Abstract
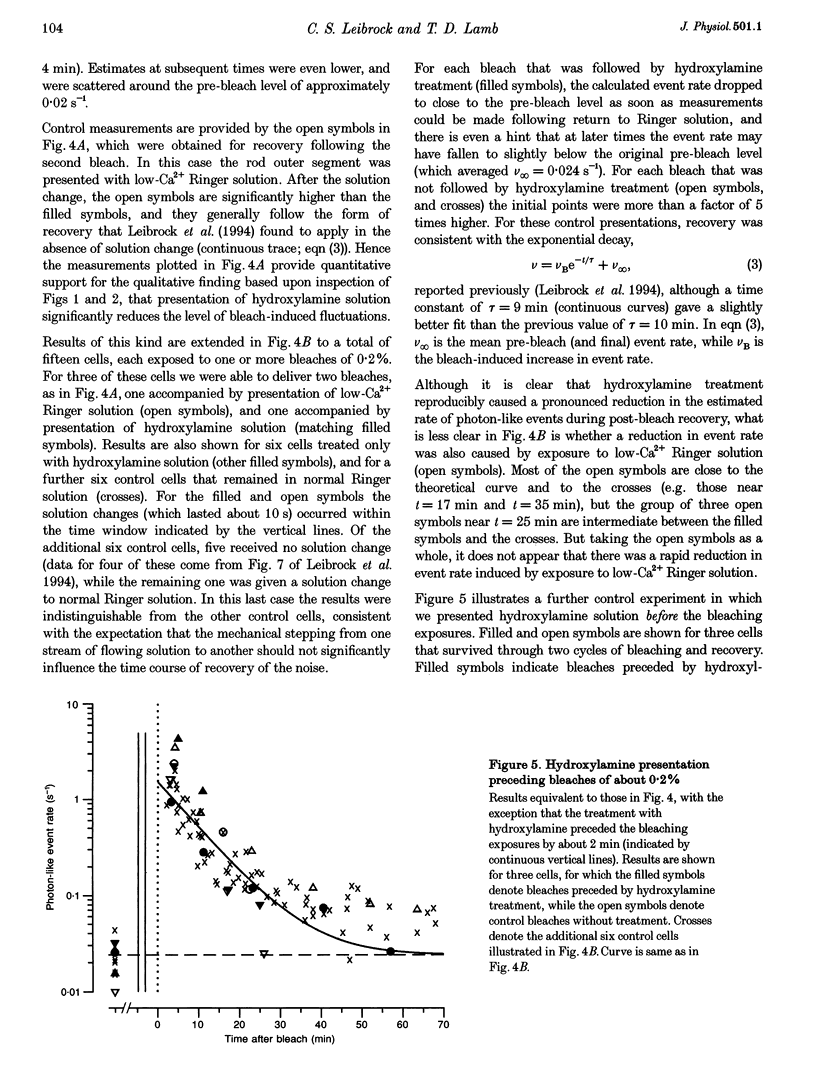
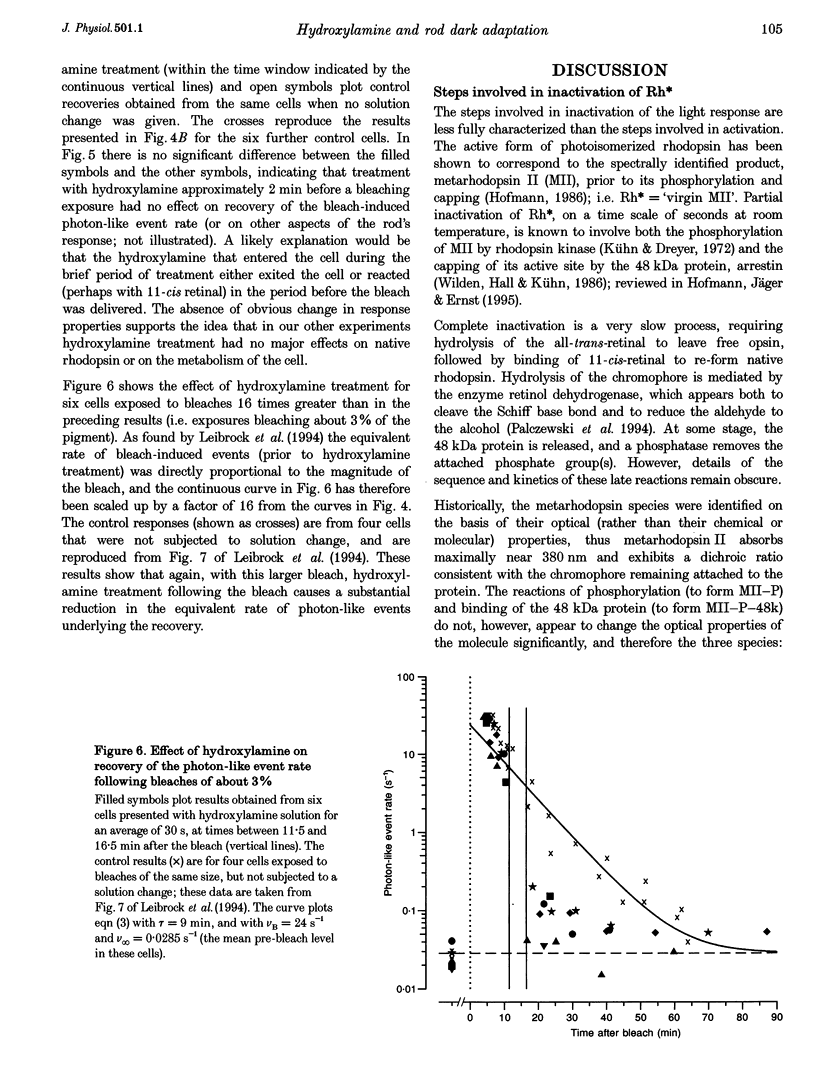
1. The suction pipette technique was used to investigate the recovery of toad rod photoreceptors following small bleaches of 0.2-3% of the rhodopsin. 2. The reduction in sensitivity and the increase in noise elicited by bleaches were measured, and from these measurements the underlying rate of occurrence of photon-like events was calculated as a function of time after the bleach. 3. Exposure to hydroxylamine solution was used to hasten the decomposition of the metarhodopsin photoproducts. The outer segment was exposed to 110 mM hydroxylamine in a low-Ca2+ Ringer solution for a period of 10-50 s beginning 10-17 min after the bleaching exposure. 4. By the time of the hydroxylamine exposure, the flash sensitivity and response kinetics had returned almost to normal, and were not significantly altered by the exposure. 5. Following hydroxylamine exposure, the rate of spontaneous photon-like events in the rods declined rapidly to near pre-bleach levels. 6. We conclude that hydroxylamine reduces the rate of occurrence of photon-like events induced by a bleach, and we postulate that this reduction results from the removal of metarhodopsin (most likely metarhodopsin II) from the outer segment. 