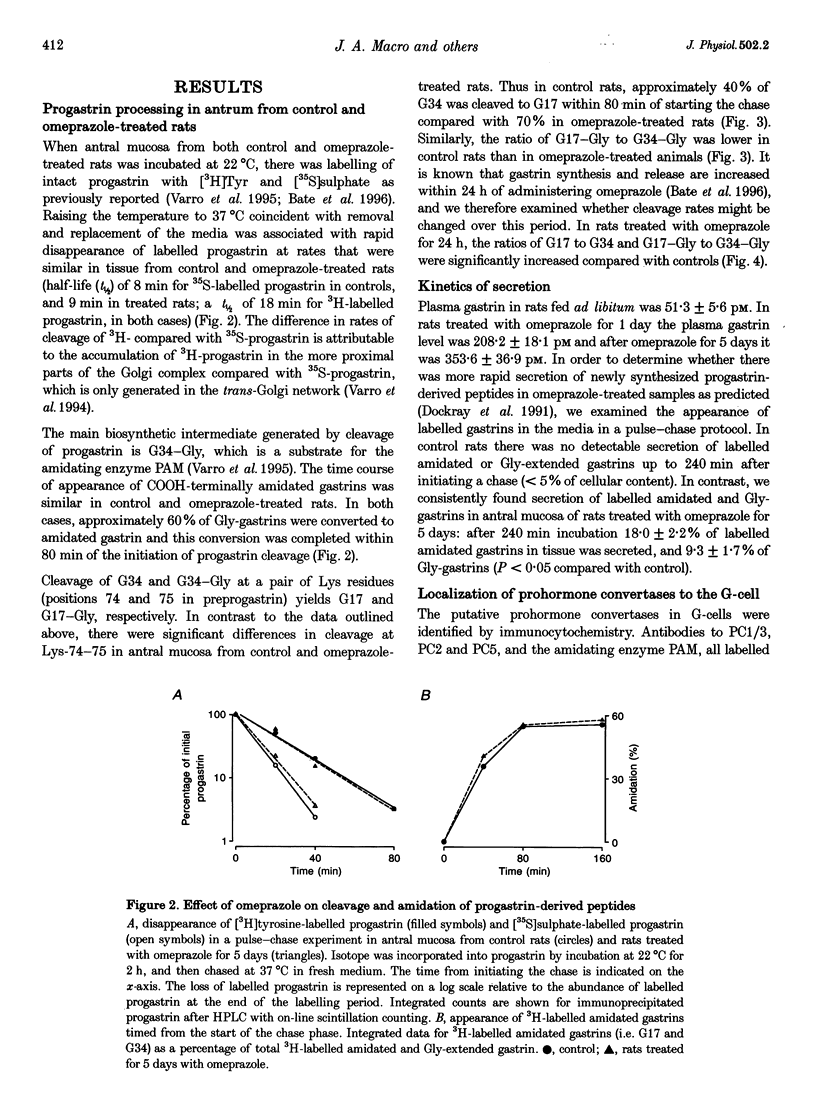
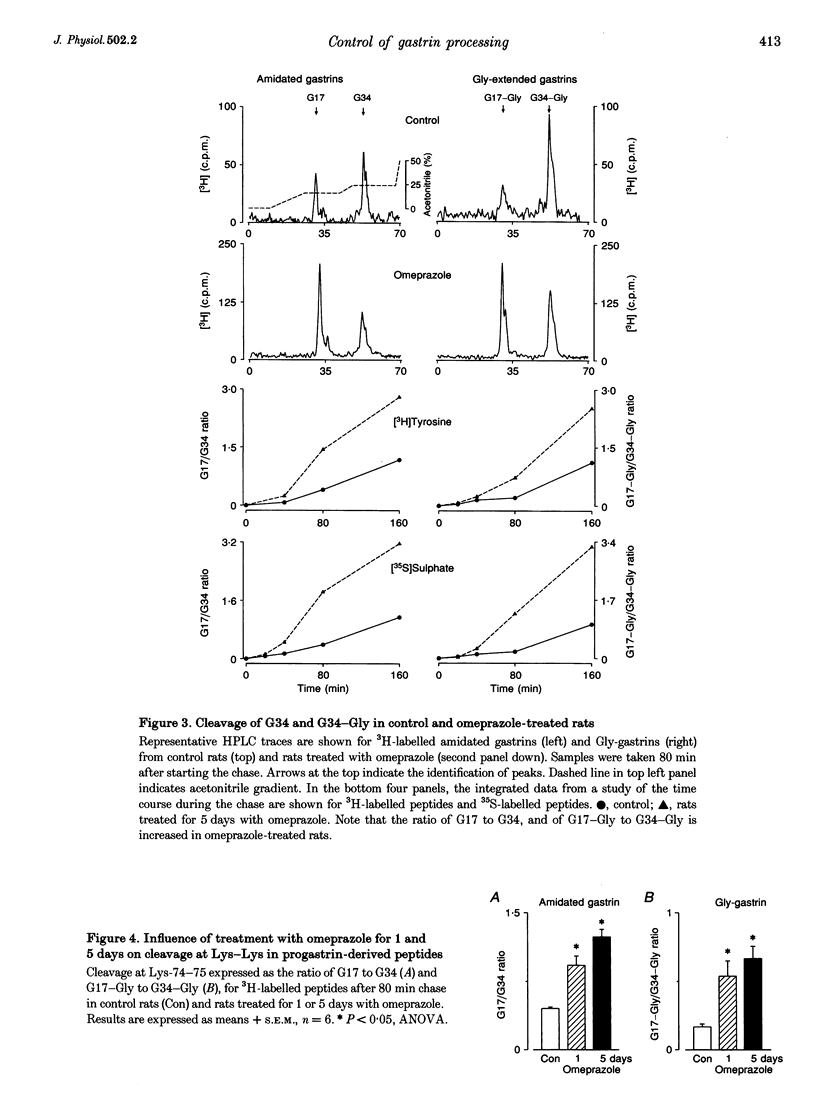
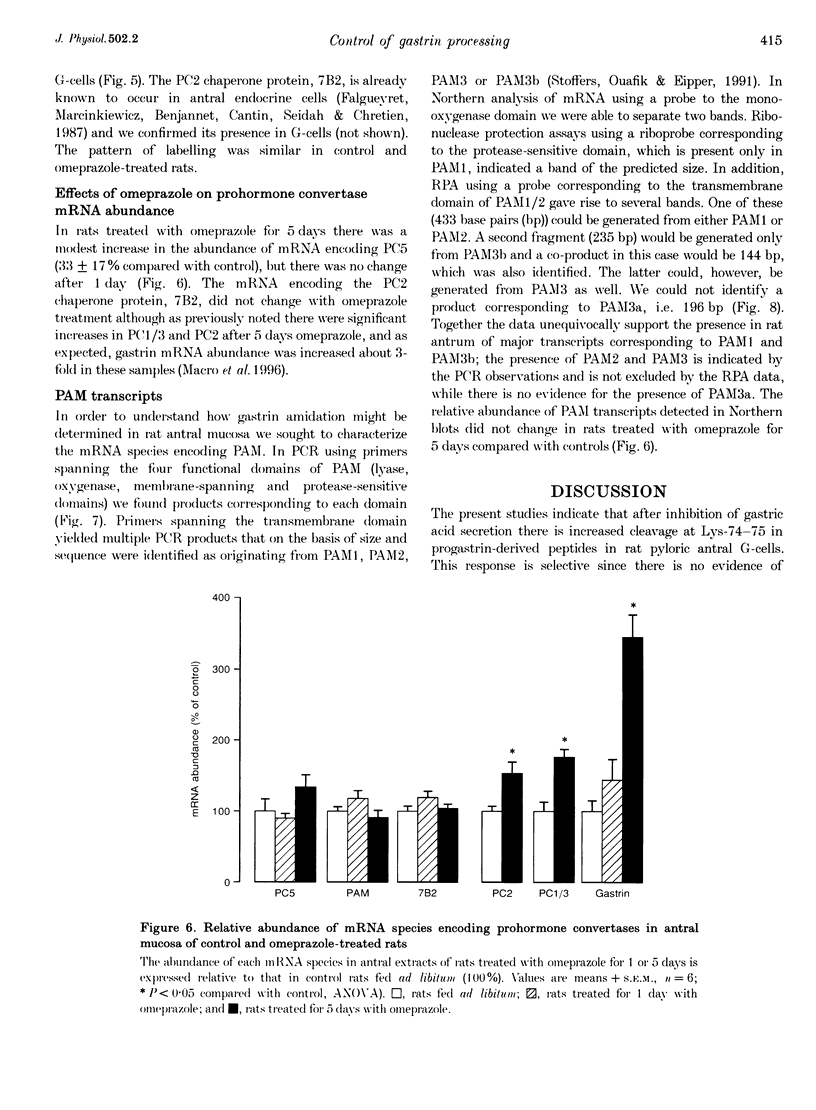
Abstract
1. Inhibition of gastric acid secretion by proton pump inhibitors like omeprazole increases the synthesis and secretion of the pyloric antral hormone gastrin. We report here how omeprazole influences the conversion of the gastrin precursor to its final products, and the abundance of mRNAs encoding proteins associated with progastrin processing in rat antral mucosa. 2. Progastrin processing was studied using a pulse-chase protocol in antral mucosa, incubated in vitro, from rats treated with omeprazole for up to 5 days. Labelled peptides were detected by on-line scintillation counting after immunoprecipitation and HPLC. The mRNAs encoding prohormone-processing enzymes were identified by Northern blot, polymerase chain reaction or ribonuclease protection assay, and their cellular origins identified by immunocytochemistry. 