Abstract
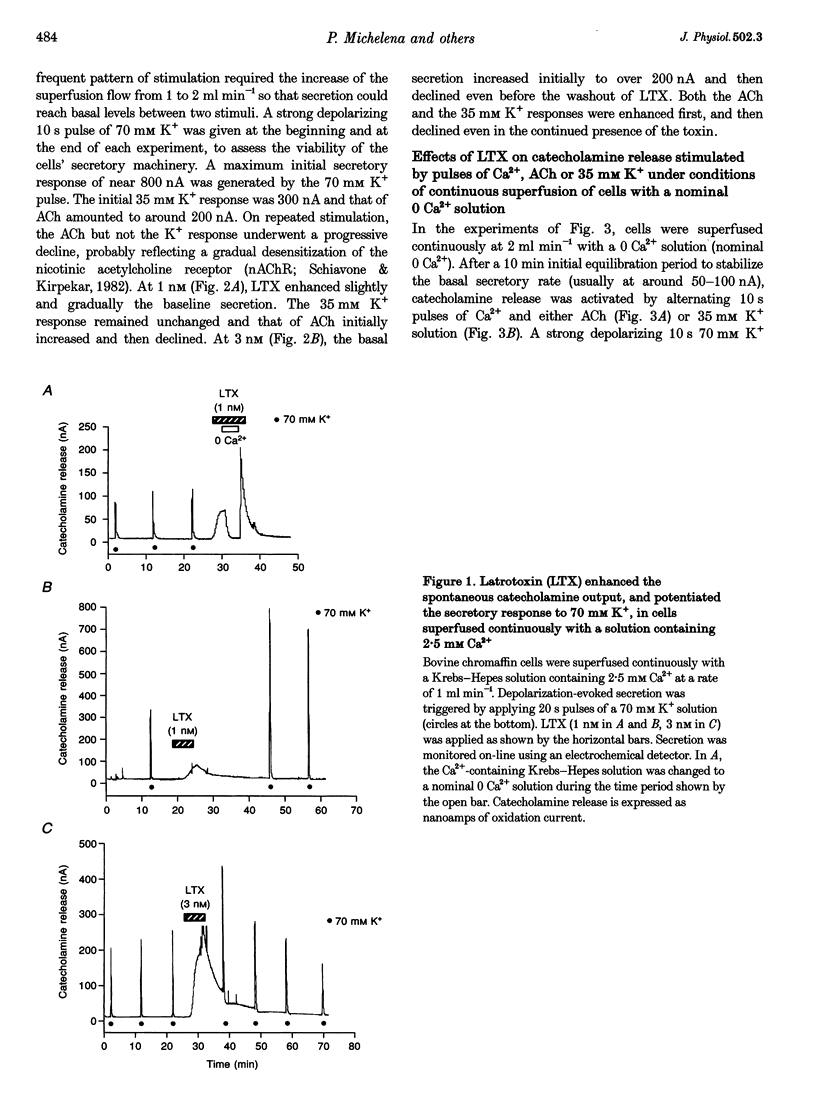
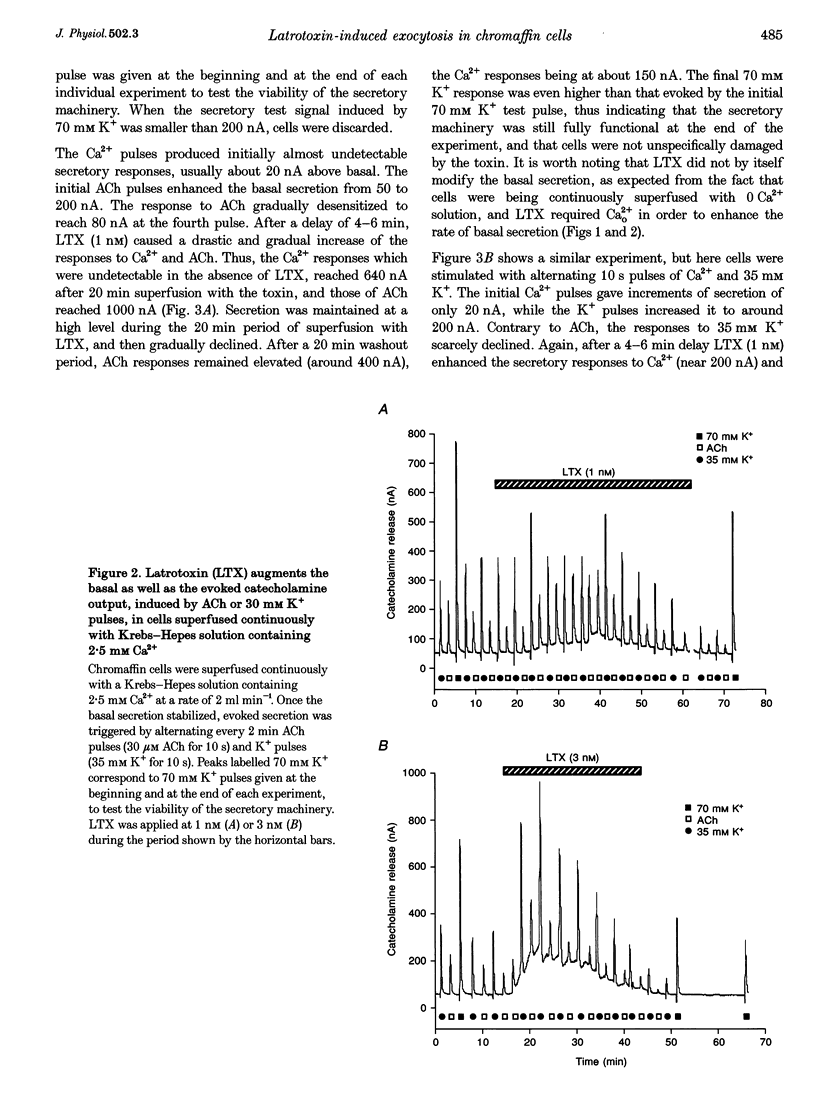
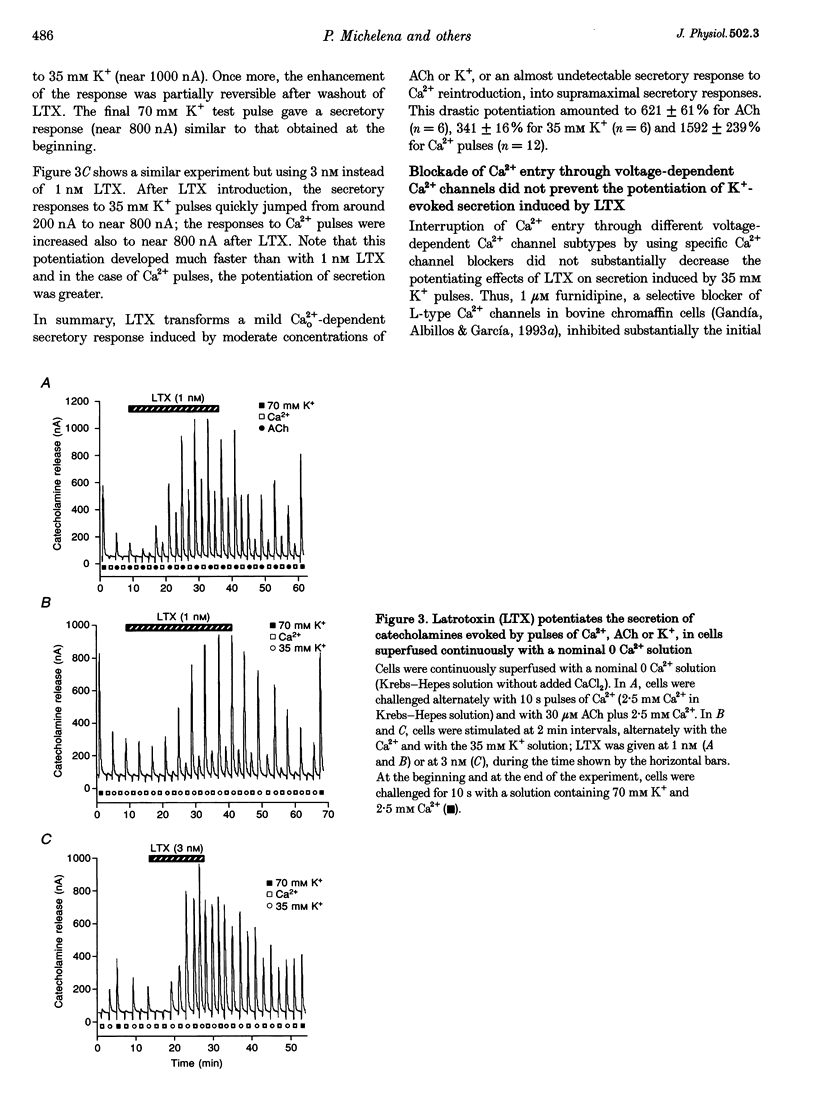
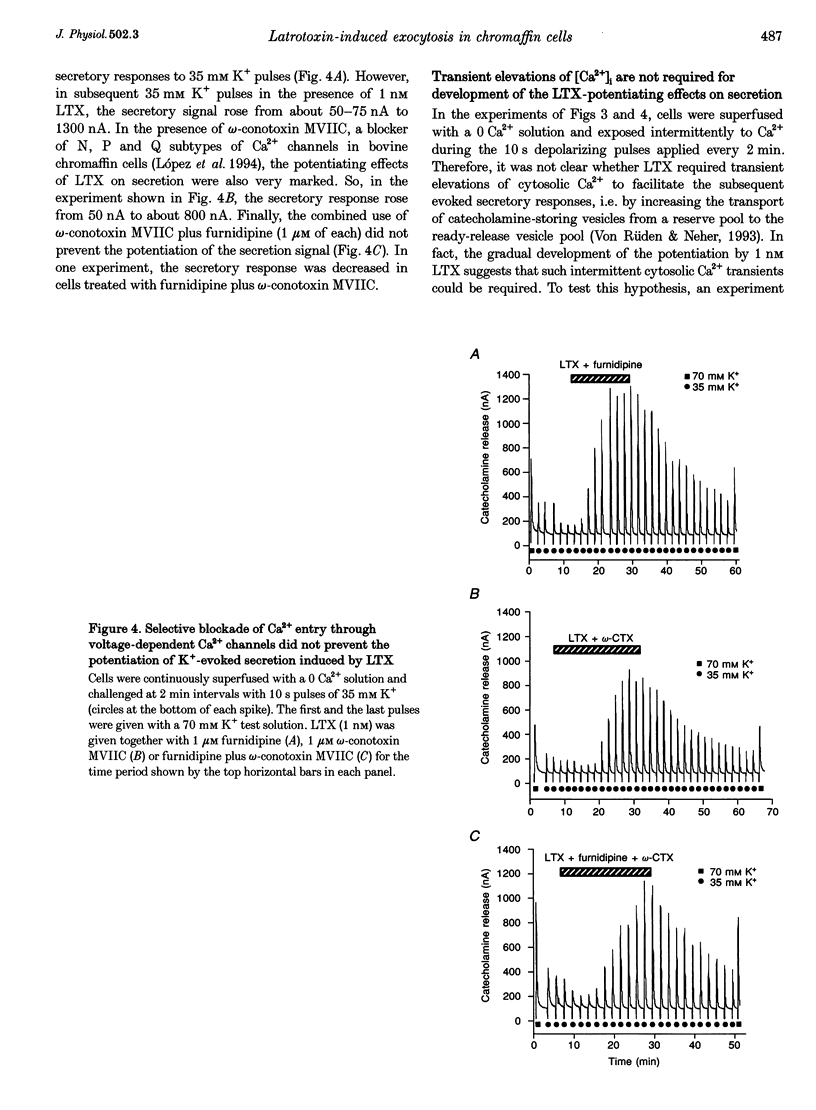
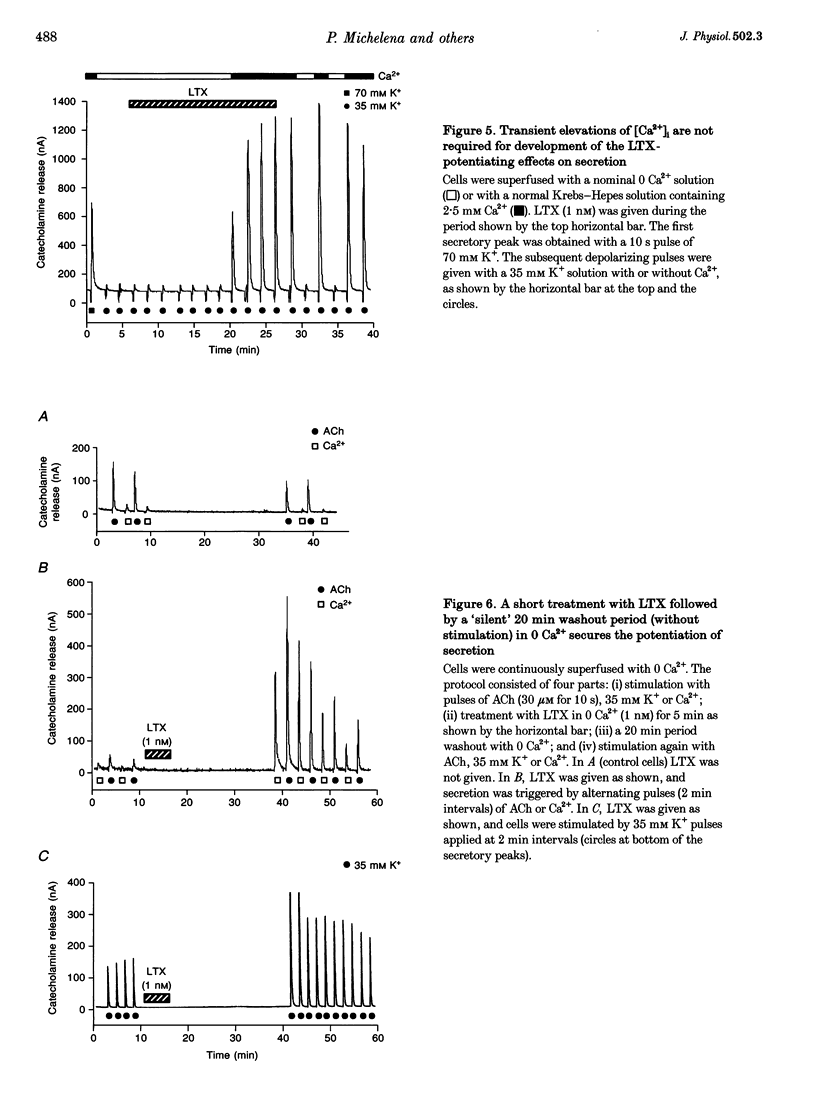
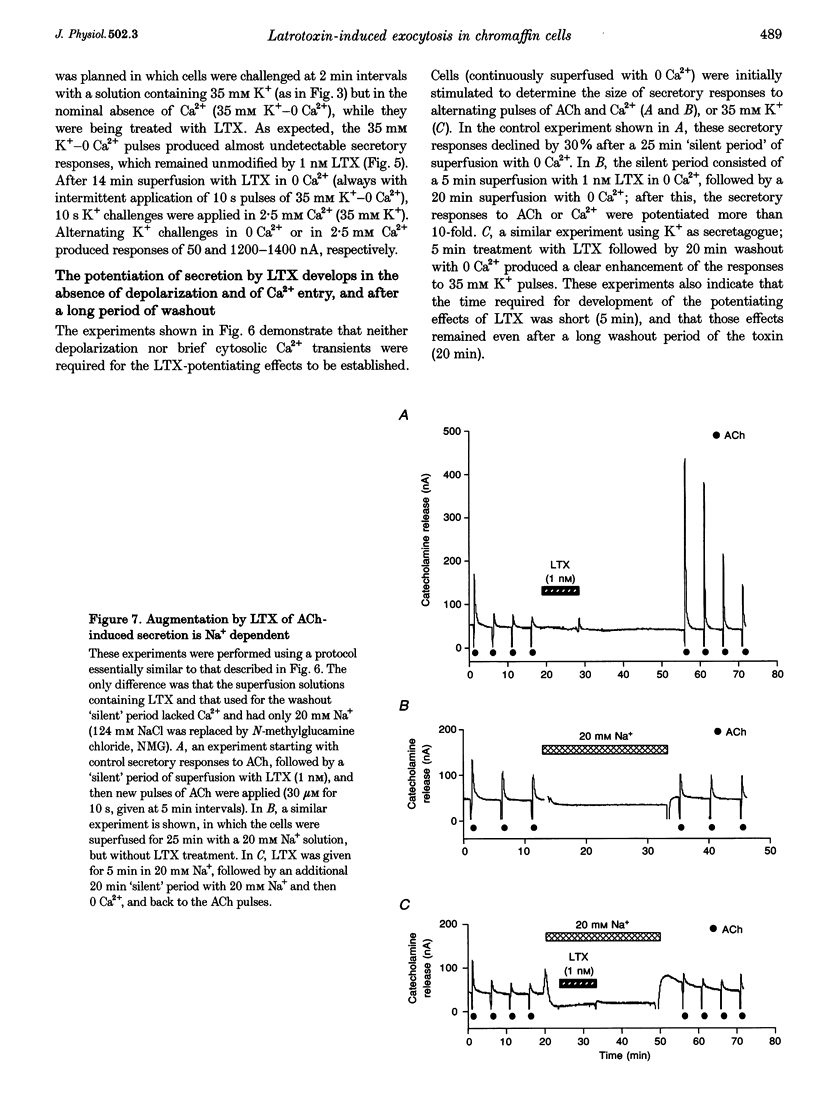
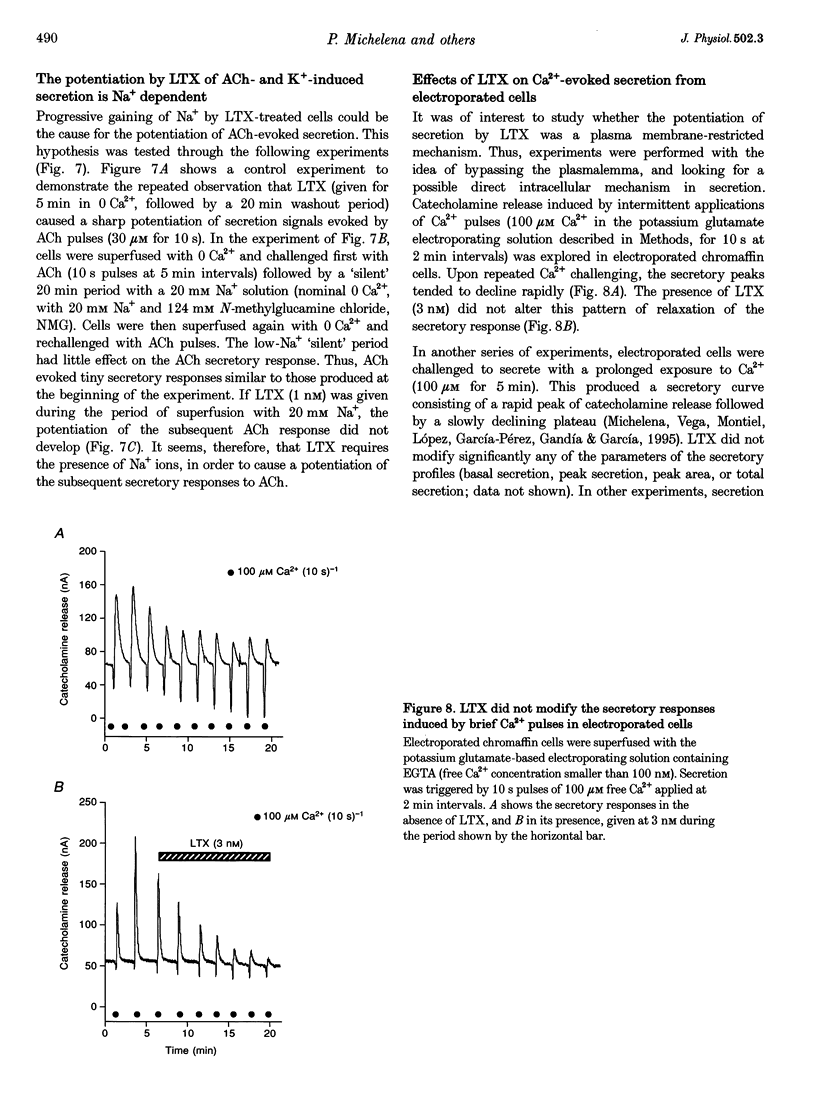
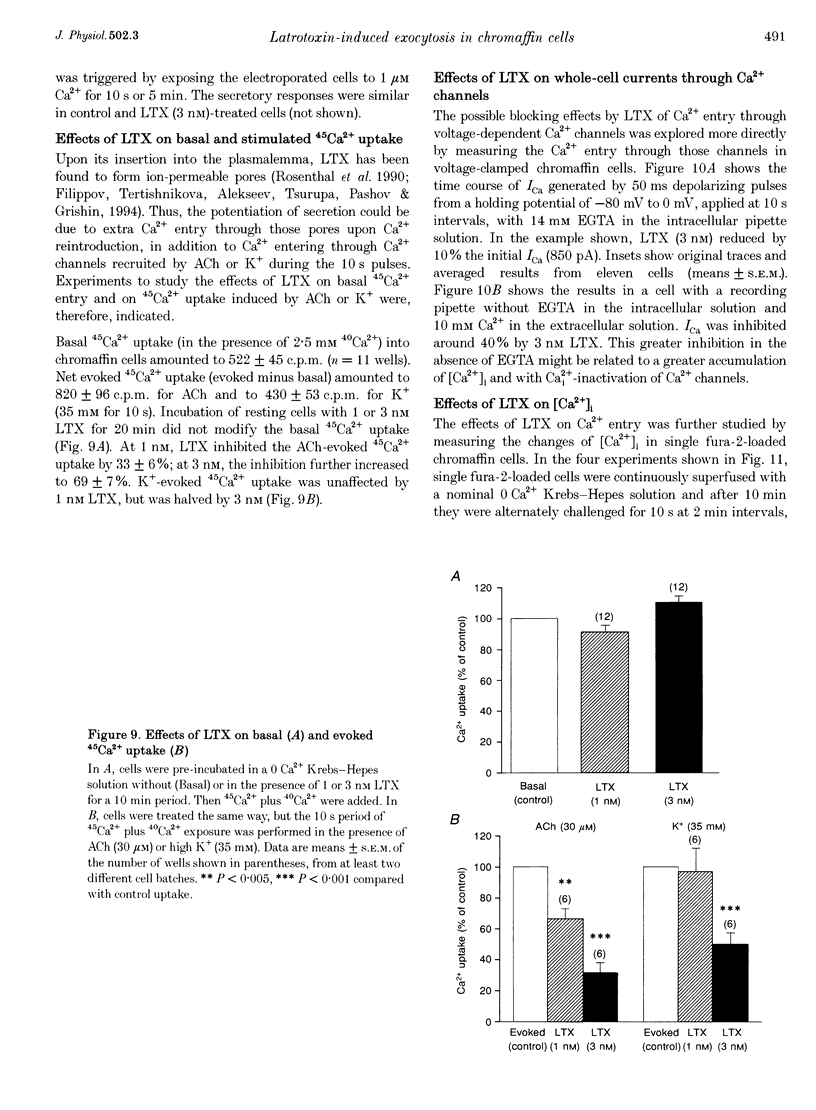
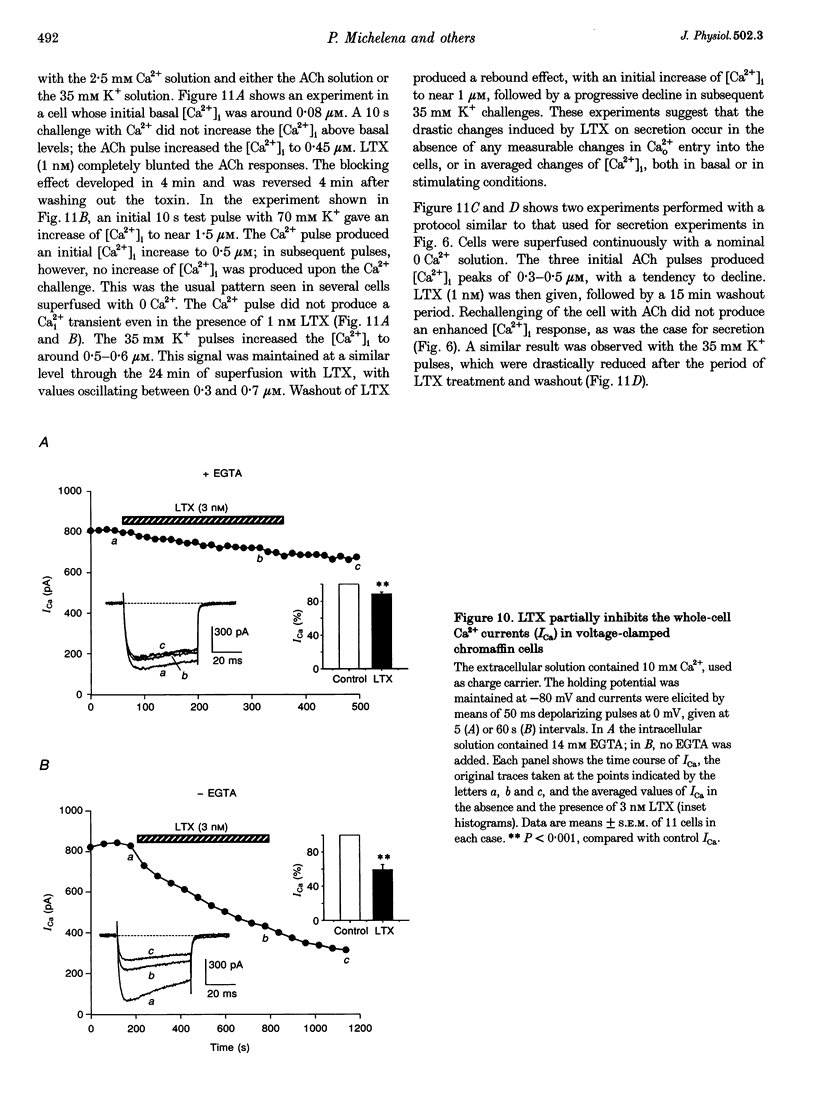
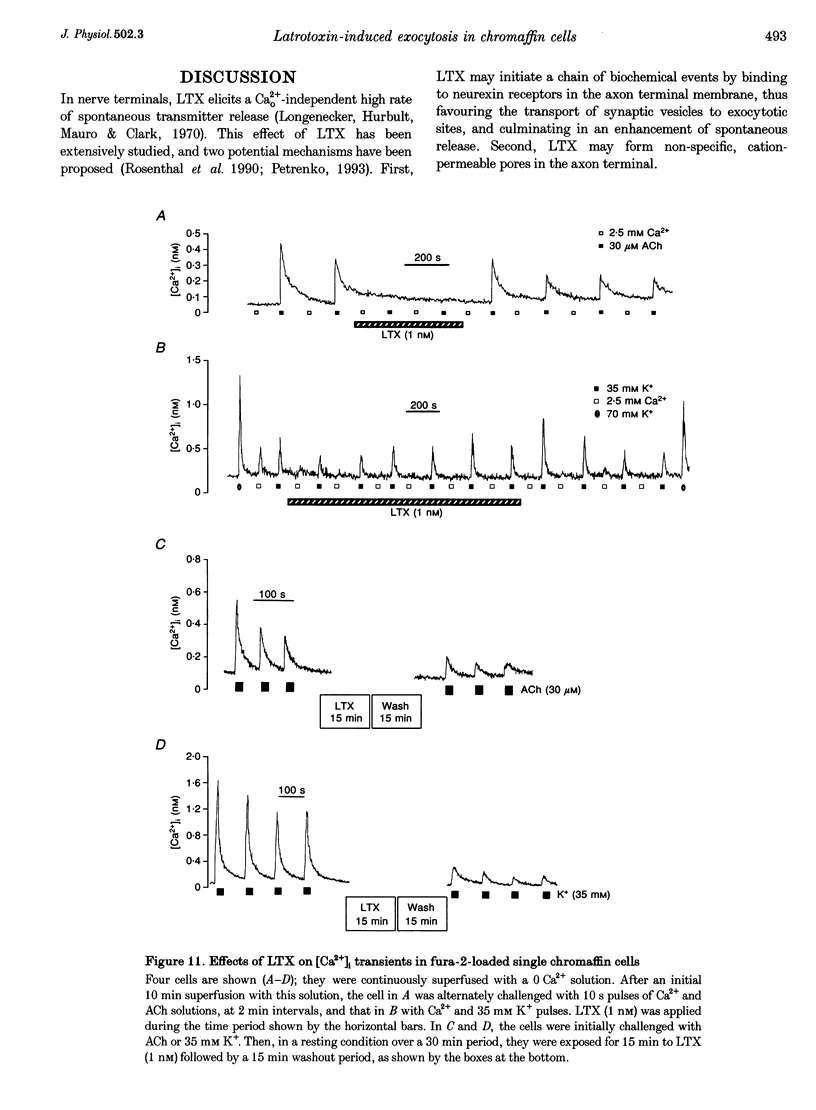
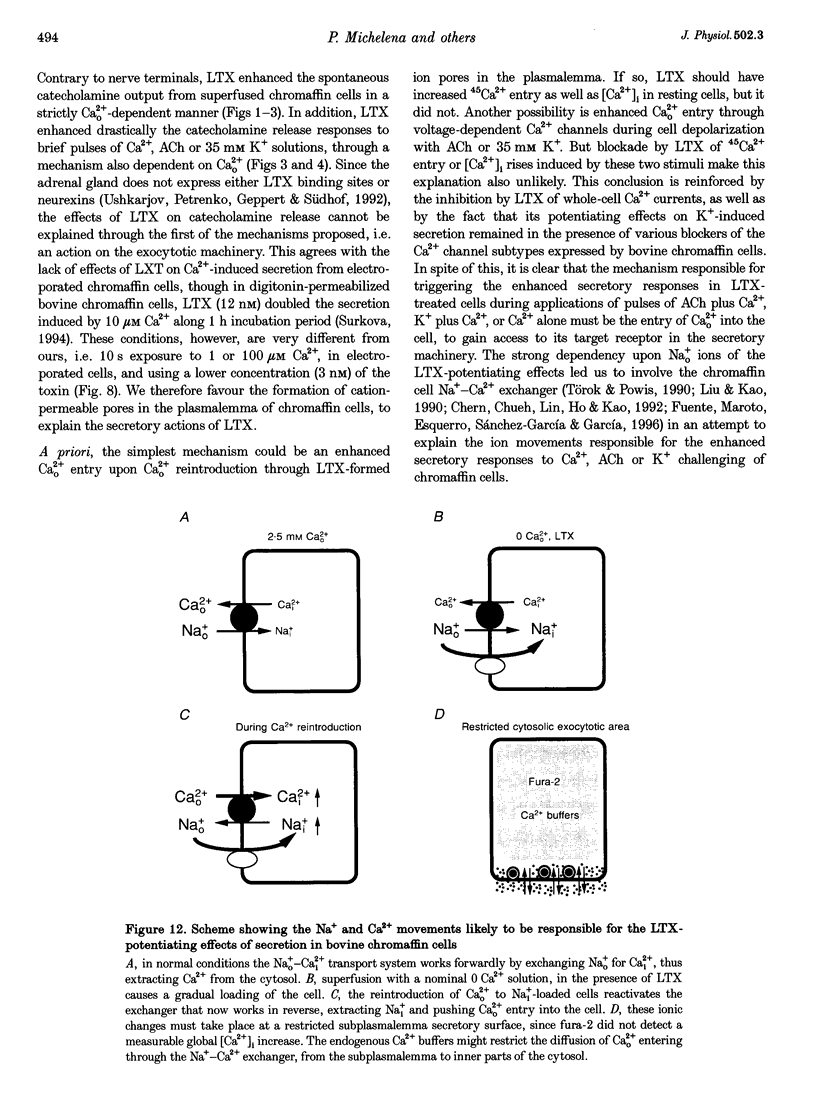
1. Latrotoxin (LTX, 1-3 nM) caused a gradual increase of the spontaneous catecholamine release rate in bovine adrenal chromaffin cells superfused with normal Krebs-Hepes solution containing 2.5 mM Ca2+. Ca2+ removal abolished this effect. LTX enhanced also the secretory responses to high K+ (35 or 70 mM) and to acetylcholine (ACh, 30 microM). 2. The application of Ca2+ pulses to cells previously superfused with a 0 Ca2+ solution (Krebs-Hepes deprived of CaCl2) induced secretory responses that gradually reached 400-800 nA of catecholamines, provided that LTX was present. The responses to ACh or 35 mM K+ pulses (in the presence of Ca2+) were also enhanced by LTX, from around 100-200 nA to over 1000 nA. Though such enhancement remained in the presence of Ca2+ channel blockers, it disappeared upon the lowering of [Na+]o or in electroporated cells. 