Abstract
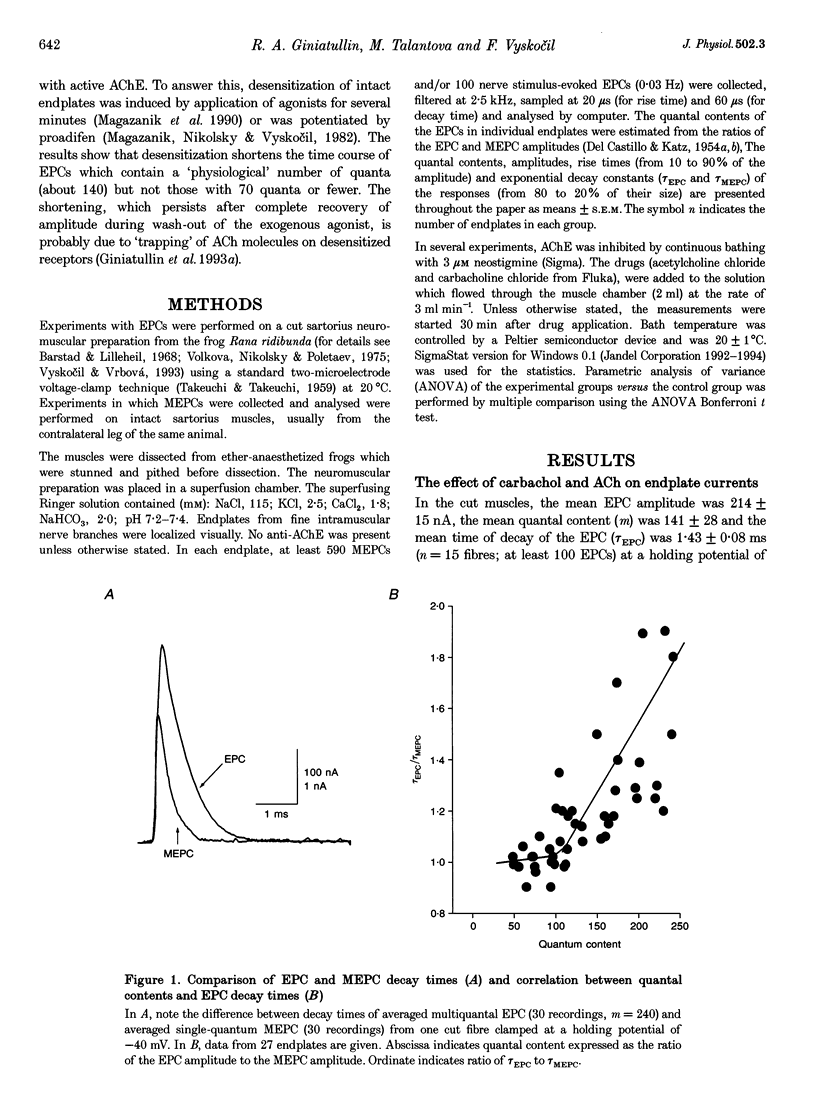
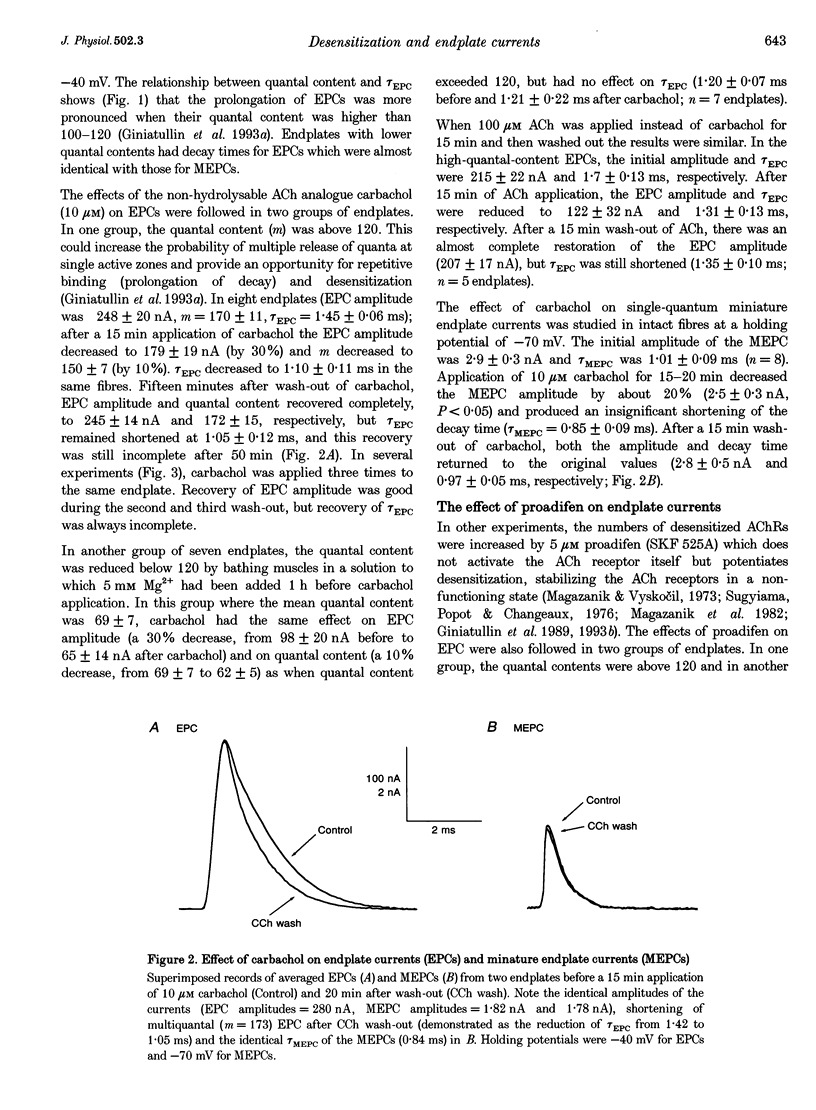
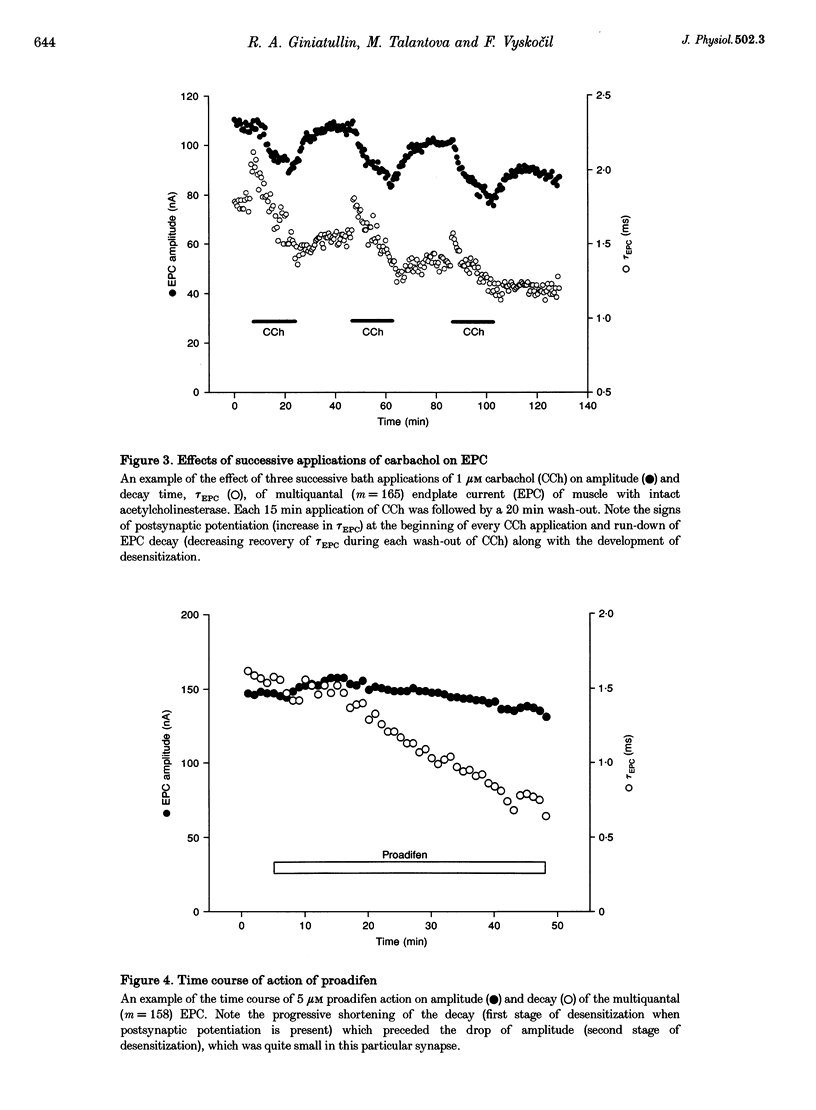
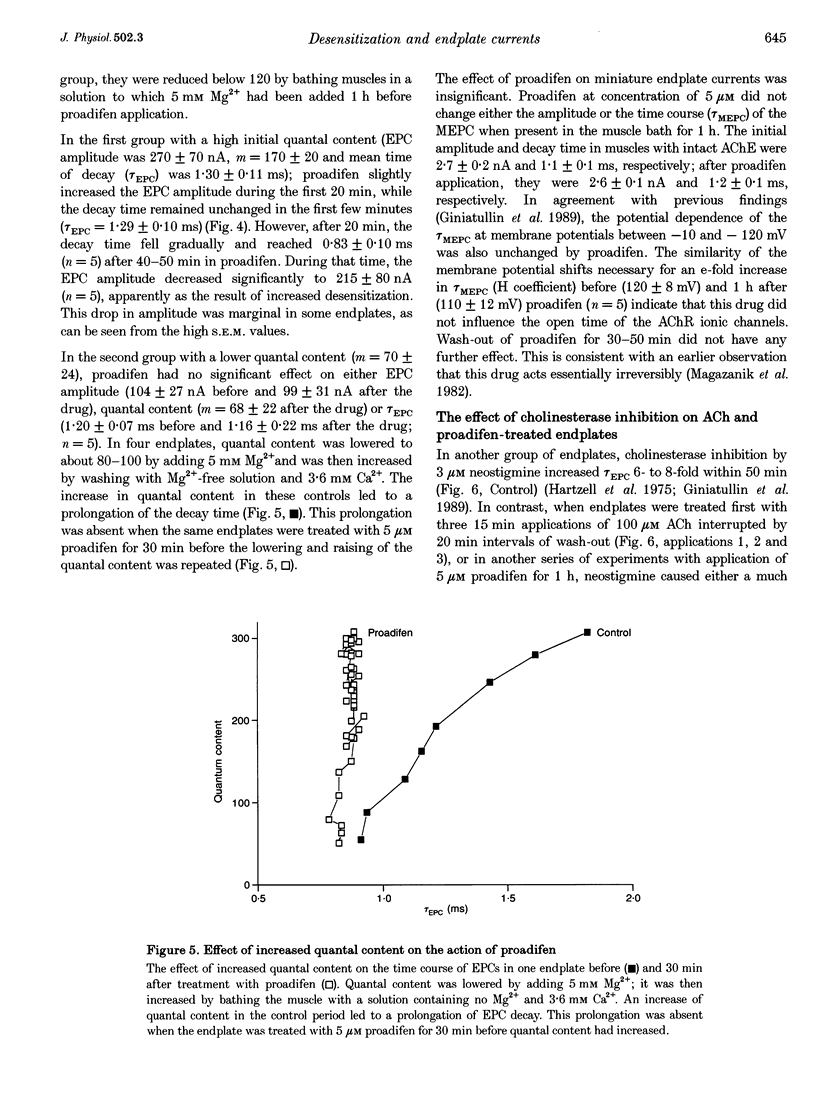
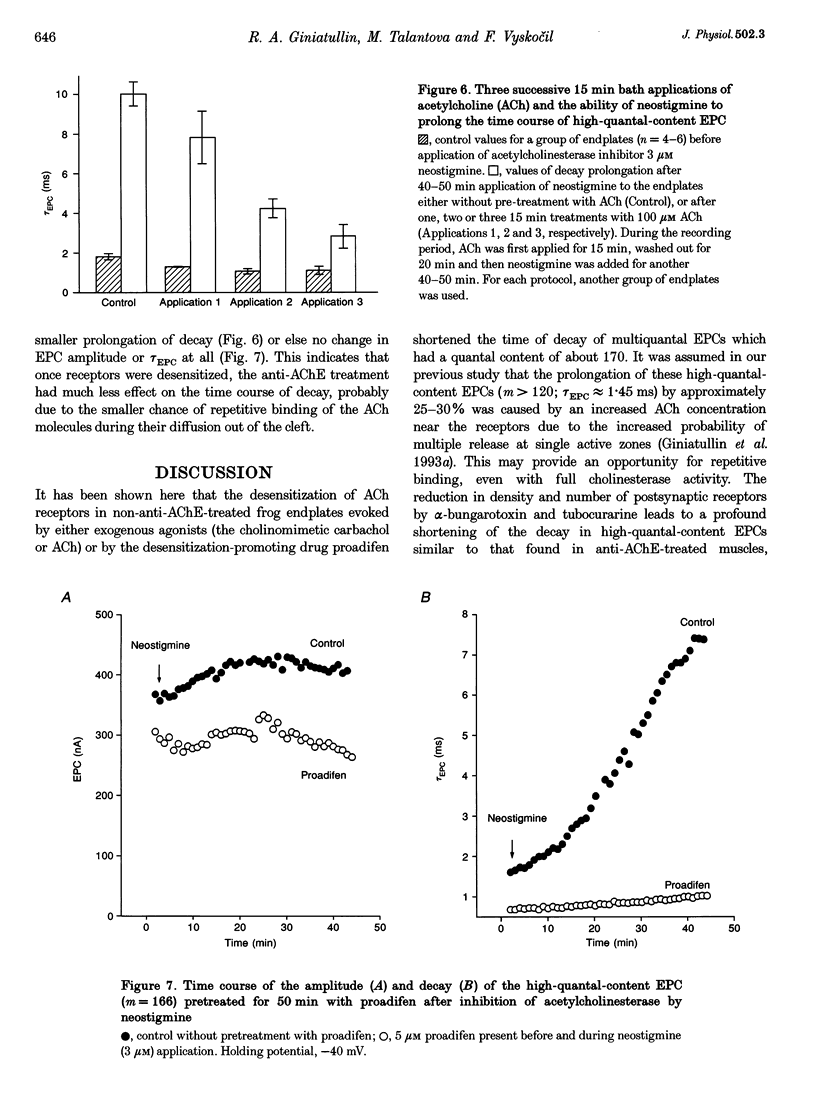
1. The desensitization induced by bath-applied carbachol or acetylcholine (ACh) and potentiated by proadifen (SKF 525A) was studied in the frog sartorius with intact synaptic acetylcholinesterase (AChE). 2. The reduction in the density and number of postsynaptic receptors produced by desensitization lowered the amplitude of the endplate currents (EPCs) and shortened the EPC decay when the quantal content (m) of the EPC was about 170 and when multiple release of quanta at single active zones was highly probably. The shortening of high-quantal-content EPCs persisted for at least 15 min after the wash-out of agonists, at a time when the amplitude had recovered fully. 3. The decay times of the low-quantal-content EPCs recorded from preparations pretreated with 5 mM Mg2+ (m approximately 70) and single-quantum miniature endplate currents (MEPCs) were not affected by carbachol, ACh or proadifen. 4. The desensitization of ACh receptors potentiated by proadifen, prevented completely the 6- to 8-fold prolongation of EPC which was induced by neostigmine inhibition of synaptic AChE. 5. It is assumed that high-quantal-content EPCs increase the incidence of multiple quanta release at single active zones and the