3. Cleavage of [3H]- and [35S]-labelled progastrins at Arg-94-95 or Arg-57-58, and amidation at Phe-92 were not influenced by pretreatment with omeprazole. In contrast, cleavage of G34 (the thirty-four amino acid amidated gastrin) at Lys-74-75 to give G17 (the seventeen amino acid amidated gastrin), and of G34-Gly to G17-Gly (G34 and G17 with COOH-terminal glycine), was increased 3-fold after treatment with omeprazole for either 1 or 5 days. 4. Approximately 20% of newly synthesized amidated and Gly-extended gastrins were secreted within 240 min of the labelling period in omeprazole-treated samples, but secretion of labelled gastrins from control tissue was undetectable over a comparable period. 5. The amidating enzyme, peptidyglycine alpha-amidating mono-oxygenase (PAM), the prohormone convertases PC1/3, PC2, PC5 and the PC2 chaperone 7B2 were localized to rat antral gastrin cells by immunocytochemistry. The relative abundance of mRNA species encoding 7B2, PC5 and PAM were unchanged after treatment with omeprazole for 5 days, whereas gastrin, PC1/3 and PC2 mRNAs are known to increase at this time. 6. The main consequence of increased cleavage at Lys-74-75 is the production of G17 and G17-Gly at the expense of G34 and G34-Gly, respectively. The latter have longer plasma half-lives, and so their increased cleavage may serve to limit the rise in plasma gastrin concentration after inhibition of acid secretion. Changes in the abundance of mRNAs encoding prohormone-processing enzymes cannot account for the rapidity of the changes in cleavage of progastrin at Lys residues after omeprazole.
