Abstract
1. We have described and analysed the movements of the isolated stomach during distension by correlating intragastric pressure with video recordings, and investigated the presence of intrinsic inhibitory and excitatory reflexes. 2. Isolated guinea-pig stomachs, placed in an organ bath, were slowly distended with Krebs solution using a syringe pump via a cannula through the pylorus. The changes in intragastric pressure during cycles of distension were monitored by pressure transducers connected to both oesophageal and pyloric cannulae. The resistivity of the gastric wall (change in pressure with volume, delta P/delta V) and the amplitude and frequency of phasic pressure events were calculated from pressure recordings. 3. The movements of the stomach were also recorded onto videotape. The motion of the gastric wall during distension cycles was analysed to establish the patterns of contractions, their propagation and the distribution of fluid in the stomach. During filling, fluid was preferentially accommodated in the fundus. Propagating (peristaltic) contractions, often starting in the fundus, moved aborally towards the pylorus. The peak of the phasic pressure event was observed when a contraction reached the orad antrum. As it reached the pylorus, intragastric pressure was at its minimum. 4. During the initial phase of distension, intragastric pressure increased steeply. Tetrodotoxin and hyoscine reduced both the resistivity and amplitude of phasic pressure events. Hexamethonium had a similar effect. Thus distension appears to activate an excitatory reflex pathway, involving nicotinic ganglionic transmission. This reflex increases wall tension and enhances myogenic peristaltic contractions. 5. In control preparations, with larger distension volumes, the intragastric pressure decreased, despite the continued infusion of Krebs solution. L-NAME and apamin abolished this drop in pressure, indicating that gastric enteric inhibitory mechanisms prevail at larger distension volumes. After blockade of the excitatory reflex, hexamethonium antagonized the inhibitory response, indicating that activation of inhibitory mechanisms involves nicotinic transmission, probably on enteric inhibitory motoneurons. 6. Both the excitatory and inhibitory reflexes in the isolated stomach operate within a physiological range of gastric volumes. The excitatory reflex predominates at small distension volumes, leading to large phasic propagated contractions that mix the contents and may lead to emptying of the stomach. The inhibitory reflex, described previously as adaptive relaxation, can maximally relax the stomach and is activated preferentially at higher distension volumes to accommodate the contents. The interplay of these reflex pathways in the isolated stomach produces a rich repertoire of gastric movements. 