probability of repetitive binding of ACh molecules which leads to EPC prolongation. The shortening which persists after complete recovery of the amplitude during wash-out of the exogenous agonist is probably due to 'trapping' of ACh molecules onto rapidly desensitized receptors and the reduced density of functional AChRs during the quantum action.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barstad J. A., Lilleheil G. Transversaly cut diaphragm preparation from rat. An adjuvant tool in the study of the physiology and pbarmacology of the myoneural junction. Arch Int Pharmacodyn Ther. 1968 Oct;175(2):373–390. [PubMed] [Google Scholar]
- Bartol T. M., Jr, Land B. R., Salpeter E. E., Salpeter M. M. Monte Carlo simulation of miniature endplate current generation in the vertebrate neuromuscular junction. Biophys J. 1991 Jun;59(6):1290–1307. doi: 10.1016/S0006-3495(91)82344-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIANI S., EDWARDS C. THE EFFECT OF ACETYLCHOLINE ON NEUROMUSCULAR TRANSMISSION IN THE FROG. J Pharmacol Exp Ther. 1963 Oct;142:21–23. [PubMed] [Google Scholar]
- Cachelin A. B., Colquhoun D. Desensitization of the acetylcholine receptor of frog end-plates measured in a Vaseline-gap voltage clamp. J Physiol. 