Abstract
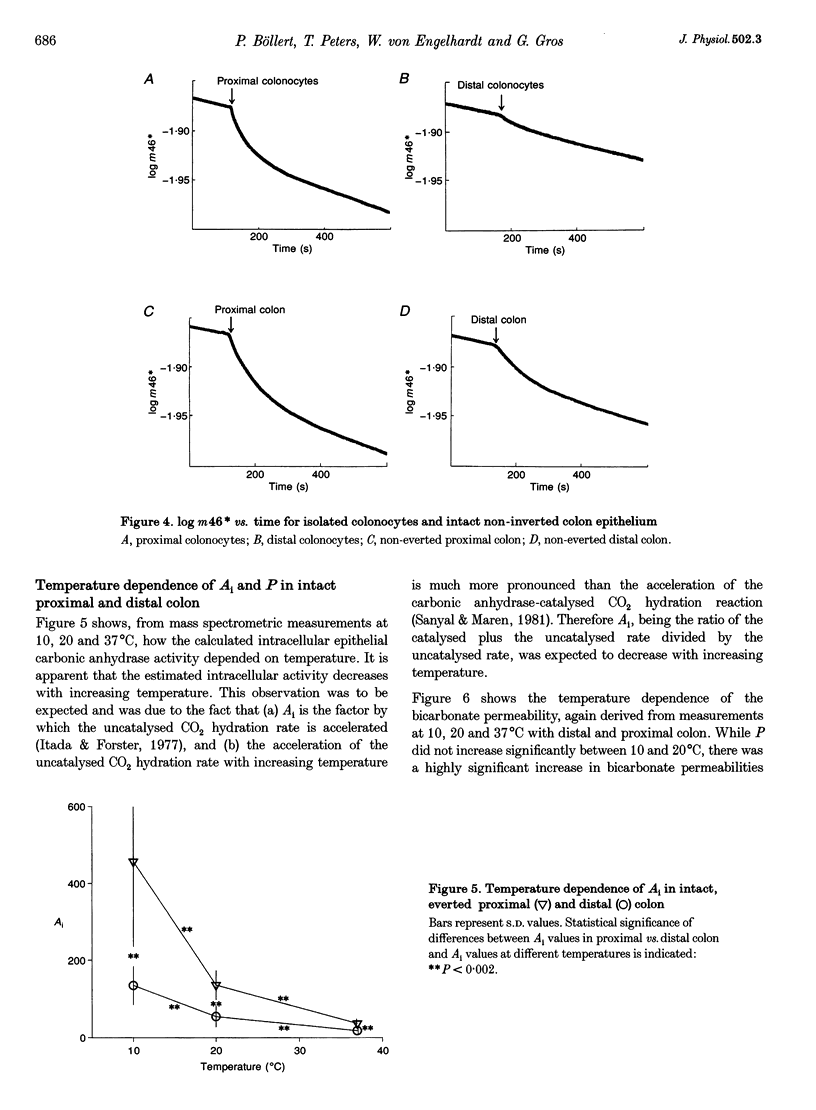
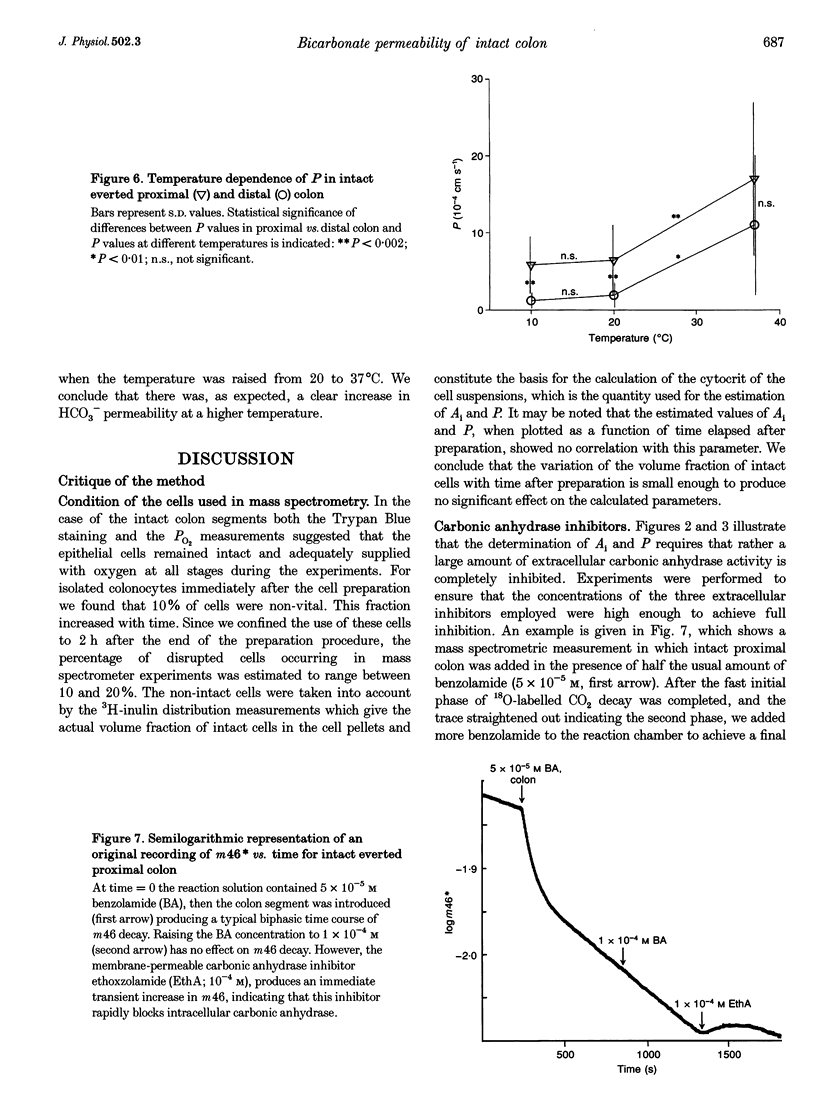
1. A mass spectrometric method originally used in red blood cells was applied to suspensions of isolated colonocytes and intact colonic epithelium to measure the exchange of 18O between HCO3-, CO2 and H2O to determine intracellular carbonic anhydrase activity (Ai) and membrane bicarbonate permeability (P). 2. In suspensions of isolated guinea-pig colon epithelial cells, colonocytes, we found significantly higher values of Ai and P for cells derived from the proximal colon than for cells from the distal colon. In the case of Ai, this confirms earlier reports. 3. When the 18O exchange process was observed across the mucosal (apical) side of intact colon mucosa, the estimated values of Ai were identical to those obtained for isolated colonocytes, for both the proximal and the distal part of the colon. This is considered to be strong evidence that this method can be applied to a layer of intact epithelium as well as to cell suspensions. 4. The values of P obtained from the apical side of intact colon mucosa were 6 times higher than those estimated from measurements with isolated colonocytes. This indicates that the basolateral membrane of colon epithelium, which participates in the 18O exchange process in isolated colonocytes but not in the 18O exchange process across the apical side of intact mucosa, has a markedly lower bicarbonate permeability than the apical membrane. 5. When the 18O exchange process was observed across the serosal (basolateral) side of intact colon mucosa, the P values, as expected, were low compared with the apical side of intact mucosa. However, rather unexpectedly, the Ai values derived from these measurements were 2-3 times lower than those obtained with isolated colonocytes. It appears possible that the latter finding is an artifact due to the submucosal tissue markedly slowing down CO2 diffusion from the bathing medium into the epithelial cells, thus causing an apparent fall in Ai. 6. Ai decreased and P increased with increasing temperature, as expected, when studied on the mucosal side of intact colon. This provides additional support for the validity of the method.