Full text
PDF



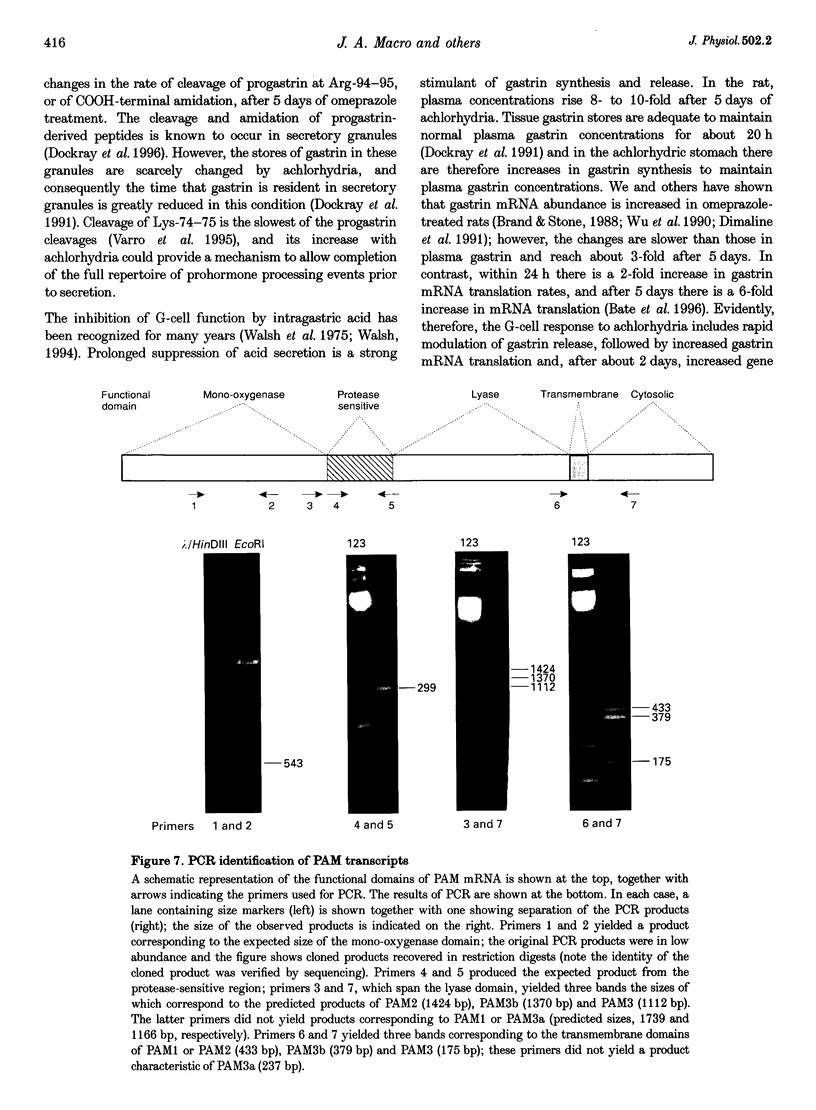
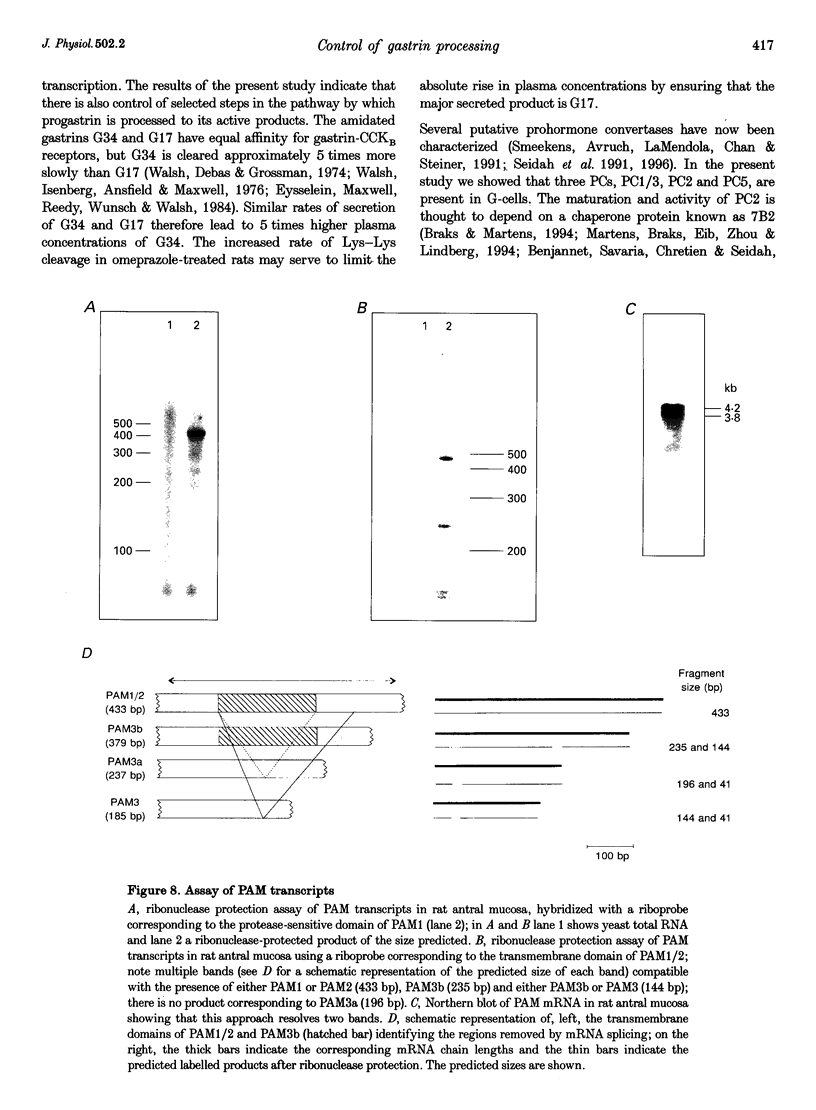


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcón C., Lincoln B., Rhodes C. J. The biosynthesis of the subtilisin-related proprotein convertase PC3, but no that of the PC2 convertase, is regulated by glucose in parallel to proinsulin biosynthesis in rat pancreatic islets. J Biol Chem. 1993 Feb 25;268(6):4276–4280. [PubMed] [Google Scholar]
- Bate G. W., Varro A., Dimaline R., Dockray G. J. Control of preprogastrin messenger RNA translation by gastric acid in the rat. Gastroenterology. 1996 Nov;111(5):1224–1229. doi: 10.1053/gast.1996.v111.pm8898636. [DOI] [PubMed] [Google Scholar]
- Benjannet S., Savaria D., Chrétien M., Seidah N. G. 7B2 is a specific intracellular binding protein of the prohormone convertase PC2. J Neurochem. 1995 May;64(5):2303–2311. doi: 10.1046/j.1471-4159.1995.64052303.x. [DOI] [PubMed] [Google Scholar]
- Braks J. A., Martens G. J. 7B2 is a neuroendocrine chaperone that transiently interacts with prohormone convertase PC2 in the secretory pathway. Cell. 1994 Jul 29;78(2):263–273. doi: 10.1016/0092-8674(94)90296-8. [DOI] [PubMed] [Google Scholar]
- Brand S. J., Stone D. Reciprocal regulation of antral gastrin and somatostatin gene expression by omeprazole-induced achlorhydria. J Clin Invest. 1988 Sep;82(3):1059–1066. doi: 10.1172/JCI113662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day R., Schafer M. K., Watson S. J., Chrétien M., Seidah N. G. Distribution and regulation of the prohormone convertases PC1 and PC2 in the rat pituitary. Mol Endocrinol. 1992 Mar;6(3):485–497. doi: 10.1210/mend.6.3.1316544. [DOI] [PubMed] [Google Scholar]
- Dimaline R., Evans D., Varro A., Dockray G. J. Reversal by omeprazole of the depression of gastrin cell function by fasting in the rat. J Physiol. 1991 Feb;433:483–493. doi: 10.1113/jphysiol.1991.sp018439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray G. J., Hamer C., Evans D., Varro A., Dimaline R. The secretory kinetics of the G cell in omeprazole-treated rats. Gastroenterology. 1991 May;100(5 Pt 1):1187–1194. [PubMed] [Google Scholar]
- Dockray G. J., Varro A., Dimaline R. Gastric endocrine cells: gene expression, processing, and targeting of active products. Physiol Rev. 1996 Jul;76(3):767–798. doi: 10.1152/physrev.1996.76.3.767. [DOI] [PubMed] [Google Scholar]
