Abstract
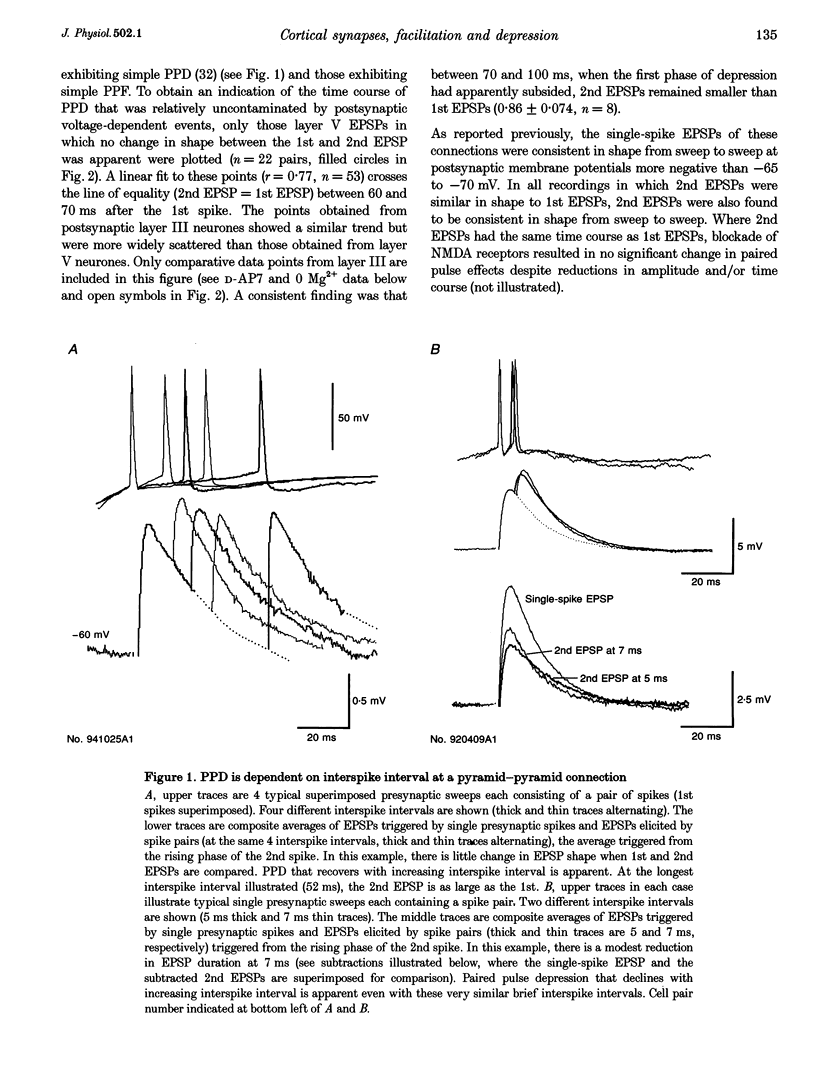
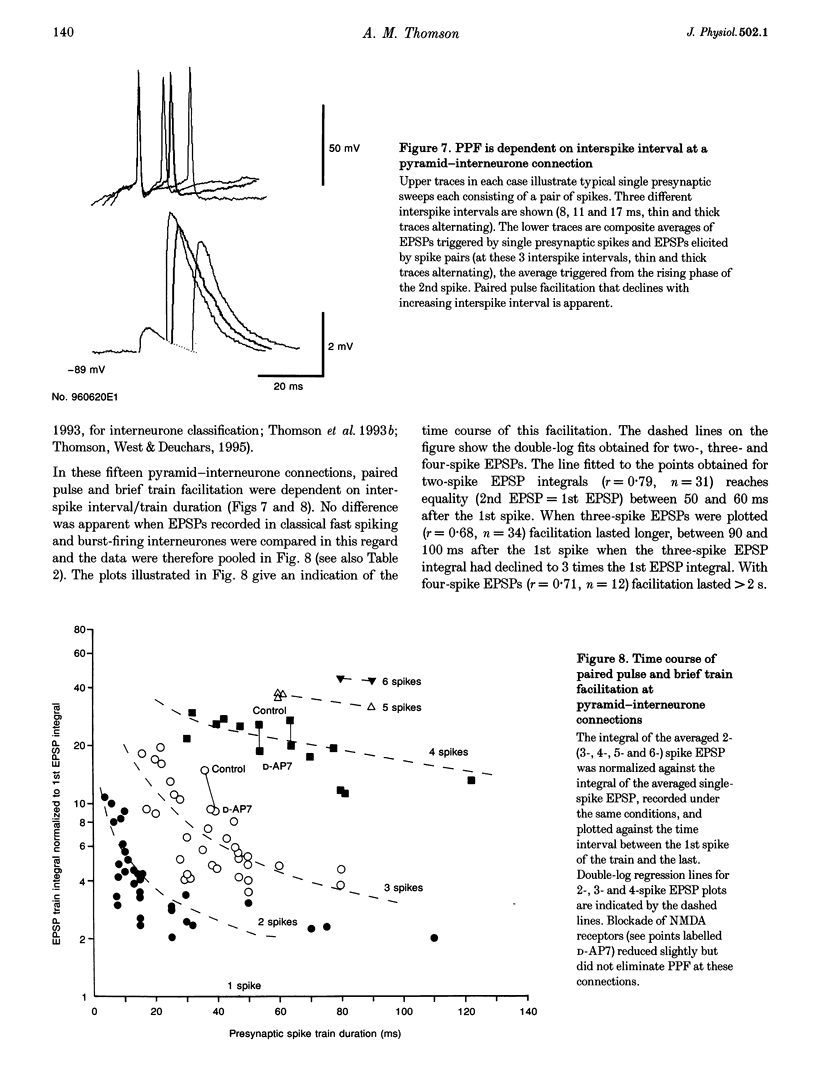
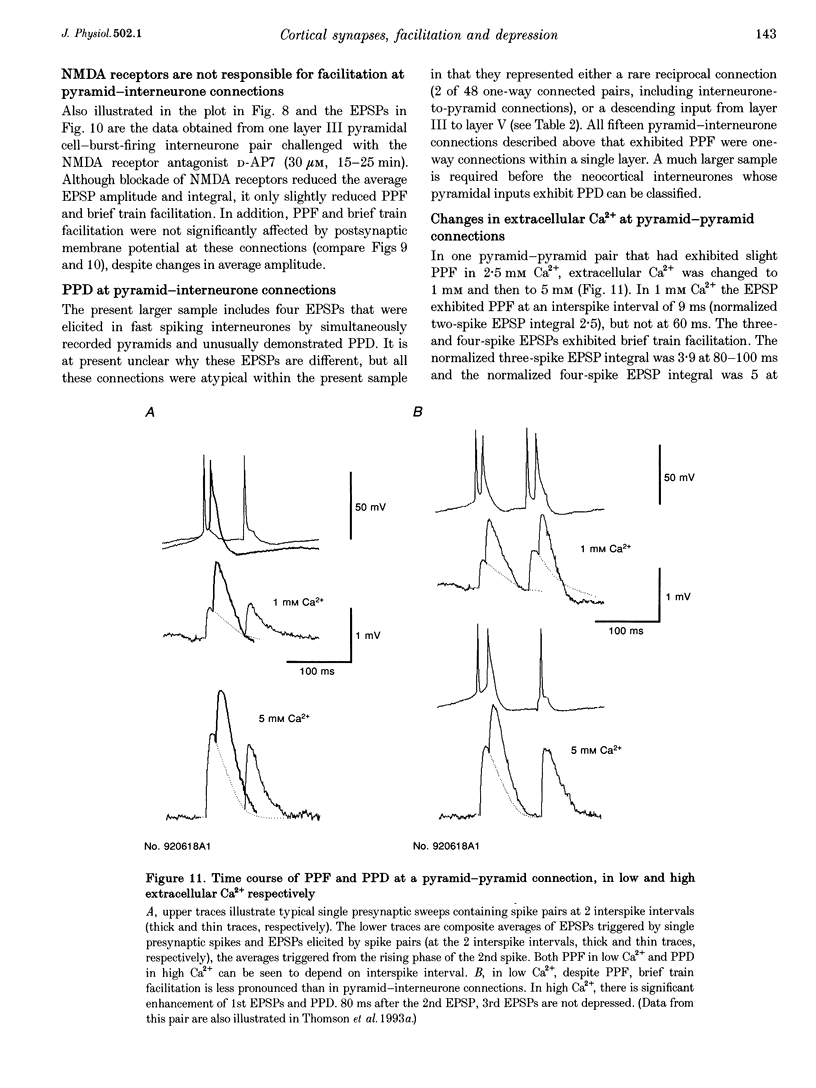
1. To compare the dynamics of synaptic transmission at different types of connection, dual intracellular recordings were made from pairs of neurones in slices of adult rat neocortex. Excitatory postsynaptic potentials (EPSPs) were elicited by single spikes, spike pairs and brief spike trains in presynaptic pyramidal cells and responses recorded in postsynaptic pyramidal cells and in interneurones. 2. Pyramid-pyramid EPSPs were strongly voltage dependent and this resulted in a range of paired pulse effects. At thirty-two of sixty-nine pyramid-pyramid connections, the 2nd EPSP was the same shape as the 1st, indicating minimal interaction between active synapses. In these thirty-two connections, paired pulse depression (PPD) was apparent (2nd EPSP integral 46 +/- 21% of the 1st, at 5-20 ms), which recovered within 60-70 ms. 3. In eleven additional pyramid-pyramid pairs, the 2nd EPSP was also the same shape as the 1st, but paired pulse facilitation (PPF, 149 +/- 32%) decaying within 50-60 ms was apparent. Even these connections displayed frequency-dependent depression, however, as 3rd EPSPs were smaller than 1st EPSPs at intervals < 100 ms. 4. At twenty-five pyramid-pyramid connections, 2nd EPSPs were broader than 1st EPSPs and in sixteen of these, voltage- and NMDA receptor-dependent enhancement was large enough to obscure the underlying PPD. PPD was revealed by postsynaptic hyperpolarization (4 pairs), N-methyl-D-aspartate (NMDA) receptor blockade (3 paris), or if Mg2+ was removed (in the one case studied). If synapse location allowed significant depolarization of one active site by another, voltage-dependent enhancement could produce supralinear EPSP summation and overcome PPD. Third EPSPs were, however, consistently smaller than 1st EPSPs. 5. In striking contrast, profound frequency-dependent facilitation, independent of voltage or NMDA receptors was seen at fifteen connections involving two classes of postsynaptic interneurones. 6. At these pyramid-interneurone connections, facilitation of the 2nd EPSP (655 +/- 380% at 5-20 ms) decayed rapidly, within 50-60 ms. Third and fourth EPSPs showed additional facilitation which decayed more slowly, within 90 ms and 2 s, respectively. Facilitation due to five to six spike trains was still apparent at 3 s. Therefore, once initiated by a brief high frequency spike train, facilitation was maintained at lower frequencies.