1989 Aug;415:159–188. doi: 10.1113/jphysiol.1989.sp017717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol. 1982 Jan;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniatullin R. A., Bal'tser S. K., Nikol'skii E. E., Magazanik L. G. Issledovanie postsinapticheskoi potentsiatsii i desensitizatsii v mionevral'nom sinapse liagushki, vyzvannykh ritmicheskoi stimuliatsiei dvigatel'nogo nerva. Neirofiziologiia. 1986;18(5):645–654. [PubMed] [Google Scholar]
- Giniatullin R. A., Khamitov G., Khazipov R., Magazanik L. G., Nikolsky E. E., Snetkov V. A., Vyskocil F. Development of desensitization during repetitive end-plate activity and single end-plate currents in frog muscle. J Physiol. 1989 May;412:113–122. doi: 10.1113/jphysiol.1989.sp017606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniatullin R. A., Khazipov R. N., Oranska T. I., Nikolsky E. E., Voronin V. A., Vyskocil F. The effect of non-quantal acetylcholine release on quantal miniature currents at mouse diaphragm. J Physiol. 1993 Jul;466:105–114. [PMC free article] [PubMed] [Google Scholar]
- Giniatullin R. A., Khazipov R. N., Vyskocil F. A correlation between quantal content and decay time of endplate currents in frog muscles with intact cholinesterase. J Physiol. 1993 Jul;466:95–103. [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Fast kinetic studies on the interaction of a fluorescent agonist with the membrane-bound acetylcholine receptor from Torpedo marmorata. Eur J Biochem. 1979 Feb 15;94(1):255–279. doi: 10.1111/j.1432-1033.1979.tb12893.x. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazanik L. G., Nikolsky E., Vyskocil F. Effect of the desensitization-potentiating agent SKF-525a on frog end-plate currents. Eur J Pharmacol. 1982 May 7;80(1):115–119. doi: 10.1016/0014-2999(82)90185-6. [DOI] [PubMed] [Google Scholar]
- Magazanik L. G., Snetkov V. A., Giniatullin R. A., Khazipov R. N. Changes in the time course of miniature endplate currents induced by bath-applied acetylcholine. Neurosci Lett. 1990 Jun 8;113(3):281–285. doi: 10.1016/0304-3940(90)90598-4. [DOI] [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocil F. Dependence of acetylcholine desensitization on the membrane potential of frog muscle fibre and on the ionic changes in the medium. J Physiol. 1970 Oct;210(3):507–518. doi: 10.1113/jphysiol.1970.sp009223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocit F. The effect of temperature on desensitization kinetics at the post-synaptic membrane of the frog muscle fibre. J Physiol. 1975 Jul;249(2):285–300. doi: 10.1113/jphysiol.1975.sp011016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S. A study of desensitization of acetylcholine receptors using nerve-released transmitter in the frog. J Physiol. 1981 Jul;316:225–250. doi: 10.1113/jphysiol.1981.sp013784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström B. Postnatal development of motor nerve terminals in "slow-red" and "fast-white" cat muscles. Acta Neurol Scand. 1968;44(3):363–383. doi: 10.1111/j.1600-0404.1968.tb05578.x. [DOI] [PubMed] [Google Scholar]