3. Using protocols similar to those of secretion, LTX did not enhance basal 45Ca2+ uptake, whole-cell Ca2+ currents or basal [Ca2+]i. In fact, LTX attenuated the K(+)- or ACh-evoked increases in 45Ca2+ uptake and [Ca2+]i. 4. It is proposed that the secretory response to brief periods of Ca2+ reintroductions is triggered by local subplasmalemmal Ca2+i transients, produced by the Na(+)-Ca2+ exchanger of the plasma membrane working in the reverse mode. This situation might be physiologically reproduced during ACh stimulation of chromaffin cells, which is followed by the firing of Na(+)-dependent action potentials.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Carvalho M. H., Prat J. C., Garcia A. G., Kirpekar S. M. Ionomycin stimulates secretion of catecholamines from cat adrenal gland and spleen. Am J Physiol. 1982 Mar;242(3):E137–E145. doi: 10.1152/ajpendo.1982.242.3.E137. [DOI] [PubMed] [Google Scholar]
- Ceña V., Nicolas G. P., Sanchez-Garcia P., Kirpekar S. M., Garcia A. G. Pharmacological dissection of receptor-associated and voltage-sensitive ionic channels involved in catecholamine release. Neuroscience. 1983 Dec;10(4):1455–1462. doi: 10.1016/0306-4522(83)90126-4. [DOI] [PubMed] [Google Scholar]
- Chern Y. J., Chueh S. H., Lin Y. J., Ho C. M., Kao L. S. Presence of Na+/Ca2+ exchange activity and its role in regulation of intracellular calcium concentration in bovine adrenal chromaffin cells. Cell Calcium. 1992 Feb;13(2):99–106. doi: 10.1016/0143-4160(92)90003-b. [DOI] [PubMed] [Google Scholar]
- Cochrane D. E., Douglas W. W. Calcium-induced extrusion of secretory granules (exocytosis) in mast cells exposed to 48-80 or the ionophores A-23187 and X-537A. Proc Natl Acad Sci U S A. 1974 Feb;71(2):408–412. doi: 10.1073/pnas.71.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Fuente M. T., Maroto R., Esquerro E., Sánchez-García P., García A. G. The actions of ouabain and lithium chloride on cytosolic Ca2+ in single chromaffin cells. Eur J Pharmacol. 1996 Jun 13;306(1-3):219–226. doi: 10.1016/0014-2999(96)00191-4. [DOI] [PubMed] [Google Scholar]