7. Our results are consistent with a model in which photon-like events result from reversal of the reactions (phosphorylation and capping by arrestin) that lead to inactivation of the activated form of rhodopsin, Rh*.
Full text
PDF


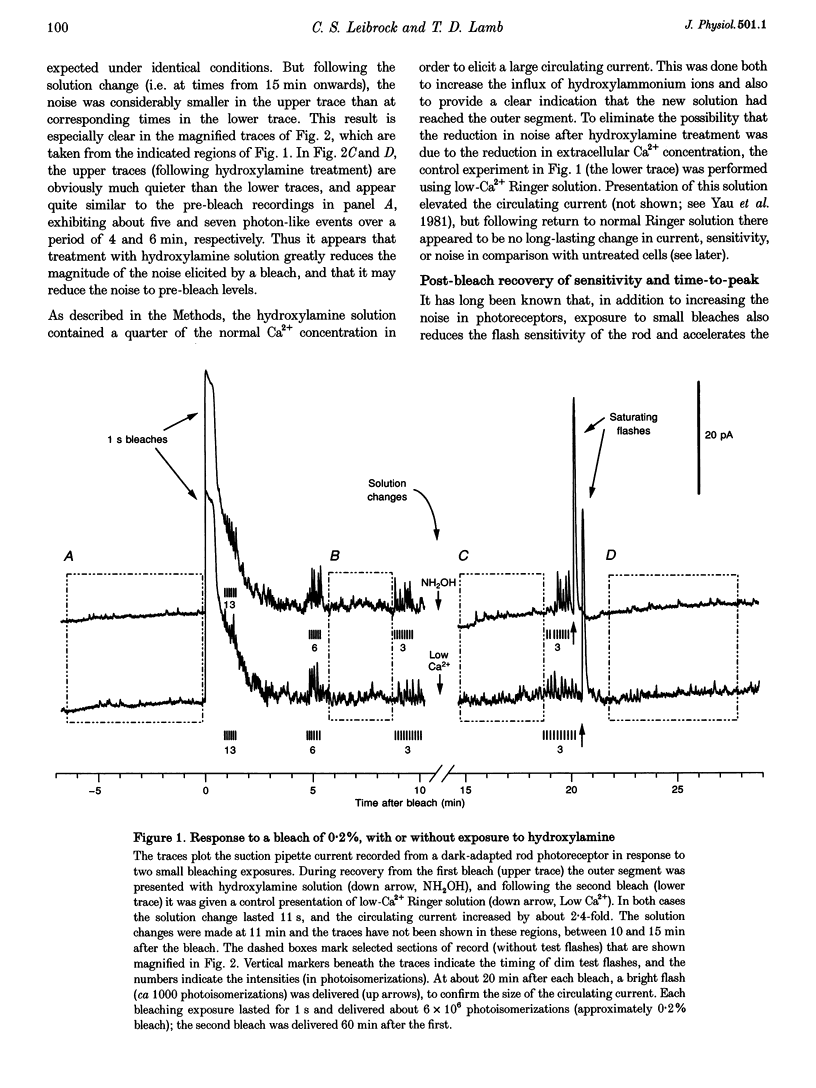
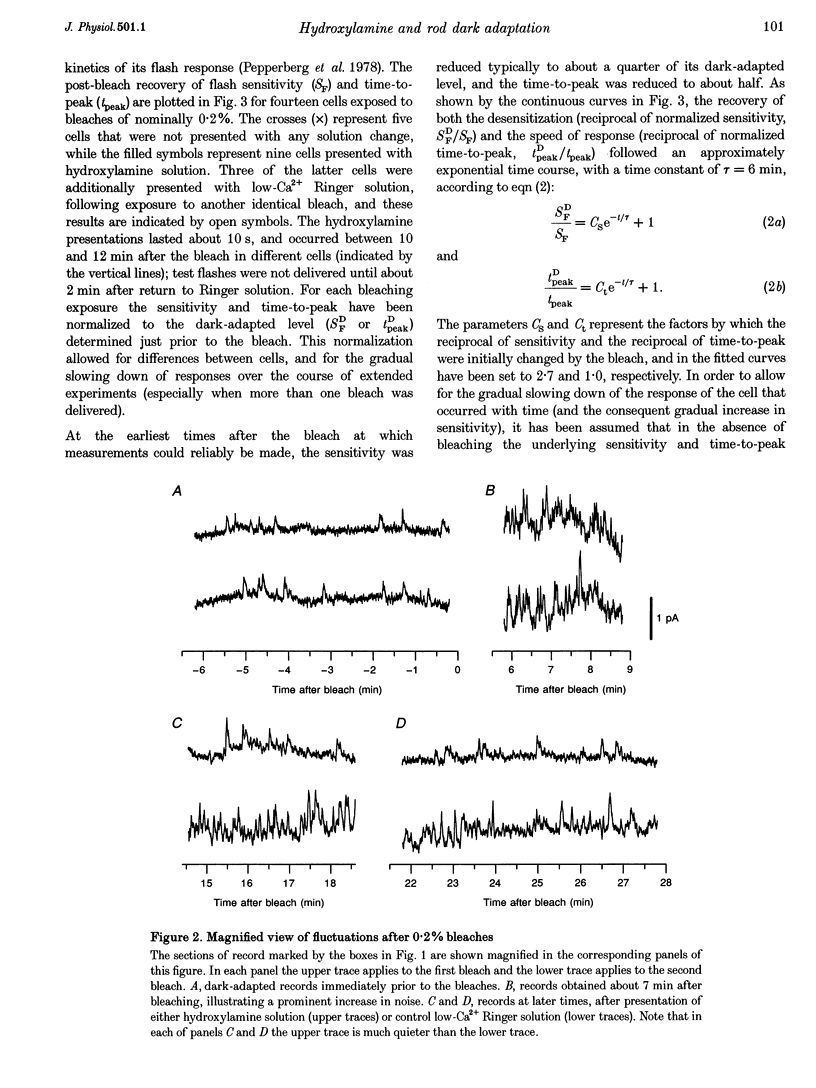
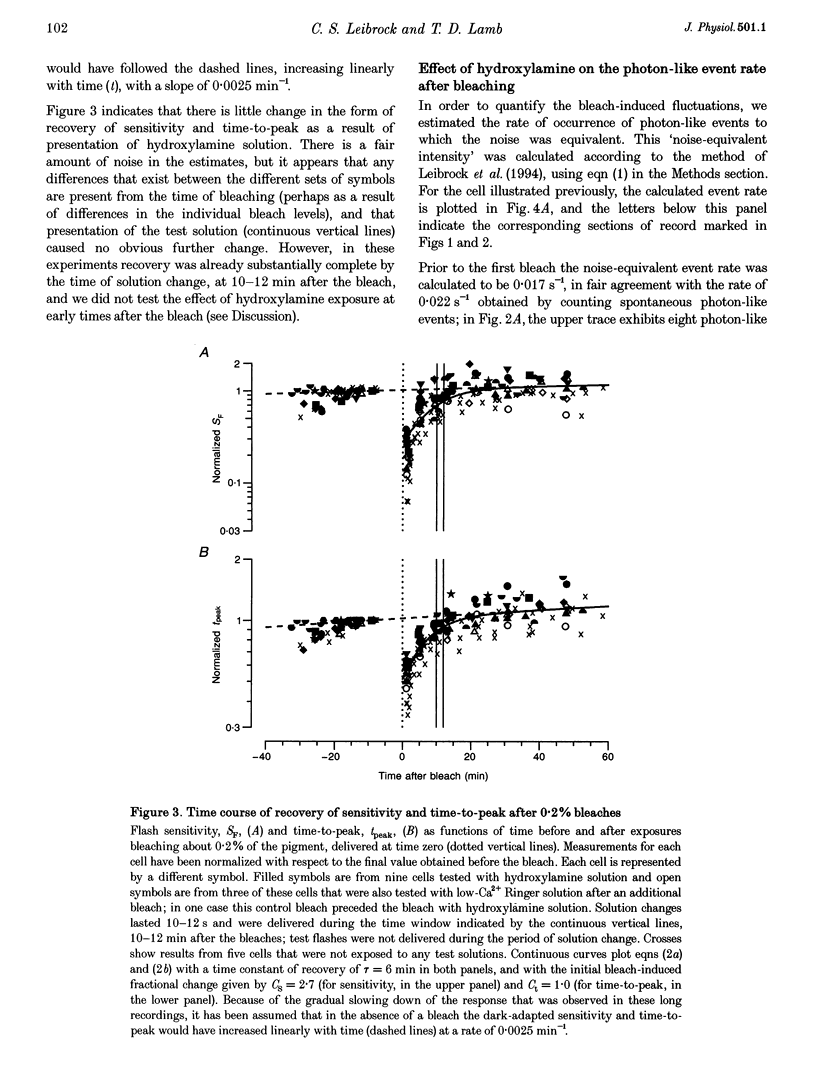
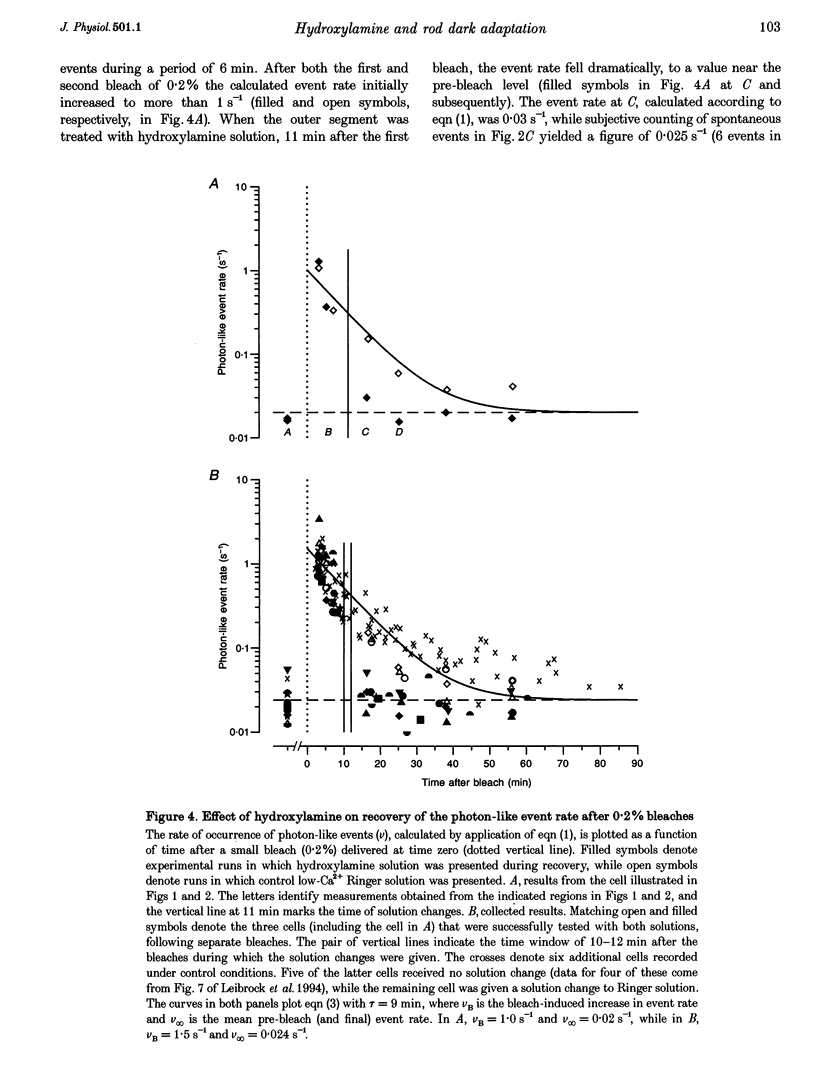




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aho A. C., Donner K., Hydén C., Larsen L. O., Reuter T. Low retinal noise in animals with low body temperature allows high visual sensitivity. Nature. 1988 Jul 28;334(6180):348–350. doi: 10.1038/334348a0. [DOI] [PubMed] [Google Scholar]
- Azuma K., Azuma M., Sickel W. Regeneration of rhodopsin in frog rod outer segments. J Physiol. 1977 Oct;271(3):747–759. doi: 10.1113/jphysiol.1977.sp012024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann C. Kinetics of slow thermal reactions during the bleaching of rhodopsin in the perfused frog retina. J Physiol. 1972 May;222(3):643–663. doi: 10.1113/jphysiol.1972.sp009819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J., Schnapf J. L. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984 Dec;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brin K. P., Ripps H. Rhodopsin photoproducts and rod sensitivity in the skate retina. J Gen Physiol. 