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreasen M., Lambert J. D. The excitability of CA1 pyramidal cell dendrites is modulated by a local Ca(2+)-dependent K(+)-conductance. Brain Res. 1995 Nov 6;698(1-2):193–203. doi: 10.1016/0006-8993(95)00910-i. [DOI] [PubMed] [Google Scholar]
- Betz W. J. Depression of transmitter release at the neuromuscular junction of the frog. J Physiol. 1970 Mar;206(3):629–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Biophysical aspects of neuro-muscular transmission. Prog Biophys Biophys Chem. 1956;6:121–170. [PubMed] [Google Scholar]
- Davis G. W., Murphey R. K. A role for postsynaptic neurons in determining presynaptic release properties in the cricket CNS: evidence for retrograde control of facilitation. J Neurosci. 1993 Sep;13(9):3827–3838. doi: 10.1523/JNEUROSCI.13-09-03827.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz R. A., Fortin G., Zieglgänsberger W. Voltage dependence of excitatory postsynaptic potentials of rat neocortical neurons. J Neurophysiol. 1991 Feb;65(2):371–382. doi: 10.1152/jn.1991.65.2.371. [DOI] [PubMed] [Google Scholar]
- Deuchars J., Thomson A. M. Innervation of burst firing spiny interneurons by pyramidal cells in deep layers of rat somatomotor cortex: paired intracellular recordings with biocytin filling. Neuroscience. 1995 Dec;69(3):739–755. doi: 10.1016/0306-4522(95)00288-t. [DOI] [PubMed] [Google Scholar]
- Deuchars J., West D. C., Thomson A. M. Relationships between morphology and physiology of pyramid-pyramid single axon connections in rat neocortex in vitro. J Physiol. 1994 Aug 1;478(Pt 3):423–435. doi: 10.1113/jphysiol.1994.sp020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind C. K., Pearce J., Wojtowicz J. M., Atwood H. L. "Strong" and "weak" synaptic differentiation in the crayfish opener muscle: structural correlates. Synapse. 1994 Jan;16(1):45–58. doi: 10.1002/syn.890160106. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Further study of the role of calcium in synaptic transmission. J Physiol. 