7. The isolated stomach preparation, used with a combination of kinematic, kinetic and pharmacological methods, provides a highly suitable means of investigating the mechanisms of gastric motility.
Full text
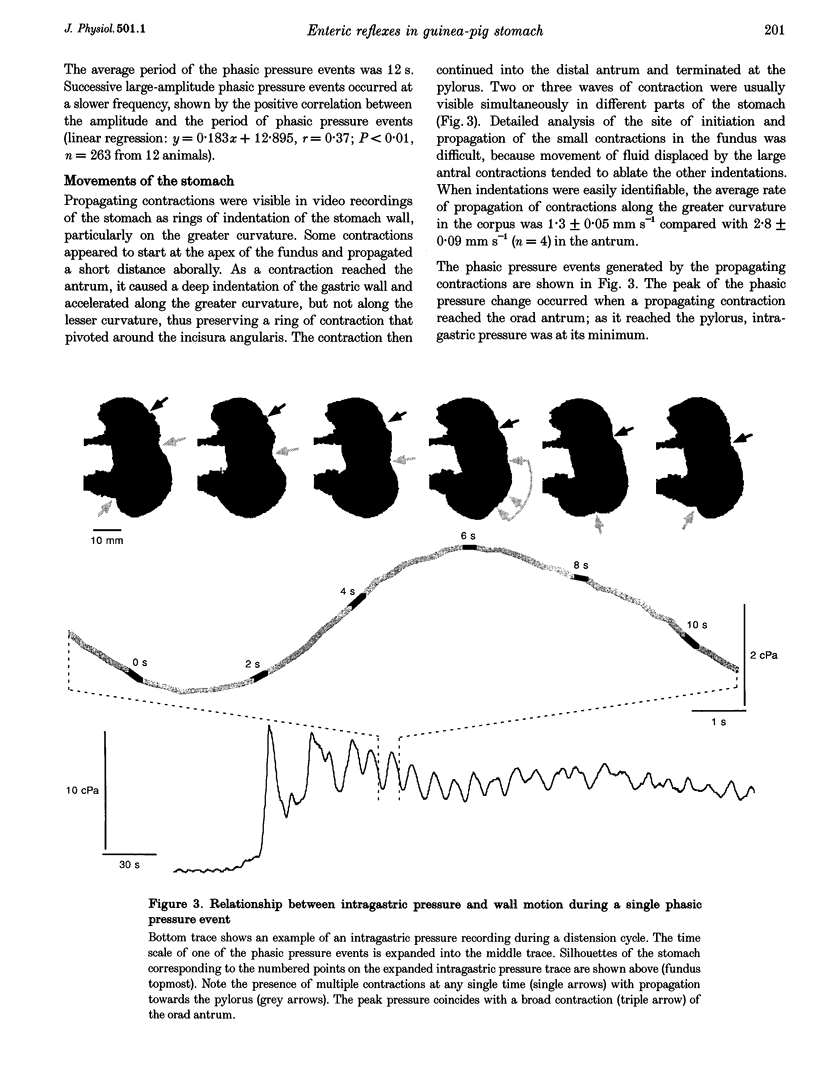
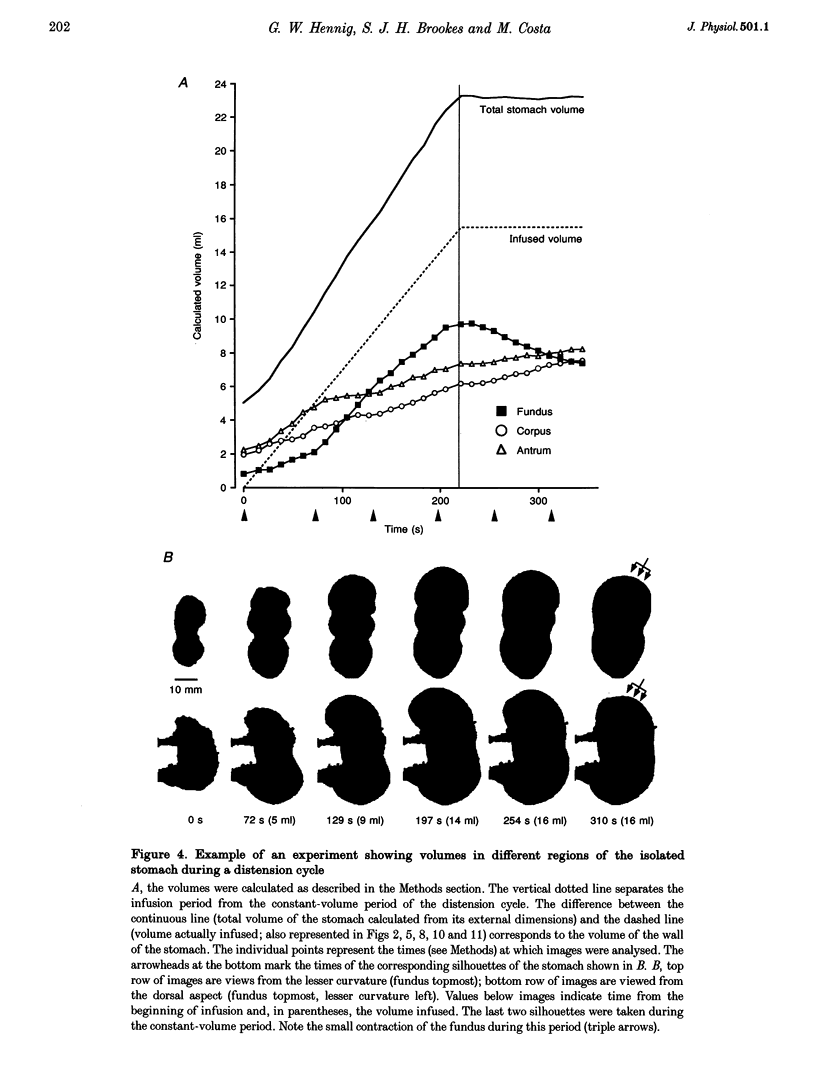
PDF

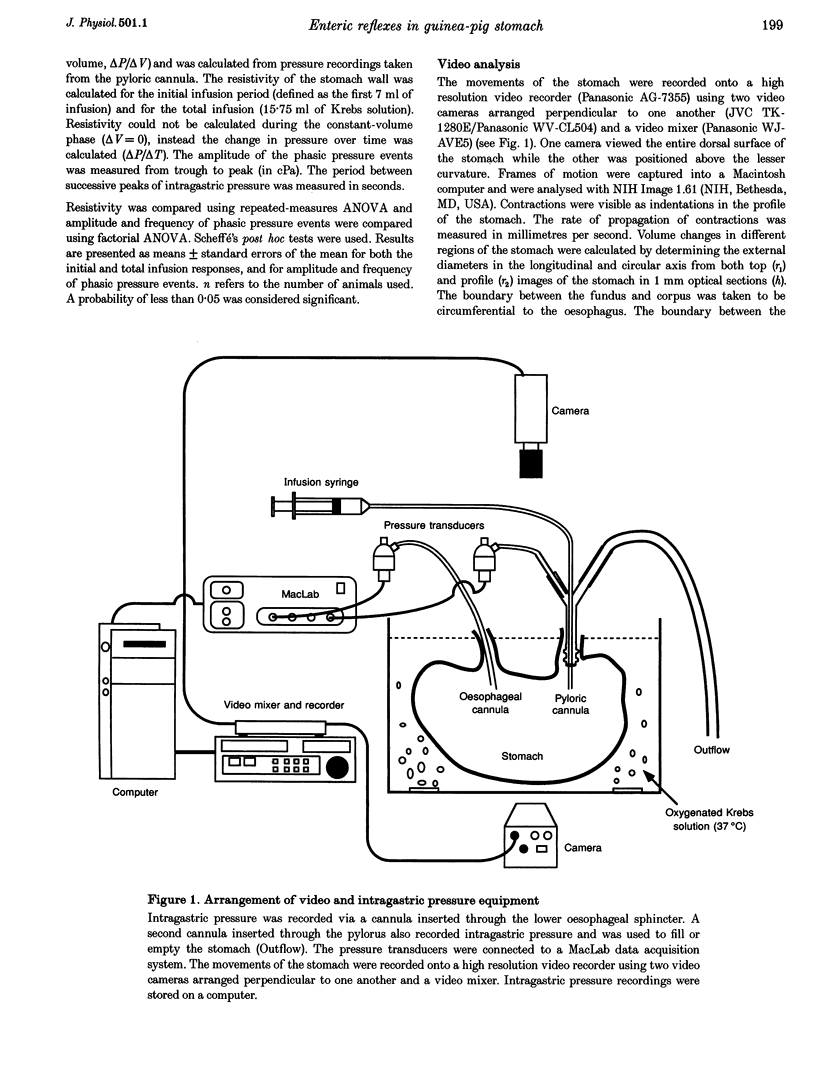
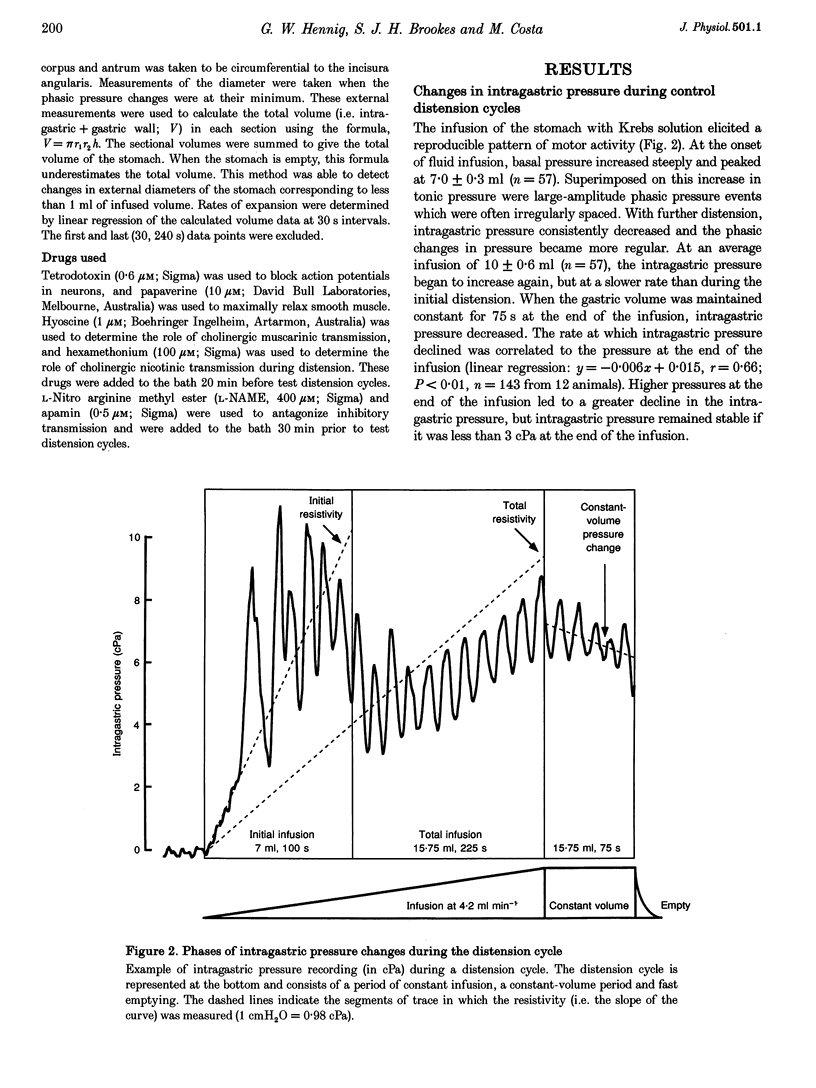
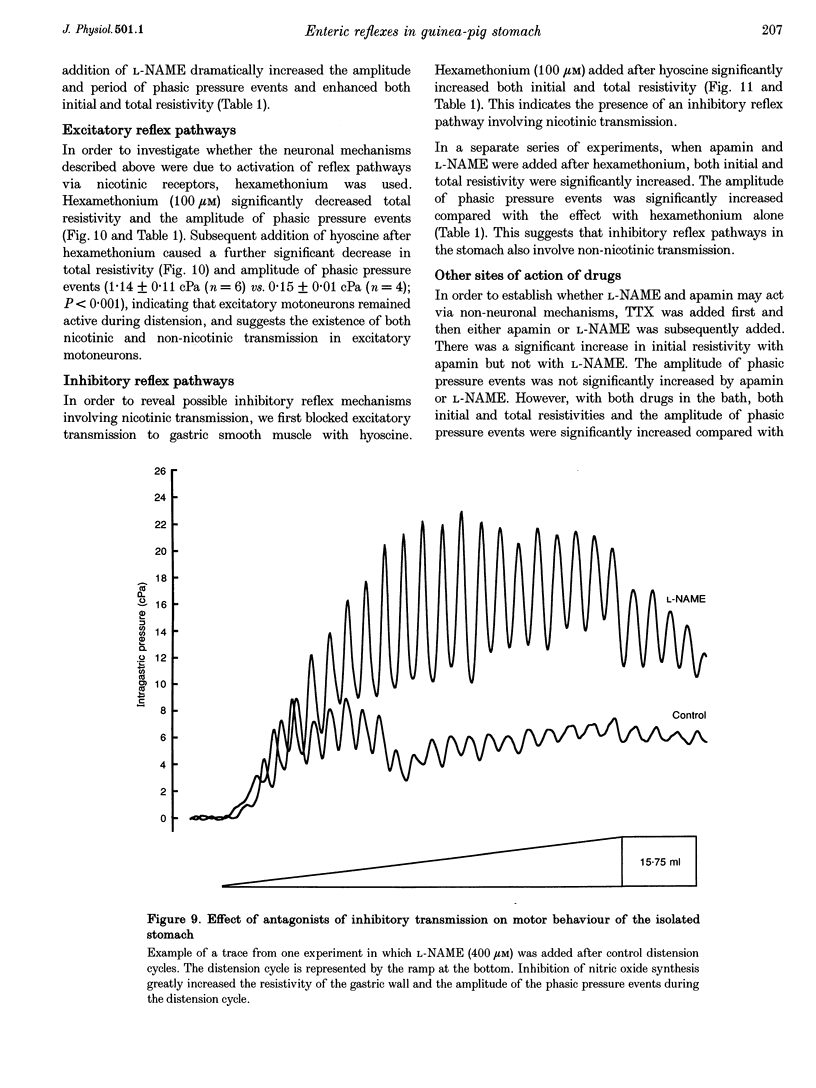
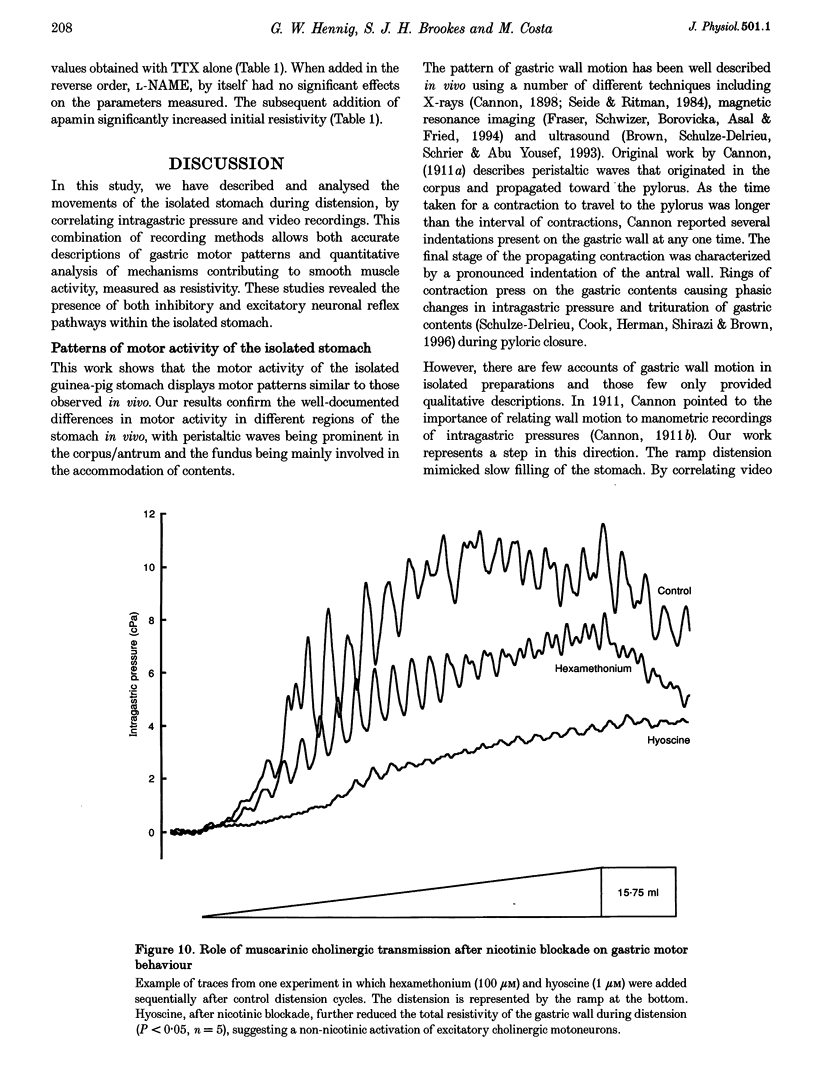
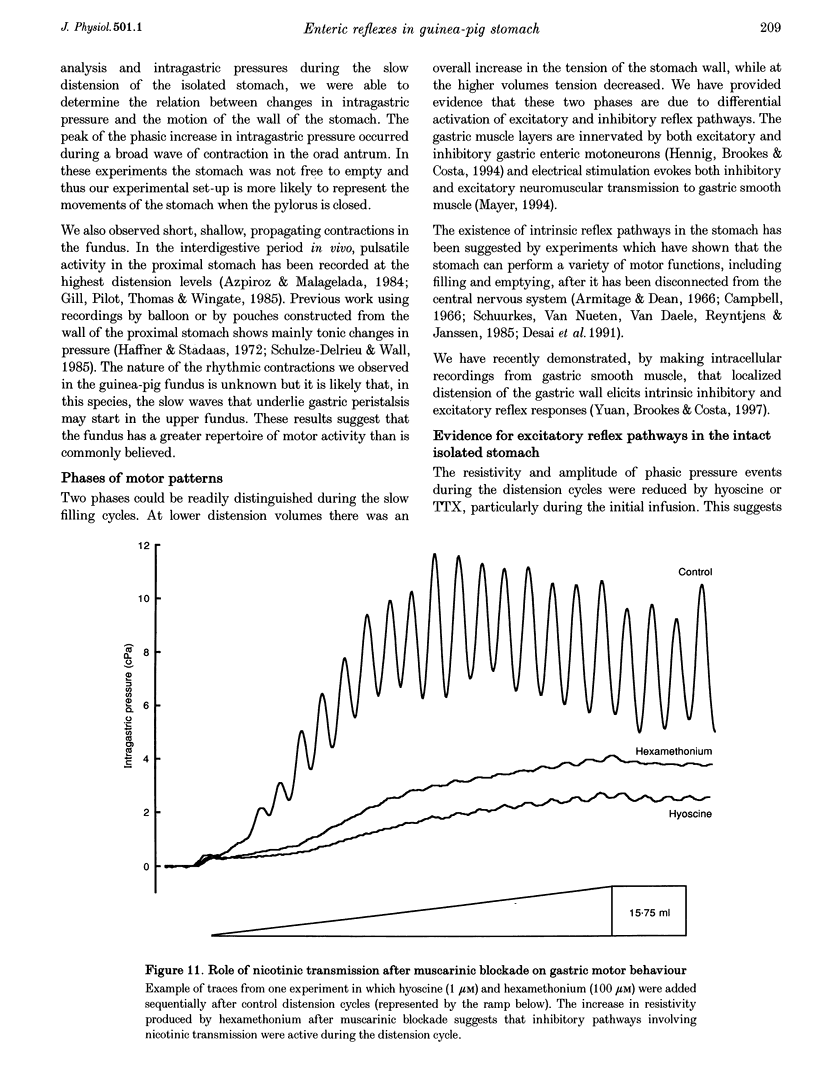



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. L., Bingham S. Adaptation of the mechanisms controlling gastric motility following chronic vagotomy in the ferret. Exp Physiol. 1990 Nov;75(6):811–825. doi: 10.1113/expphysiol.1990.sp003463. [DOI] [PubMed] [Google Scholar]
- Armitage A. K., Dean A. C. The effects of pressure and pharmacologically active substances on gastric peristalsis in a transmurally stimulated rat soomach-duodenum preparation. J Physiol. 1966 Jan;182(1):42–56. doi: 10.1113/jphysiol.1966.sp007807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpiroz F., Malagelada J. R. Pressure activity patterns in the canine proximal stomach: response to distension. Am J Physiol. 1984 Sep;247(3 Pt 1):G265–G272. doi: 10.1152/ajpgi.1984.247.3.G265. [DOI] [PubMed] [Google Scholar]