1977 Jan;69(1):97–120. doi: 10.1085/jgp.69.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocozza J. D., Ostroy S. E. Factors affecting the regeneration of rhodopsin in the isolated amphibian retina. Vision Res. 1987;27(7):1085–1091. doi: 10.1016/0042-6989(87)90023-x. [DOI] [PubMed] [Google Scholar]
- Copenhagen D. R., Donner K., Reuter T. Ganglion cell performance at absolute threshold in toad retina: effects of dark events in rods. J Physiol. 1987 Dec;393:667–680. doi: 10.1113/jphysiol.1987.sp016847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall M. C., Fain G. L. Bleached pigment activates transduction in isolated rods of the salamander retina. J Physiol. 1994 Oct 15;480(Pt 2):261–279. doi: 10.1113/jphysiol.1994.sp020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOWLING J. E. NEURAL AND PHOTOCHEMICAL MECHANISMS OF VISUAL ADAPTATION IN THE RAT. J Gen Physiol. 1963 Jul;46:1287–1301. doi: 10.1085/jgp.46.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartnall H. J. The photosensitivities of visual pigments in the presence of hydroxylamine. Vision Res. 1968 Apr;8(4):339–358. doi: 10.1016/0042-6989(68)90104-1. [DOI] [PubMed] [Google Scholar]