- Pennefather P., Quastel D. M. Relation between subsynaptic receptor blockade and response to quantal transmitter at the mouse neuromuscular junction. J Gen Physiol. 1981 Sep;78(3):313–344. doi: 10.1085/jgp.78.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama H., Popot J. L., Changeux J. P. Studies on the electrogenic action of acetylcholine with Torpedo marmorata electric organ. III. Pharmocological desensitization in vitro of the receptor-rich membrane fragments by cholinergic agonists. J Mol Biol. 1976 Sep 25;106(3):485–496. doi: 10.1016/0022-2836(76)90248-5. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]
- Volkova I. N., Nikol'skii E. E., Poletaev G. I. Blokirovanie potentsialov deistviia i sokrashchenii skeletnoi myshtsy liagushki poperechnym rassecheniem myshechnykh volokon. Fiziol Zh SSSR Im I M Sechenova. 1975 Sep;61(9):1433–1436. [PubMed] [Google Scholar]
- Vyskocil F., Nikolsky E., Edwards C. An analysis of the mechanisms underlying the non-quantal release of acetylcholine at the mouse neuromuscular junction. Neuroscience. 1983 Jun;9(2):429–435. doi: 10.1016/0306-4522(83)90305-6. [DOI] [PubMed] [Google Scholar]
- Vyskocil F., Vrbová G. Non-quantal release of acetylcholine affects polyneuronal innervation on developing rat muscle fibres. Eur J Neurosci. 1993 Dec 1;5(12):1677–1683. doi: 10.1111/j.1460-9568.1993.tb00235.x. [DOI] [PubMed] [Google Scholar]