1970 May;207(3):789–801. doi: 10.1113/jphysiol.1970.sp009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz P. S., Kirk M. D., Govind C. K. Facilitation and depression at different branches of the same motor axon: evidence for presynaptic differences in release. J Neurosci. 1993 Jul;13(7):3075–3089. doi: 10.1523/JNEUROSCI.13-07-03075.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Groupings of nonpyramidal and pyramidal cells with specific physiological and morphological characteristics in rat frontal cortex. J Neurophysiol. 1993 Feb;69(2):416–431. doi: 10.1152/jn.1993.69.2.416. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Kubota Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J Neurophysiol. 1993 Jul;70(1):387–396. doi: 10.1152/jn.1993.70.1.387. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. J Neurosci. 1995 Apr;15(4):2638–2655. doi: 10.1523/JNEUROSCI.15-04-02638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDBERG A., QUILISCH H. Presynaptic potentiation and depression of neuromuscular transmission in frog and rat. Acta Physiol Scand Suppl. 1953;111:111–120. [PubMed] [Google Scholar]
- Lübke J., Markram H., Frotscher M., Sakmann B. Frequency and dendritic distribution of autapses established by layer 5 pyramidal neurons in the developing rat neocortex: comparison with synaptic innervation of adjacent neurons of the same class. J Neurosci. 1996 May 15;16(10):3209–3218. doi: 10.1523/JNEUROSCI.16-10-03209.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee J. C., Christofi G., Miyakawa H., Christie B., Lasser-Ross N., Johnston D. Subthreshold synaptic activation of voltage-gated Ca2+ channels mediates a localized Ca2+ influx into the dendrites of hippocampal pyramidal neurons. J Neurophysiol. 1995 Sep;74(3):1335–1342. doi: 10.1152/jn.1995.74.3.1335. [DOI] [PubMed] [Google Scholar]
- Magee J. C., Johnston D. Synaptic activation of voltage-gated channels in the dendrites of hippocampal pyramidal neurons. Science. 1995 Apr 14;268(5208):301–304. doi: 10.1126/science.7716525. [DOI] [PubMed] [Google Scholar]