- Deri Z., Adam-Vizi V. Detection of intracellular free Na+ concentration of synaptosomes by a fluorescent indicator, Na(+)-binding benzofuran isophthalate: the effect of veratridine, ouabain, and alpha-latrotoxin. J Neurochem. 1993 Sep;61(3):818–825. doi: 10.1111/j.1471-4159.1993.tb03592.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg M., Hall J. E., Mead C. A. The nature of the voltage-dependent conductance induced by alamethicin in black lipid membranes. J Membr Biol. 1973 Dec 31;14(2):143–176. doi: 10.1007/BF01868075. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov A. K., Tertishnikova S. M., Alekseev A. E., Tsurupa G. P., Pashkov V. N., Grishin E. V. Mechanism of alpha-latrotoxin action as revealed by patch-clamp experiments on Xenopus oocytes injected with rat brain messenger RNA. Neuroscience. 1994 Jul;61(1):179–189. doi: 10.1016/0306-4522(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Fonteríz R. I., López M. G., García-Sancho J., García A. G. Alamethicin channel permeation by Ca2+, Mn2+ and Ni2+ in bovine chromaffin cells. FEBS Lett. 1991 May 20;283(1):89–92. doi: 10.1016/0014-5793(91)80560-p. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L., Gomperts B. D. Calcium ionophores and movement of calcium ions following the physiological stimulus to a secretory process. Nature. 1973 Oct 5;245(5423):249–251. doi: 10.1038/245249a0. [DOI] [PubMed] [Google Scholar]
- Gandía L., Albillos A., García A. G. Bovine chromaffin cells possess FTX-sensitive calcium channels. Biochem Biophys Res Commun. 1993 Jul 30;194(2):671–676. doi: 10.1006/bbrc.1993.1874. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hurlbut W. P., Chieregatti E., Valtorta F., Haimann C. Alpha-latrotoxin channels in neuroblastoma cells. J Membr Biol. 1994 Feb;138(1):91–102. doi: 10.1007/BF00211072. [DOI] [PubMed] [Google Scholar]
- Latorre R., Alvarez O. Voltage-dependent channels in planar lipid bilayer membranes. Physiol Rev. 1981 Jan;61(1):77–150. doi: 10.1152/physrev.1981.61.1.77. [DOI] [PubMed] [Google Scholar]
- Liu P. S., Kao L. S. Na(+)-dependent Ca2+ influx in bovine adrenal chromaffin cells. Cell Calcium. 1990 Oct;11(9):573–579. doi: 10.1016/0143-4160(90)90011-i. [DOI] [PubMed] [Google Scholar]
- Livett B. G. Adrenal medullary chromaffin cells in vitro. Physiol Rev. 1984 Oct;64(4):1103–1161. doi: 10.1152/physrev.1984.64.4.1103. [DOI] [PubMed] [Google Scholar]