Full text
PDF

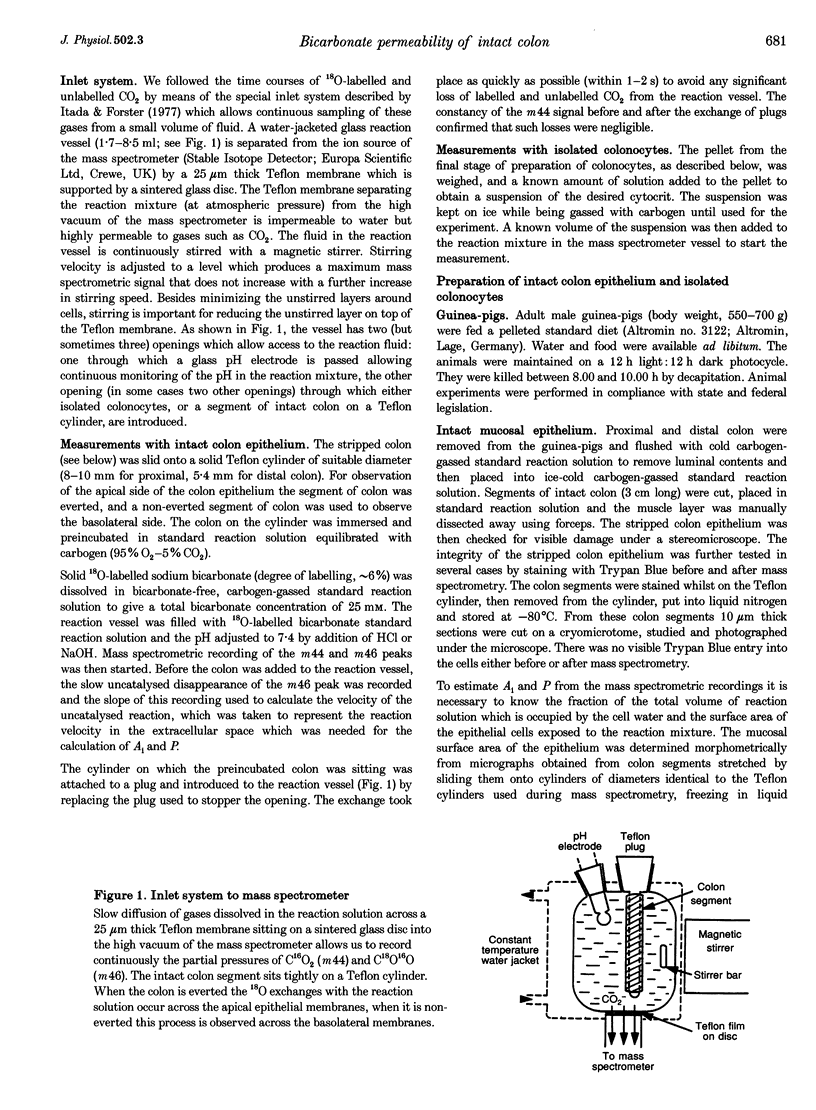

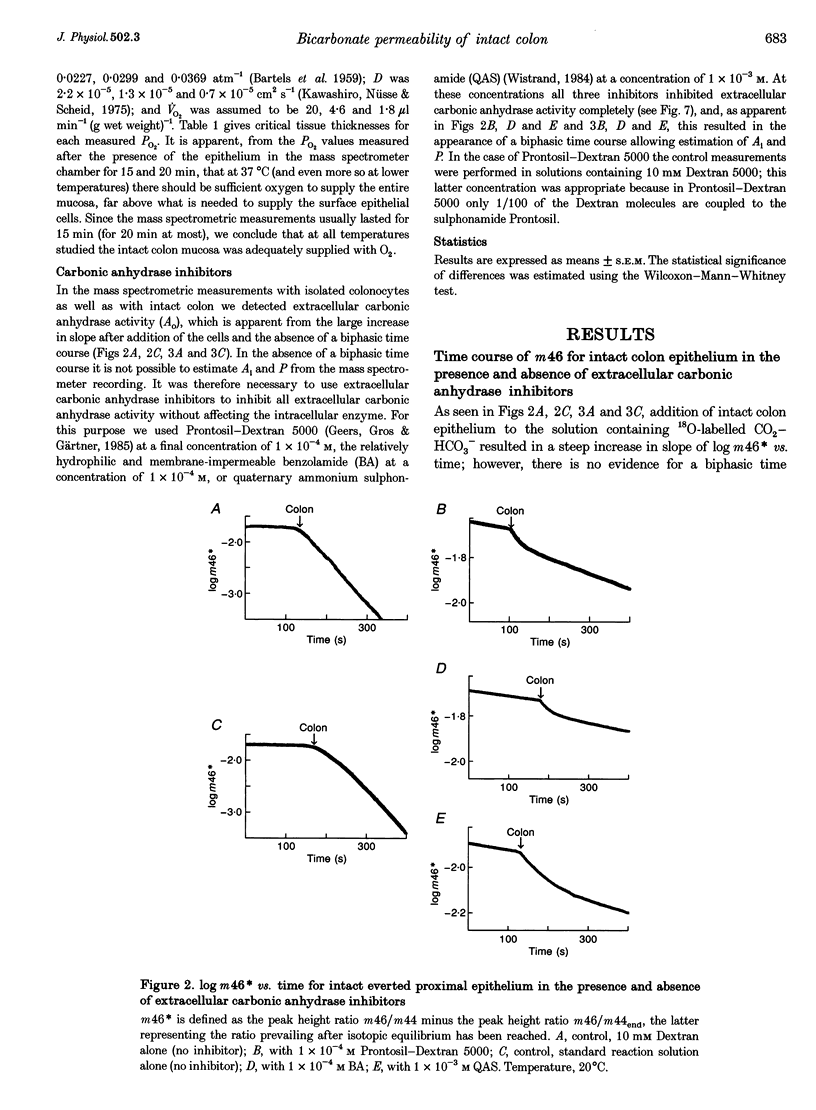
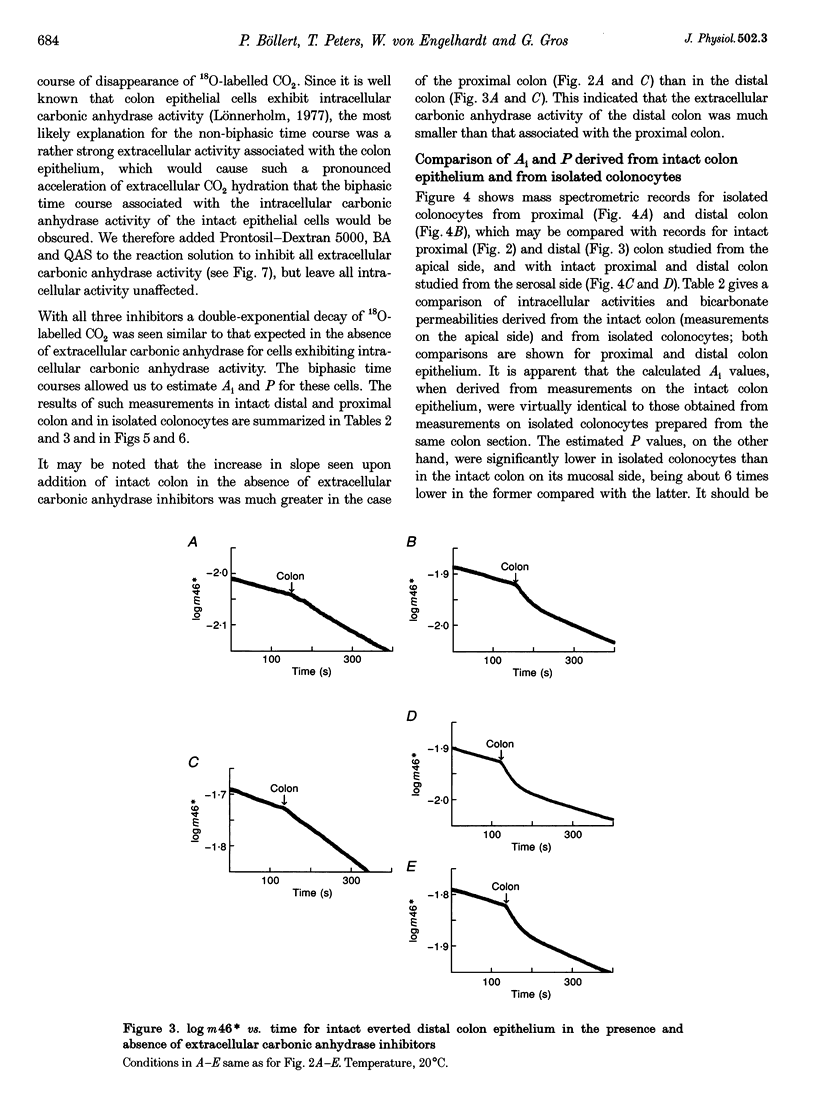
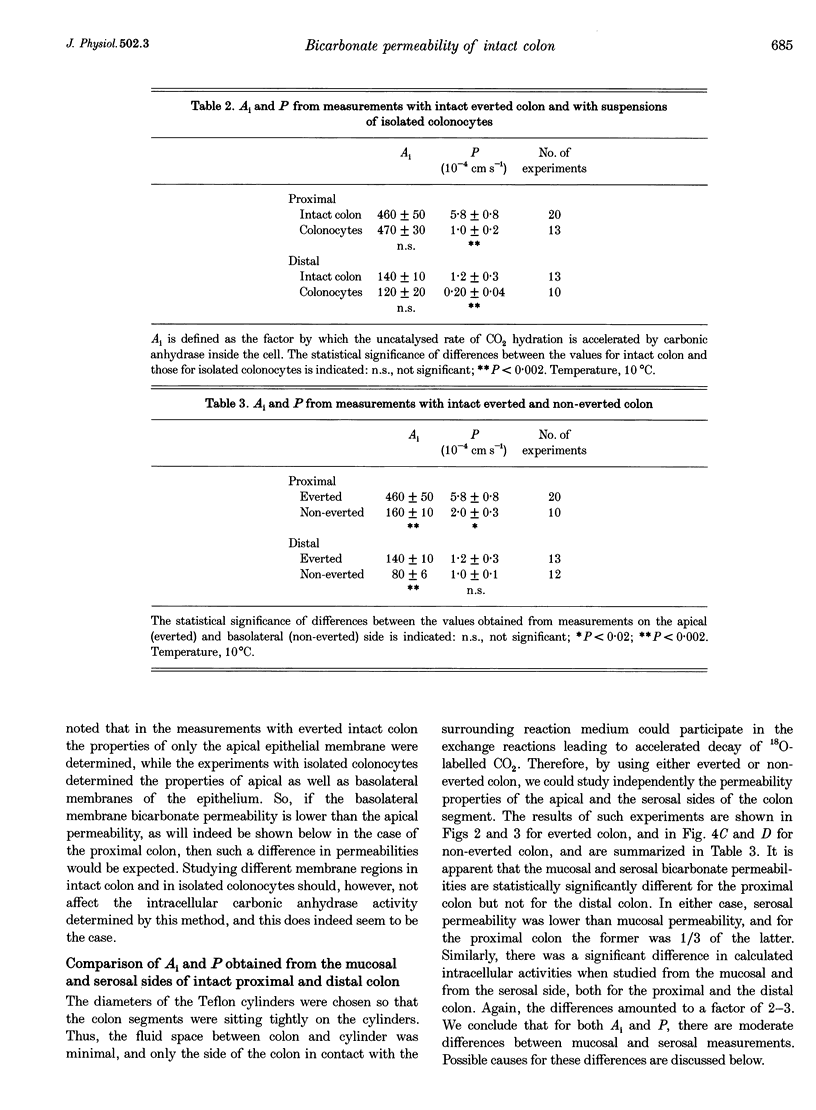




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter M. J., Parsons D. S. The carbonic anhydrases of some guinea-pig tissues. Biochim Biophys Acta. 1970 Apr 22;206(1):190–192. doi: 10.1016/0005-2744(70)90099-9. [DOI] [PubMed] [Google Scholar]
- Carter M. J., Parsons D. S. The purification and properties of carbonic anhydrases from guinea-pig erythrocytes and mucosae of the gastrointestinal tract. Biochem J. 1970 Dec;120(4):797–808. doi: 10.1042/bj1200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow E. I., Crandall E. D., Forster R. E. Kinetics of bicarbonate-chloride exchange across the human red blood cell membrane. J Gen Physiol. 1976 Dec;68(6):633–652. doi: 10.1085/jgp.68.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers C., Gros G., Gärtner A. Extracellular carbonic anhydrase of skeletal muscle associated with the sarcolemma. J Appl Physiol (1985) 1985 Aug;59(2):548–558. doi: 10.1152/jappl.1985.59.2.548. [DOI] [PubMed] [Google Scholar]