- Markram H., Sakmann B. Calcium transients in dendrites of neocortical neurons evoked by single subthreshold excitatory postsynaptic potentials via low-voltage-activated calcium channels. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5207–5211. doi: 10.1073/pnas.91.11.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H., Tsodyks M. Redistribution of synaptic efficacy between neocortical pyramidal neurons. Nature. 1996 Aug 29;382(6594):807–810. doi: 10.1038/382807a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Crill W. E. Amplification of synaptic current by persistent sodium conductance in apical dendrite of neocortical neurons. J Neurophysiol. 1995 Nov;74(5):2220–2224. doi: 10.1152/jn.1995.74.5.2220. [DOI] [PubMed] [Google Scholar]
- Silver R. A., Traynelis S. F., Cull-Candy S. G. Rapid-time-course miniature and evoked excitatory currents at cerebellar synapses in situ. Nature. 1992 Jan 9;355(6356):163–166. doi: 10.1038/355163a0. [DOI] [PubMed] [Google Scholar]
- Smith S. J., Augustine G. J., Charlton M. P. Transmission at voltage-clamped giant synapse of the squid: evidence for cooperativity of presynaptic calcium action. Proc Natl Acad Sci U S A. 1985 Jan;82(2):622–625. doi: 10.1073/pnas.82.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. F. Calcium currents in a vertebrate presynaptic nerve terminal: the chick ciliary ganglion calyx. Brain Res. 1989 Dec 29;505(2):341–345. doi: 10.1016/0006-8993(89)91465-0. [DOI] [PubMed] [Google Scholar]
- Stanley E. F. Presynaptic calcium channels and the transmitter release mechanism. Ann N Y Acad Sci. 1993 Jun 21;681:368–372. doi: 10.1111/j.1749-6632.1993.tb22915.x. [DOI] [PubMed] [Google Scholar]
- Stevens C. F., Wang Y. Facilitation and depression at single central synapses. Neuron. 1995 Apr;14(4):795–802. doi: 10.1016/0896-6273(95)90223-6. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., Deuchars J., West D. C. Large, deep layer pyramid-pyramid single axon EPSPs in slices of rat motor cortex display paired pulse and frequency-dependent depression, mediated presynaptically and self-facilitation, mediated postsynaptically. J Neurophysiol. 1993 Dec;70(6):2354–2369. doi: 10.1152/jn.1993.70.6.2354. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., Deuchars J., West D. C. Single axon excitatory postsynaptic potentials in neocortical interneurons exhibit pronounced paired pulse facilitation. Neuroscience. 1993 May;54(2):347–360. doi: 10.1016/0306-4522(93)90257-g. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., Girdlestone D., West D. C. A local circuit neocortical synapse that operates via both NMDA and non-NMDA receptors. Br J Pharmacol. 