- Eysselein V. E., Maxwell V., Reedy T., Wünsch E., Walsh J. H. Similar acid stimulatory potencies of synthetic human big and little gastrins in man. J Clin Invest. 1984 May;73(5):1284–1290. doi: 10.1172/JCI111330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falgueyret J. P., Marcinkiewicz M., Benjannet S., Cantin M., Seidah N. G., Chrétien M. Immunocytochemical localization of a novel pituitary polypeptide "7B2" in the gastro-intestinal tract of the rat. Cell Tissue Res. 1987 Sep;249(3):707–709. doi: 10.1007/BF00217343. [DOI] [PubMed] [Google Scholar]
- GREGORY R. A., TRACY H. J. THE CONSTITUTION AND PROPERTIES OF TWO GASTRINS EXTRACTED FROM HOG ANTRAL MUCOSA. Gut. 1964 Apr;5:103–114. [PMC free article] [PubMed] [Google Scholar]
- Gregory R. A., Tracy H. J. Isolation of two "big gastrins" from Zollinger-Ellison tumour tissue. Lancet. 1972 Oct 14;2(7781):797–799. doi: 10.1016/s0140-6736(72)92151-4. [DOI] [PubMed] [Google Scholar]
- Higashide S., Gomez G., Greeley G. H., Jr, Townsend C. M., Jr, Thompson J. C. Glycine-extended gastrin potentiates gastrin-stimulated gastric acid secretion in rats. Am J Physiol. 1996 Jan;270(1 Pt 1):G220–G224. doi: 10.1152/ajpgi.1996.270.1.G220. [DOI] [PubMed] [Google Scholar]
- Larsson H., Carlsson E., Mattsson H., Lundell L., Sundler F., Sundell G., Wallmark B., Watanabe T., Håkanson R. Plasma gastrin and gastric enterochromaffinlike cell activation and proliferation. Studies with omeprazole and ranitidine in intact and antrectomized rats. Gastroenterology. 1986 Feb;90(2):391–399. doi: 10.1016/0016-5085(86)90938-8. [DOI] [PubMed] [Google Scholar]
- Lusson J., Vieau D., Hamelin J., Day R., Chrétien M., Seidah N. G. cDNA structure of the mouse and rat subtilisin/kexin-like PC5: a candidate proprotein convertase expressed in endocrine and nonendocrine cells. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6691–6695. doi: 10.1073/pnas.90.14.6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens G. J., Braks J. A., Eib D. W., Zhou Y., Lindberg I. The neuroendocrine polypeptide 7B2 is an endogenous inhibitor of prohormone convertase PC2. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5784–5787. doi: 10.1073/pnas.91.13.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyarce A. M., Hand T. A., Mains R. E., Eipper B. A. Dopaminergic regulation of secretory granule-associated proteins in rat intermediate pituitary. J Neurochem. 1996 Jul;67(1):229–241. doi: 10.1046/j.1471-4159.1996.67010229.x. [DOI] [PubMed] [Google Scholar]