- Berthoud H. R., Powley T. L. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol. 1992 May 8;319(2):261–276. doi: 10.1002/cne.903190206. [DOI] [PubMed] [Google Scholar]
- Brown B. P., Schulze-Delrieu K., Schrier J. E., Abu-Yousef M. M. The configuration of the human gastroduodenal junction in the separate emptying of liquids and solids. Gastroenterology. 1993 Aug;105(2):433–440. doi: 10.1016/0016-5085(93)90717-q. [DOI] [PubMed] [Google Scholar]
- Campbell G. The inhibitory nerve fibres in the vagal supply to the guinea-pig stomach. J Physiol. 1966 Aug;185(3):600–612. doi: 10.1113/jphysiol.1966.sp008004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J., Torres E. I. Three layers of the opossum stomach: responses to nerve stimulation. Gastroenterology. 1975 Sep;69(3):641–648. [PubMed] [Google Scholar]
- Delbro D., Fändriks L., Lisander B., Andersson S. A. Gastric atropine-sensitive excitation by peripheral vagal stimulation after hexamethonium. Antidromic activation of afferents? Acta Physiol Scand. 1982 Mar;114(3):433–440. doi: 10.1111/j.1748-1716.1982.tb07006.x. [DOI] [PubMed] [Google Scholar]