- Gros G., Moll W. The diffusion of carbon dioxide in erythrocytes and hemoglobin solutions. Pflugers Arch. 1971;324(3):249–266. doi: 10.1007/BF00586422. [DOI] [PubMed] [Google Scholar]
- Itada N., Forster R. E. Carbonic anhydrase activity in intact red blood cells measured with 18O exchange. J Biol Chem. 1977 Jun 10;252(11):3881–3890. [PubMed] [Google Scholar]
- Kawashiro T., Nüsse W., Scheid P. Determination of diffusivity of oxygen and carbon dioxide in respiring tissue: results in rat skeletal muscle. Pflugers Arch. 1975 Sep 9;359(3):231–251. doi: 10.1007/BF00587382. [DOI] [PubMed] [Google Scholar]
- Luciano L., Reale E., Rechkemmer G., von Engelhardt W. Structure of zonulae occludentes and the permeability of the epithelium to short-chain fatty acids in the proximal and the distal colon of guinea pig. J Membr Biol. 1984;82(2):145–156. doi: 10.1007/BF01868939. [DOI] [PubMed] [Google Scholar]
- Maren T. H. The general physiology of reactions catalyzed by carbonic anhydrase and their inhibition by sulfonamides. Ann N Y Acad Sci. 1984;429:568–579. doi: 10.1111/j.1749-6632.1984.tb12389.x. [DOI] [PubMed] [Google Scholar]
- Mascolo N., Rajendran V. M., Binder H. J. Mechanism of short-chain fatty acid uptake by apical membrane vesicles of rat distal colon. Gastroenterology. 1991 Aug;101(2):331–338. doi: 10.1016/0016-5085(91)90008-9. [DOI] [PubMed] [Google Scholar]
- Rechkemmer G., Rönnau K., von Engelhardt W. Fermentation of polysaccharides and absorption of short chain fatty acids in the mammalian hindgut. Comp Biochem Physiol A Comp Physiol. 1988;90(4):563–568. doi: 10.1016/0300-9629(88)90668-8. [DOI] [PubMed] [Google Scholar]
- Sanyal G., Maren T. H. Thermodynamics of carbonic anhydrase catalysis. A comparison between human isoenzymes B and C. J Biol Chem. 1981 Jan 25;256(2):608–612. [PubMed] [Google Scholar]
- Stieger B., Marxer A., Hauri H. P. Isolation of brush-border membranes from rat and rabbit colonocytes: is alkaline phosphatase a marker enzyme? J Membr Biol. 1986;91(1):19–31. doi: 10.1007/BF01870211. [DOI] [PubMed] [Google Scholar]
- von Engelhardt W., Burmester M., Hansen K., Becker G., Rechkemmer G. Effects of amiloride and ouabain on short-chain fatty acid transport in guinea-pig large intestine. J Physiol. 1993 Jan;460:455–466. doi: 10.1113/jphysiol.1993.sp019481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt W., Gros G., Burmester M., Hansen K., Becker G., Rechkemmer G. Functional role of bicarbonate in propionate transport across guinea-pig isolated caecum and proximal colon. J Physiol. 1994 Jun 1;477(Pt 2):365–371. doi: 10.1113/jphysiol.1994.sp020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt W., Rechkemmer G. Segmental differences of short-chain fatty acid transport across guinea-pig large intestine. Exp Physiol. 1992 May;77(3):491–499. doi: 10.1113/expphysiol.1992.sp003609. [DOI] [PubMed] [Google Scholar]