- Donner K. O., Hemilä S. Kinetics of long-lived rhodopsin photoproducts in the frog retina as a function of the amount bleached. Vision Res. 1975 Aug-Sep;15:985–995. doi: 10.1016/0042-6989(75)90241-2. [DOI] [PubMed] [Google Scholar]
- Donner K. O., Reuter T. Dark-adaptation processes in the rhodopsin rods of the frog's retina. Vision Res. 1967 Jan;7(1):17–41. doi: 10.1016/0042-6989(67)90023-5. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol. 1985 Jan;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann K. P., Emeis D., Schnetkamp P. P. Interplay between hydroxylamine, metarhodopsin II and GTP-binding protein in bovine photoreceptor membranes. Biochim Biophys Acta. 1983 Oct 31;725(1):60–70. doi: 10.1016/0005-2728(83)90224-4. [DOI] [PubMed] [Google Scholar]
- Hofmann K. P., Pulvermüller A., Buczyłko J., Van Hooser P., Palczewski K. The role of arrestin and retinoids in the regeneration pathway of rhodopsin. J Biol Chem. 1992 Aug 5;267(22):15701–15706. [PubMed] [Google Scholar]
- Kühn H., Dreyer W. J. Light dependent phosphorylation of rhodopsin by ATP. FEBS Lett. 1972 Jan 15;20(1):1–6. doi: 10.1016/0014-5793(72)80002-4. [DOI] [PubMed] [Google Scholar]
- Lamb T. D., Matthews H. R., Torre V. Incorporation of calcium buffers into salamander retinal rods: a rejection of the calcium hypothesis of phototransduction. J Physiol. 