- Desai K. M., Sessa W. C., Vane J. R. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature. 1991 Jun 6;351(6326):477–479. doi: 10.1038/351477a0. [DOI] [PubMed] [Google Scholar]
- Fraser R., Schwizer W., Borovicka J., Asal K., Fried M. Gastric motility measurement by MRI. Dig Dis Sci. 1994 Dec;39(12 Suppl):20S–23S. doi: 10.1007/BF02300363. [DOI] [PubMed] [Google Scholar]
- Gill R. C., Pilot M. A., Thomas P. A., Wingate D. L. Organization of fasting and postprandial myoelectric activity in stomach and duodenum of conscious dogs. Am J Physiol. 1985 Dec;249(6 Pt 1):G655–G661. doi: 10.1152/ajpgi.1985.249.6.G655. [DOI] [PubMed] [Google Scholar]
- Haffner J. F., Stadaas J. Pressure responses to cholinergic and adrenergic agents in the fundus, corpus, and antrum of isolated rabbit stomachs. Acta Chir Scand. 1972;138(7):713–719. [PubMed] [Google Scholar]
- Hartley M. N., Mackie C. R. Gastric adaptive relaxation and symptoms after vagotomy. Br J Surg. 1991 Jan;78(1):24–27. doi: 10.1002/bjs.1800780109. [DOI] [PubMed] [Google Scholar]
- Komori K., Suzuki H. Distribution and properties of excitatory and inhibitory junction potentials in circular muscle of the guinea-pig stomach. J Physiol. 1986 Jan;370:339–355. doi: 10.1113/jphysiol.1986.sp015938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N. On Inhibitory Fibres in the Vagus for the end of the OEsophagus and the Stomach. J Physiol. 