1989 Feb;96(2):406–408. doi: 10.1111/j.1476-5381.1989.tb11831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. M., Girdlestone D., West D. C. Voltage-dependent currents prolong single-axon postsynaptic potentials in layer III pyramidal neurons in rat neocortical slices. J Neurophysiol. 1988 Dec;60(6):1896–1907. doi: 10.1152/jn.1988.60.6.1896. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., West D. C., Deuchars J. Properties of single axon excitatory postsynaptic potentials elicited in spiny interneurons by action potentials in pyramidal neurons in slices of rat neocortex. Neuroscience. 1995 Dec;69(3):727–738. doi: 10.1016/0306-4522(95)00287-s. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., West D. C. Fluctuations in pyramid-pyramid excitatory postsynaptic potentials modified by presynaptic firing pattern and postsynaptic membrane potential using paired intracellular recordings in rat neocortex. Neuroscience. 1993 May;54(2):329–346. doi: 10.1016/0306-4522(93)90256-f. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., West D. C., Hahn J., Deuchars J. Single axon IPSPs elicited in pyramidal cells by three classes of interneurones in slices of rat neocortex. J Physiol. 1996 Oct 1;496(Pt 1):81–102. doi: 10.1113/jphysiol.1996.sp021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell L. O., Thio L. L., Zorumski C. F., Fischbach G. D. Rapid desensitization of glutamate receptors in vertebrate central neurons. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2834–2838. doi: 10.1073/pnas.85.8.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell L. O., Zhang S., Raman I. M. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron. 1993 Jun;10(6):1185–1196. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- Walrond J. P., Govind C. K., Huestis S. E. Two structural adaptations for regulating transmitter release at lobster neuromuscular synapses. J Neurosci. 1993 Nov;13(11):4831–4845. doi: 10.1523/JNEUROSCI.13-11-04831.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S., Fogelson A. L. Relationship between transmitter release and presynaptic calcium influx when calcium enters through discrete channels. Proc Natl Acad Sci U S A. 1986 May;83(9):3032–3036. doi: 10.1073/pnas.83.9.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]