- Schuppin G. T., Rhodes C. J. Specific co-ordinated regulation of PC3 and PC2 gene expression with that of preproinsulin in insulin-producing beta TC3 cells. Biochem J. 1996 Jan 1;313(Pt 1):259–268. doi: 10.1042/bj3130259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah N. G., Hamelin J., Mamarbachi M., Dong W., Tardos H., Mbikay M., Chretien M., Day R. cDNA structure, tissue distribution, and chromosomal localization of rat PC7, a novel mammalian proprotein convertase closest to yeast kexin-like proteinases. Proc Natl Acad Sci U S A. 1996 Apr 16;93(8):3388–3393. doi: 10.1073/pnas.93.8.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah N. G., Marcinkiewicz M., Benjannet S., Gaspar L., Beaubien G., Mattei M. G., Lazure C., Mbikay M., Chrétien M. Cloning and primary sequence of a mouse candidate prohormone convertase PC1 homologous to PC2, Furin, and Kex2: distinct chromosomal localization and messenger RNA distribution in brain and pituitary compared to PC2. Mol Endocrinol. 1991 Jan;5(1):111–122. doi: 10.1210/mend-5-1-111. [DOI] [PubMed] [Google Scholar]
- Seva C., Dickinson C. J., Yamada T. Growth-promoting effects of glycine-extended progastrin. Science. 1994 Jul 15;265(5170):410–412. doi: 10.1126/science.8023165. [DOI] [PubMed] [Google Scholar]
- Singh P., Owlia A., Espeijo R., Dai B. Novel gastrin receptors mediate mitogenic effects of gastrin and processing intermediates of gastrin on Swiss 3T3 fibroblasts. Absence of detectable cholecystokinin (CCK)-A and CCK-B receptors. J Biol Chem. 1995 Apr 14;270(15):8429–8438. doi: 10.1074/jbc.270.15.8429. [DOI] [PubMed] [Google Scholar]
- Smeekens S. P., Avruch A. S., LaMendola J., Chan S. J., Steiner D. F. Identification of a cDNA encoding a second putative prohormone convertase related to PC2 in AtT20 cells and islets of Langerhans. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):340–344. doi: 10.1073/pnas.88.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffers D. A., Green C. B., Eipper B. A. Alternative mRNA splicing generates multiple forms of peptidyl-glycine alpha-amidating monooxygenase in rat atrium. Proc Natl Acad Sci U S A. 1989 Jan;86(2):735–739. doi: 10.1073/pnas.86.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffers D. A., Ouafik L., Eipper B. A. Characterization of novel mRNAs encoding enzymes involved in peptide alpha-amidation. J Biol Chem. 1991 Jan 25;266(3):1701–1707. [PubMed] [Google Scholar]
- Todisco A., Takeuchi Y., Seva C., Dickinson C. J., Yamada T. Gastrin and glycine-extended progastrin processing intermediates induce different programs of early gene activation. J Biol Chem. 1995 Nov 24;270(47):28337–28341. doi: 10.1074/jbc.270.47.28337. [DOI] [PubMed] [Google Scholar]