1898 Dec 30;23(5):407–414. doi: 10.1113/jphysiol.1898.sp000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas A. J., Den Hertog A., Ras R., Van den Akker J. The action of apamin on guinea-pig taenia caeci. Eur J Pharmacol. 1980 Oct 17;67(2-3):265–274. doi: 10.1016/0014-2999(80)90507-5. [DOI] [PubMed] [Google Scholar]
- Minami H., McCallum R. W. The physiology and pathophysiology of gastric emptying in humans. Gastroenterology. 1984 Jun;86(6):1592–1610. [PubMed] [Google Scholar]
- Ozaki H., Blondfield D. P., Hori M., Publicover N. G., Kato I., Sanders K. M. Spontaneous release of nitric oxide inhibits electrical, Ca2+ and mechanical transients in canine gastric smooth muscle. J Physiol. 1992 Jan;445:231–247. doi: 10.1113/jphysiol.1992.sp018921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemann M., Grundy D. Electrophysiological identification of vagally innervated enteric neurons in guinea pig stomach. Am J Physiol. 1992 Nov;263(5 Pt 1):G709–G718. doi: 10.1152/ajpgi.1992.263.5.G709. [DOI] [PubMed] [Google Scholar]
- Schemann M., Schaaf C., Mäder M. Neurochemical coding of enteric neurons in the guinea pig stomach. J Comp Neurol. 1995 Mar 6;353(2):161–178. doi: 10.1002/cne.903530202. [DOI] [PubMed] [Google Scholar]
