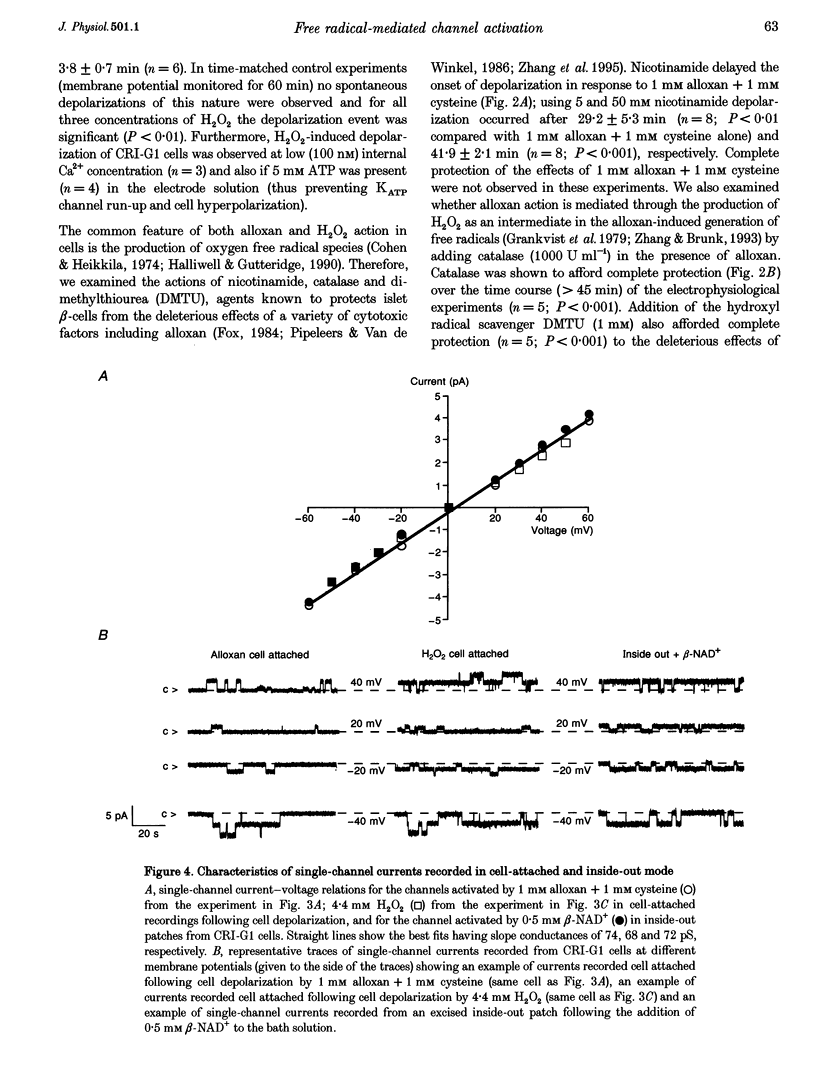
1. Alloxan and its auto-oxidation product hydrogen peroxide (H2O2) irreversibly depolarize insulinoma cells by opening a non-selective cation channel. The channel opened is characterized by a linear current-voltage relation with a conductance of approximately 70 pS and very slow kinetics (of the order of seconds). 2. Cells are protected against the alloxan-induced channel opening and consequent cell depolarization by the presence of H2O2 and hydroxyl radical scavengers. 3. The free radical-activated non-selective cation channel is not operative in isolated patches but can be activated by the application of beta-NAD+ to the cytoplasmic aspect of the membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carrington C. A., Rubery E. D., Pearson E. C., Hales C. N. Five new insulin-producing cell lines with differing secretory properties. J Endocrinol. 1986 May;109(2):193–200. doi: 10.1677/joe.0.1090193. [DOI] [PubMed] [Google Scholar]
- Cohen G., Heikkila R. E. The generation of hydrogen peroxide, superoxide radical, and hydroxyl radical by 6-hydroxydopamine, dialuric acid, and related cytotoxic agents. J Biol Chem. 1974 Apr 25;249(8):2447–2452. [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. The bioelectrical properties of pancreatic islet cells: effects of diabetogenic agents. Diabetologia. 1972 Jul;8(3):173–178. doi: 10.1007/BF01212257. [DOI] [PubMed] [Google Scholar]
- Fischer L. J., Hamburger S. A. Inhibition of alloxan action in isolated pancreatic islets by superoxide dismutase, catalase, and a metal chelator. Diabetes. 1980 Mar;29(3):213–216. doi: 10.2337/diab.29.3.213. [DOI] [PubMed] [Google Scholar]
- Fox R. B. Prevention of granulocyte-mediated oxidant lung injury in rats by a hydroxyl radical scavenger, dimethylthiourea. J Clin Invest. 1984 Oct;74(4):1456–1464. doi: 10.1172/JCI111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grankvist K., Marklund S., Sehlin J., Täljedal I. B. Superoxide dismutase, catalase and scavengers of hydroxyl radical protect against the toxic action of alloxan on pancreatic islet cells in vitro. Biochem J. 1979 Jul 15;182(1):17–25. doi: 10.1042/bj1820017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M. Free radicals in disease processes: a compilation of cause and consequence. Free Radic Res Commun. 1993;19(3):141–158. doi: 10.3109/10715769309111598. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Koliwad S. K., Elliott S. J., Kunze D. L. Oxidized glutathione mediates cation channel activation in calf vascular endothelial cells during oxidant stress. J Physiol. 1996 Aug 15;495(Pt 1):37–49. doi: 10.1113/jphysiol.1996.sp021572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomikos I. N., Prowse S. J., Carotenuto P., Lafferty K. J. Combined treatment with nicotinamide and desferrioxamine prevents islet allograft destruction in NOD mice. Diabetes. 1986 Nov;35(11):1302–1304. doi: 10.2337/diab.35.11.1302. [DOI] [PubMed] [Google Scholar]
- Pipeleers D., Van de Winkel M. Pancreatic B cells possess defense mechanisms against cell-specific toxicity. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5267–5271. doi: 10.1073/pnas.83.14.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pociot F., Reimers J. I., Andersen H. U. Nicotinamide--biological actions and therapeutic potential in diabetes prevention. IDIG Workshop, Copenhagen, Denmark, 4-5 December 1992. Diabetologia. 1993 Jun;36(6):574–576. doi: 10.1007/BF02743277. [DOI] [PubMed] [Google Scholar]
- Reale V., Hales C. N., Ashford M. L. The effects of pyridine nucleotides on the activity of a calcium-activated nonselective cation channel in the rat insulinoma cell line, CRI-G1. J Membr Biol. 1994 Dec;142(3):299–307. doi: 10.1007/BF00233437. [DOI] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- Wilson G. L., Patton N. J., McCord J. M., Mullins D. W., Mossman B. T. Mechanisms of streptozotocin- and alloxan-induced damage in rat B cells. Diabetologia. 1984 Dec;27(6):587–591. doi: 10.1007/BF00276973. [DOI] [PubMed] [Google Scholar]
- Zhang H., Brunk U. T. Alloxan cytotoxicity is highly potentiated by plasma membrane- and lysosomal-associated iron--a study on a model system of cultured J-774 cells. Diabetologia. 1993 Aug;36(8):707–715. doi: 10.1007/BF00401140. [DOI] [PubMed] [Google Scholar]
- Zhang H., Ollinger K., Brunk U. Insulinoma cells in culture show pronounced sensitivity to alloxan-induced oxidative stress. Diabetologia. 1995 Jun;38(6):635–641. doi: 10.1007/BF00401832. [DOI] [PubMed] [Google Scholar]