- Varro A., Henry J., Vaillant C., Dockray G. J. Discrimination between temperature- and brefeldin A-sensitive steps in the sulfation, phosphorylation, and cleavage of progastrin and its derivatives. J Biol Chem. 1994 Aug 12;269(32):20764–20770. [PubMed] [Google Scholar]
- Varro A., Voronina S., Dockray G. J. Pathways of processing of the gastrin precursor in rat antral mucosa. J Clin Invest. 1995 Apr;95(4):1642–1649. doi: 10.1172/JCI117839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varro A., Yegen B., Dockray G. J. Control of tissue progastrin concentrations in the rat. Exp Physiol. 1993 May;78(3):327–336. doi: 10.1113/expphysiol.1993.sp003688. [DOI] [PubMed] [Google Scholar]
- Waldbieser G. C., Aimi J., Dixon J. E. Cloning and characterization of the rat complementary deoxyribonucleic acid and gene encoding the neuroendocrine peptide 7B2. Endocrinology. 1991 Jun;128(6):3228–3236. doi: 10.1210/endo-128-6-3228. [DOI] [PubMed] [Google Scholar]
- Walsh J. H., Debas H. T., Grossman M. I. Pure human big gastrin. Immunochemical properties, disappearance half time, and acid-stimulating action in dogs. J Clin Invest. 1974 Aug;54(2):477–485. doi: 10.1172/JCI107783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. H., Isenberg J. I., Ansfield J., Maxwell V. Clearance and acid-stimulating action of human big and little gastrins in duodenal ulcer subjects. J Clin Invest. 1976 May;57(5):1125–1131. doi: 10.1172/JCI108379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. H., Richardson C. T., Fordtran J. S. pH dependence of acid secretion and gastrin release in normal and ulcer subjects. J Clin Invest. 1975 Mar;55(3):462–468. doi: 10.1172/JCI107952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. C., Koh T. J., Varro A., Cahill R. J., Dangler C. A., Fox J. G., Dockray G. J. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest. 1996 Oct 15;98(8):1918–1929. doi: 10.1172/JCI118993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. V., Giraud A., Mogard M., Sumii K., Walsh J. H. Effects of inhibition of gastric secretion on antral gastrin and somatostatin gene expression in rats. Am J Physiol. 1990 May;258(5 Pt 1):G788–G793. doi: 10.1152/ajpgi.1990.258.5.G788. [DOI] [PubMed] [Google Scholar]
- Zhu X., Lindberg I. 7B2 facilitates the maturation of proPC2 in neuroendocrine cells and is required for the expression of enzymatic activity. J Cell Biol. 1995 Jun;129(6):1641–1650. doi: 10.1083/jcb.129.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]