- Schemann M., Wood J. D. Electrical behaviour of myenteric neurones in the gastric corpus of the guinea-pig. J Physiol. 1989 Oct;417:501–518. doi: 10.1113/jphysiol.1989.sp017815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemann M., Wood J. D. Synaptic behaviour of myenteric neurones in the gastric corpus of the guinea-pig. J Physiol. 1989 Oct;417:519–535. doi: 10.1113/jphysiol.1989.sp017816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Delrieu K., Wall J. P. Mechanical activity of muscular "patch pouches" from cat and rabbit stomachs. Gastroenterology. 1985 Apr;88(4):1012–1019. doi: 10.1016/s0016-5085(85)80022-6. [DOI] [PubMed] [Google Scholar]
- Schuurkes J. A., Van Nueten J. M., Van Daele P. G., Reyntjens A. J., Janssen P. A. Motor-stimulating properties of cisapride on isolated gastrointestinal preparations of the guinea pig. J Pharmacol Exp Ther. 1985 Sep;234(3):775–783. [PubMed] [Google Scholar]
- Tack J. F., Wood J. D. Electrical behaviour of myenteric neurones in the gastric antrum of the guinea-pig. J Physiol. 1992 Feb;447:49–66. doi: 10.1113/jphysiol.1992.sp018990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk V., Maggi C. A. Electrophysiological evidence for different release mechanism of ATP and NO as inhibitory NANC transmitters in guinea-pig colon. Br J Pharmacol. 1994 Aug;112(4):1077–1082. doi: 10.1111/j.1476-5381.1994.tb13193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