1986 Mar;372:315–349. doi: 10.1113/jphysiol.1986.sp016011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D. Spontaneous quantal events induced in toad rods by pigment bleaching. Nature. 1980 Sep 25;287(5780):349–351. doi: 10.1038/287349a0. [DOI] [PubMed] [Google Scholar]
- Lamb T. D. The involvement of rod photoreceptors in dark adaptation. Vision Res. 1981;21(12):1773–1782. doi: 10.1016/0042-6989(81)90211-x. [DOI] [PubMed] [Google Scholar]
- Leibrock C. S., Reuter T., Lamb T. D. Dark adaptation of toad rod photoreceptors following small bleaches. Vision Res. 1994 Nov;34(21):2787–2800. doi: 10.1016/0042-6989(94)90048-5. [DOI] [PubMed] [Google Scholar]
- MATTHEWS R. G., HUBBARD R., BROWN P. K., WALD G. TAUTOMERIC FORMS OF METARHODOPSIN. J Gen Physiol. 1963 Nov;47:215–240. doi: 10.1085/jgp.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada D., Nakai T., Ikai A. Transducin activation by molecular species of rhodopsin other than metarhodopsin II. Photochem Photobiol. 1989 Feb;49(2):197–203. doi: 10.1111/j.1751-1097.1989.tb04096.x. [DOI] [PubMed] [Google Scholar]
- Palczewski K., Jäger S., Buczyłko J., Crouch R. K., Bredberg D. L., Hofmann K. P., Asson-Batres M. A., Saari J. C. Rod outer segment retinol dehydrogenase: substrate specificity and role in phototransduction. Biochemistry. 1994 Nov 22;33(46):13741–13750. doi: 10.1021/bi00250a027. [DOI] [PubMed] [Google Scholar]