- Longenecker H. E., Jr, Hurlbut W. P., Mauro A., Clark A. W. Effects of black widow spider venom on the frog neuromuscular junction. Effects on end-plate potential, miniature end-plate potential and nerve terminal spike. Nature. 1970 Feb 21;225(5234):701–703. doi: 10.1038/225701a0. [DOI] [PubMed] [Google Scholar]
- López M. G., Villarroya M., Lara B., Martínez Sierra R., Albillos A., García A. G., Gandía L. Q- and L-type Ca2+ channels dominate the control of secretion in bovine chromaffin cells. FEBS Lett. 1994 Aug 8;349(3):331–337. doi: 10.1016/0014-5793(94)00696-2. [DOI] [PubMed] [Google Scholar]
- Michelena P., Vega T., Montiel C., López M. G., García-Perez L. E., Gandía L., Garc-ia A. G. Effects of tyramine and calcium on the kinetics of secretion in intact and electroporated chromaffin cells superfused at high speed. Pflugers Arch. 1995 Dec;431(2):283–296. doi: 10.1007/BF00410202. [DOI] [PubMed] [Google Scholar]
- Moro M. A., López M. G., Gandía L., Michelena P., García A. G. Separation and culture of living adrenaline- and noradrenaline-containing cells from bovine adrenal medullae. Anal Biochem. 1990 Mar;185(2):243–248. doi: 10.1016/0003-2697(90)90287-j. [DOI] [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual R., Horga J. F., Sánchez-García P., García A. G. Release of noradrenaline by the ionophore X537A from normal and reserpinized guinea-pig atrium. Naunyn Schmiedebergs Arch Pharmacol. 1977 Dec;301(1):57–64. doi: 10.1007/BF00501264. [DOI] [PubMed] [Google Scholar]
- Petrenko A. G. alpha-Latrotoxin receptor. Implications in nerve terminal function. FEBS Lett. 1993 Jun 28;325(1-2):81–85. doi: 10.1016/0014-5793(93)81418-y. [DOI] [PubMed] [Google Scholar]
- Picotti G. B., Bondiolotti G. P., Meldolesi J. Peripheral catecholamine release by alpha-latrotoxin in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1982 Sep;320(3):224–229. doi: 10.1007/BF00510132. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Properties of ionophores with broad range cation selectivity. Fed Proc. 1973 Jun;32(6):1698–1703. [PubMed] [Google Scholar]
- Rosenthal L., Zacchetti D., Madeddu L., Meldolesi J. Mode of action of alpha-latrotoxin: role of divalent cations in Ca2(+)-dependent and Ca2(+)-independent effects mediated by the toxin. Mol Pharmacol. 1990 Dec;38(6):917–923. [PubMed] [Google Scholar]
- Schiavone M. T., Kirpekar S. M. Inactivation of secretory responses to potassium and nicotine in the cat adrenal medulla. J Pharmacol Exp Ther. 1982 Dec;223(3):743–749. [PubMed] [Google Scholar]
- Surkova I. Can exocytosis induced by alpha-latrotoxin be explained solely by its channel-forming activity? Ann N Y Acad Sci. 1994 Mar 9;710:48–64. doi: 10.1111/j.1749-6632.1994.tb26613.x. [DOI] [PubMed] [Google Scholar]
- Török T. L., Powis D. A. Catecholamine release from bovine chromaffin cells: the role of sodium-calcium exchange in ouabain-evoked release. Exp Physiol. 1990 Jul;75(4):573–586. doi: 10.1113/expphysiol.1990.sp003433. [DOI] [PubMed] [Google Scholar]
- Ushkaryov Y. A., Petrenko A. G., Geppert M., Südhof T. C. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992 Jul 3;257(5066):50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- von Rüden L., Neher E. A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science. 1993 Nov 12;262(5136):1061–1065. doi: 10.1126/science.8235626. [DOI] [PubMed] [Google Scholar]