- Pepperberg D. R., Brown P. K., Lurie M., Dowling J. E. Visual pigment and photoreceptor sensitivity in the isolated skate retina. J Gen Physiol. 1978 Apr;71(4):369–396. doi: 10.1085/jgp.71.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperberg D. R., Morrison D. F., O'Brien P. J. Depalmitoylation of rhodopsin with hydroxylamine. Methods Enzymol. 1995;250:348–361. doi: 10.1016/0076-6879(95)50084-7. [DOI] [PubMed] [Google Scholar]
- Pepperberg D. R., Okajima T. I. Hydroxylamine-dependent inhibition of rhodopsin phosphorylation in the isolated retina. Exp Eye Res. 1992 Mar;54(3):369–376. doi: 10.1016/0014-4835(92)90049-x. [DOI] [PubMed] [Google Scholar]
- Picco C., Menini A. The permeability of the cGMP-activated channel to organic cations in retinal rods of the tiger salamander. J Physiol. 1993 Jan;460:741–758. doi: 10.1113/jphysiol.1993.sp019497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilden U., Hall S. W., Kühn H. Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1174–1178. doi: 10.1073/pnas.83.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., McNaughton P. A., Hodgkin A. L. Effect of ions on the light-sensitive current in retinal rods. Nature. 1981 Aug 6;292(5823):502–505. doi: 10.1038/292502a0. [DOI] [PubMed] [Google Scholar]


